Abstract
Background: This retrospective study aimed to evaluate the characteristics of patients with long COVID syndrome. Methods: This study included 457 adults who had at least one persistent symptom after COVID-19 infection. Results: The median time interval between the last SARS-CoV-2 infection and emergency room presentation was 3 months. Older patients had comorbidities (61.7 vs. 44.9 years, p < 0.0001), moderate or severe forms of COVID-19 (61.2 vs. 50.9 years, p < 0.0001), and respiratory symptoms (56.1 vs. 52.0 years, p = 0.0027). Non-vaccinated patients were older than vaccinated patients (56.0 vs. 51.5 years, p = 0.0008) and had residual lung abnormalities following COVID-19 infection (51.5% vs. 36.8%, p < 0.003). The time interval between the last SARS-CoV-2 infection and the hospital evaluation was shorter for vaccinated patients (3.2 vs. 3.9 months, p < 0.0001) and those with mild forms (3.3 vs. 4.12 months, p = 0.0001) versus non-vaccinated individuals. After the last SARS-CoV-2 infection, 107 patients developed impaired fasting glucose, impaired glucose tolerance, or diabetes mellitus, being patients with already known chronic diseases (p = 0.0002), or hypertension (p = 0.001). Conclusions: Our study pointed out the heterogeneity of symptoms following COVID-19, and they are associated with age, vaccination status, or severity of SARS-CoV-2 infection.
1. Introduction
COVID-19, caused by the SARS-CoV-2 virus [1], is responsible for the latest pandemic SARS-CoV-2 enters the cells using the angiotensin-converting enzyme 2 (ACE2) [2] to bind with its Spike protein receptor. The spike protein contains the receptor-binding domain (RBD), which attaches to the ACE2 receptor by its six amino acid residues [3]. To complete entry into the cell, SARS-CoV-2 uses the active protease TMPRSS2 to ensure the link between spike protein and ACE2 [4]. Further, the infection induces a complex immune response associated with the synthesis of interleukins IL-6, IL-1β, IL-1α, IL-2, IL-7, IL-8, tumor necrosis factor (TNF-α), interferon-γ (IFN-γ), and C-reactive protein (CRP) [5,6]. Their release causes a cytokine storm, which leads to taste bud stem cell apoptosis, disrupting their renewal and thus affecting the taste function [7]. The majority (~80%) of the affected individuals develop mild to moderate forms of the disease, but some patients (5%) develop a critical or even fatal form of illness [1,8]. An acute COVID-19 episode lasts around five weeks, during which viral infection disables host cellular machinery, leading to cell death. These molecular events will lead to systemic hyperinflammation, followed by various injuries such as thrombosis, multi-organ failure, and even death [9]. ACE2 receptors are found in the epithelia of the respiratory, cardiovascular, and urogenital systems, gastrointestinal tract, liver, gallbladder, and nervous system [10]. The severity of this virus infection is closely related to the ACE2 capacity for binding and maturity [2]. In 70–80% of coronavirus-infected patients, it was reported that severe respiratory distress was observed by radiologic examination [11]. Regarding cardiac symptoms after COVID-19 infection, chest pain, palpitations, and postural tachycardia syndrome were recorded in patients after discharge from the hospital after an acute episode of infection [12]. Pre-existing health pathologies, such as cardiovascular diseases (CVDs), diabetes, obesity, liver and kidney damage, cancer, vitamin D deficiency, Epstein–Barr virus, and Parkinson’s disease, may enhance COVID-19 severity in both young and older populations [13,14]. Usually, this viral infection has a worse evolution when older age, male sex, and race (particularly black, Hispanic, and South Asian) are associated with various pre-existing pathologies [15]. Studies have shown that ongoing symptoms after the COVID-19 infection were more frequently reported in hospitalised patients than non-hospitalised patients, who reported only fatigue and cognitive impairment [16]. Interestingly, in patients who experienced long COVID-19 syndrome, neurological, neuropsychiatric, cardiopulmonary, gastrointestinal, and other complications (primary rheumatological complications) were significantly more often observed in female than in male patients [17,18,19,20].
SARS-CoV-2 may cause a post-acute syndrome with heterogeneous manifestations [21]. Long-term COVID-19 can affect a wide range of patients, from those with mild/moderate issues to those with severe forms, and involves various organs and systems such as the respiratory, cardiovascular, gastrointestinal, musculoskeletal, and neurological systems. Moreover, between 10 and 65% of patients who had mild or moderate COVID-19 present post-COVID-19 syndrome, characterised by fatigue, anxiety, depression, dyspnea, and impaired attention, concentration, and sleep [22,23,24,25,26]. Patients with persistent cognitive impairment syndrome, also called COVID-19 brain fog, reported perturbations regarding attention, memory, concentration, executive function, and information processing speed. Neuroinflammation is responsible for this situation, causing glial and neuronal cell dysregulation [27].
The risk of developing severe or fatal forms of the disease or persistent symptoms was significantly reduced by immunization with COVID-19 vaccines [17,28,29]. The high cumulative prevalence (9–63%) of long COVID has placed more than 100 million people in front of healthcare professionals [17,18,19,20]. The increased number of patients who complained of a decrease in the quality of life after SARS-CoV-2 infection and the large number of unscheduled return visits or repeat consultations led to the opening of the Centre for Evaluation of COVID-19 in the Carol Davila Military Emergency Hospital (CEC-CD) at the beginning of December 2021.
This retrospective study evaluated the characteristics of patients with persistent COVID-19 symptoms and unscheduled visits to the CEC-CD.
2. Materials and Methods
2.1. Medical Records
In this retrospective study, we evaluated medical records for 457 adults with at least one persisting symptom after COVID-19 and unscheduled visits to the CEC-CD between December 2021–June 2022, after the approval of the ethics committee of the CEC-CD (No. 620/09.08.2023). The methodology of this study is in accordance with the Helsinki Declaration.
The records refer to the different clinical characteristics of the patients, including those related to COVID-19 disease (e.g., disease severity, treatment, vaccination status). The most important persisting symptoms with onset during or after SARS-CoV-2 infection (e.g., fatigue, dyspnea, coughing, and headache, consisting of palpitations, chest pain, allergic reactions, and muscle and joint pain) were considered based on self-report. The data regarding myocarditis (based on angina, dyspnea, fatigue, elevated high sensitivity Troponin I-hs-TnI values, and suggestive echocardiography) and cardiovascular symptoms (e.g., palpitations, precordial pains, dyspnea), the presence of inflammatory syndrome (based on CRP values, increased fibrinogen levels, and erythrocyte sedimentation rate-ESR values), and residual lung involvement (identified by conventional radiology or CT) were also evaluated. Hs-TnI was determined by chemiluminescence assay, CRP using an automatic analyzer, ESR on an automatic reader, and fibrinogen by the Clauss method. The dataset used for this study did not include data from patients with mental illnesses, chronic dyspnea, or fatigue before COVID-19; an incomplete immunization schedule (e.g., one dose of vaccine from BioNTech (Mainz, Germany) or MODERNA (Cambridge, MA, USA)); or vaccination less than 2 weeks before infection with COVID-19.
2.2. Statistical Analysis
Statistical analysis was primarily performed by using RStudio for Windows. The Student’s t-test or Mann–Whitney U-test were used to compare the groups of data. The age of patients or the interval between the last SARS-CoV-2 infection and unscheduled return visits to the CEC-CD that exceed the mean value ± 3 standard deviations were considered outliers and were excluded from the analysis. The Bonferroni correction was used to adjust probability (p) values for 16 variables, and we considered the differences to be statistically significant if p< 0.003.
3. Results
This dataset includes medical records for adults who had at least one persisting symptom after COVID-19 which caused an unscheduled visit to the CEC-CD (Table 1).

Table 1.
Characteristics of subjects involved in the study.
The mean age of patients at presentation in the CEC-CD was 53.85 (range 18–91) years old; most of them belonged to the age group 40–69 years (73.4% of women; 63.9% of men) and had mild clinical COVID-19 symptoms (Figure 1).
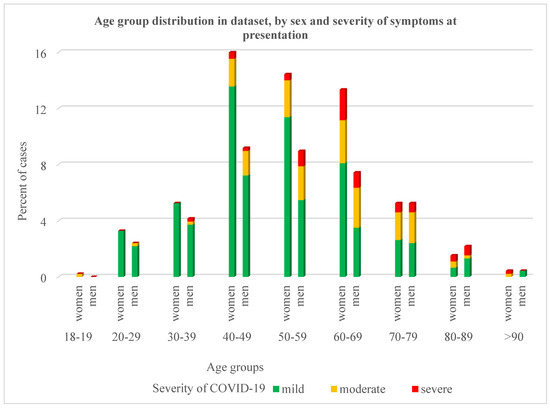
Figure 1.
The distribution of cases from the dataset by age group, gender, and severity of symptoms at presentation to the CEC-CD.
The age at presentation was similar in both genders (p > 0.05) and in the subgroup of subjects under (women vs. men: 50.06 ± 11.58 vs. 49.3± 11.7 years old) or over (women vs. men: 76.67 ± 5.18 vs. 76.86 ± 6.32) the age of 70 years old. However, age presented significant differences between different subgroups of subjects. Patients who had comorbidities, moderate or severe forms of COVID-19, and respiratory symptoms (dyspnea, cough) were older compared to those who did not have these characteristics (Table 2).

Table 2.
Age differences between subjects, stratified according to different characteristics.
The age of patients who had myocarditis (58.0 vs. 53.4 years old) or inflammatory syndrome (57.2 vs. 53.1 years old) tends to be higher (p = 0.02) than in those without these syndromes. Lung damage was present in 92 males and 111 females (44.42% of patients) from our dataset; 50.2% of them (43 males and 59 females) reported respiratory problems at the moment of presentation at the CEC-CD. Patients with post-COVID residual pulmonary involvement were older than those without such manifestations (58.78 vs. 49.91 years, p< 0.0001); they were more frequently identified in the non-vaccinated than in the vaccinated lot (51.48% vs. 36.82%, p = 0.002). An X-ray or CT scan performed during evaluation in the CEC-CD revealed that patients who had moderate forms of COVID-19 have different degrees of pulmonary fibrosis, whereas those with severe forms of SARS-CoV-2 infection showed significant pulmonary fibrosis, which may be associated with persistent ground-glass opacities. At least two signs or symptoms at the CEC-CD presentation were reported more frequently by patients who had chronic diseases compared to those without medical history (59.02% vs. 42.72%, p = 0.0005). A different distribution of medical history was identified in patients stratified according to the clinical severity of COVID-19 (Table 3).

Table 3.
Significant differences between patients with moderate or severe forms and those with a mild form of COVID-19.
Atopies were more frequently reported by women than by men (30.29% vs. 15.85%, p = 0.0004). The symptoms noted consisted of significant oculo-nasal catarrh and skin erythema in individuals without an allergic history. The age of patients with such symptoms seems lower compared to those who denied the existence of these manifestations (51.40 vs. 54.64 years old, p < 0.05).
The median time interval between the last SARS-CoV-2 infection and the CEC-CD presentation was 3 months (interval 1–15 months). For sixteen patients, this interval was considered an outlier. The distribution of remaining values was different in subjects stratified according to vaccination status, hypertension, the clinical form of COVID-19, or the treatment administered (Table 4).

Table 4.
Time interval between the last SARS-CoV-2 infection and admission to CEC-CD.
Vaccinated patients, compared with those unvaccinated, were significantly younger (51.5 vs. 56.0 years, p < 0.001), had a mild form of COVID-19 (77.72% vs. 64.97%, p = 0.003), and had a shorter time interval between clinical onset of this disease to unscheduled visits to the CEC-CD (3.2 vs. 3.9 months, p < 0.0001). This time interval was also shorter in patients who had mild forms of COVID-19 compared to those with more severe forms of the disease (3.31 vs. 4.12 months, p = 0.0001).
Fifty patients were diagnosed with hypertension after COVID-19. They represent 22.22% of the patients with previous CVDs and 7.45% from the sublot of patients without these health problems (p < 0.0001). Patients with new-onset diabetes mellitus were more frequently detected in the subgroup of patients who had comorbidities (30.33% vs. 15.49%, p < 0.0002) or hypertension (42% vs. 21.13%, p = 0.001).
4. Discussion
There was estimated to be a significant number of cases of long COVID in Romania (2020: 79,500 cases; 2021: 415,000) [30]. The Romanian Ministry of Health decided in December 2021 to establish health assessment centres for patients with COVID-19 to relieve Emergency Units. In the CEC-CD, most patients were evaluated in the first six months after establishment, after which patient presentation decreased significantly. This observation shows why the evaluated dataset refers to patients who had unscheduled visits to the CEC-CD between December 2021–June 2022.
In a meta-analysis report, it was noted that 80% of the patients with a confirmed diagnosis of COVID-19 continued to have at least one overall effect beyond 2 weeks following acute infection [31]. A relevant physical and functional recovery during the first year after COVID-19 infection was observed for a significant percentage of patients, although in some patients, symptoms persisted for a longer period [32,33,34]. It was estimated that the post-COVID symptoms affect 7.8–35% of COVID-19 patients (this figure may be even higher in some particular groups of patients) [35,36,37,38].
We retrospectively investigated the post-COVID-19 persistent symptoms of 457 patients with mild or moderate general conditions who had unscheduled visits to the CEC-CD. Our results, like other previously published data, reveal that some manifestations of post-COVID syndrome such as cardio-vascular involvement) develop more frequently in women [39,40]. The risk of developing long COVID was estimated to be several times lower in patients in their twenties than in people in their sixties (1–2% vs. 5%) [41]. Generally, it is considered that older patients with COVID-19 (or at least until 70 years of age) have a higher risk of long-term COVID-19 and are prone to worse outcomes than younger patients [37,42,43,44]. In our study, 69 patients were at least 70 years old (36 were male; 41 were unvaccinated). Gender was not associated with statistically significant differences between subgroups of patients aged <70 or ≥70 years. However, the age of vaccinated patients is significantly lower compared to the age of unvaccinated subjects (51.5 vs. 56.03, p < 0.001).
The consensus WHO definition mentions that post-COVID-19 occurs usually 3 months from the onset of COVID-19 [18]. In our dataset, 34.57% of the unscheduled visits to the CEC-CD occurred in the first 2 months after the last episode of COVID-19. This figure can be explained by the persistence of symptoms (in particular dyspnea and fatigue) that negatively influence the quality of life after SARS-CoV-2 infection, thus causing patients to refer to the CEC-CD for medical evaluation. This interval was also shorter for patients vaccinated before the last COVID-19 outbreak compared to those not vaccinated (3.17 vs. 3.90, p = 0.0001). Also, 1.09% of the subjects presented at the CEC-CD 13–15 months after the diagnosis of the viral infection with SARS-CoV-2. During this interval, all of these patients had at least one episode of symptoms specific to SARS-CoV-2 infection, but they did not perform a diagnostic test. This result is consistent with the data from the literature that claims that post-COVID symptoms tend to decrease progressively in the first year after the infection [45,46,47].
Residual lung lesions may be correlated with functional involvement and, at least in some patients, with the respiratory symptoms reported in long COVID [48]. A prospective study described a temporary improvement in pulmonary physiology for the majority of patients; however, 24% of patients who had a severe form of COVID-19 without the requirement for mechanical ventilation did not fully resolve lung radiological changes at 12 months after discharge [49]. Our results are concordant with these findings: 44.42% of the patients had abnormal chest imaging findings, and 50.24% of them also reported respiratory complaints at presentation.
Fatigue (51.0%), respiratory (60.4%), and cognitive (35.4%) symptoms are often alleged by patients with long COVID [30,50,51]. Meta-analysis revealed that a significant proportion of individuals have persistent fatigue and/or cognitive impairment following the resolution of acute COVID-19 [52,53]. Patients with dyspnea or fatigue prior to COVID-19 were not selected for this study in an attempt to avoid bias caused by chronic fatigue syndrome [54]. General complaints (e.g., physical asthenia—65.86%) were more frequent, and neurological manifestations (e.g., paresthesia, concentration, and memory disorders—22.54%) were rarer in our dataset compared to the values reported in the literature. Respiratory manifestations (e.g., dyspnea, coughing) were reported by 44.42% of the subjects from our dataset, a value that is in the range of values reported in the literature (dyspnea in post-COVID—36.0–74.3%) [55,56]. Fatigue is not only the most common symptom of COVID-19 but also a persistent one [34,57,58]. A retrospective survey study developed between 1 October and 31 December 2020 reported that fatigue was the main persistent symptom (36%) and was several times more frequent than dyspnea (10.3%) in Romanian patients who had COVID-19 [59]. In our study, 29.54% of the subjects reported general complaints more than 3 months after the onset of COVID-19, and most of them (65.92%) were not vaccinated. Different inclusion criteria may contribute to the higher frequency of general symptoms estimated in our study.
Metabolic dysfunctions seem to be a risk factor for both severe acute and post-acute COVID-19 sequelae [60]. The relationship between type 2 diabetes mellitus and COVID-19 seems bidirectional [61,62]. In the present study, the most common conditions identified after the detection of SARS-CoV-2 infection were impaired fasting glucose, impaired glucose tolerance, and diabetes mellitus (107 cases); 33 of these cases had no other comorbidities.
It has been estimated that the risk of long COVID was up to 42% in unvaccinated individuals and decreased in those who have received a single vaccine dose (30%), two vaccine doses (17%), or a booster vaccine (16%) [63,64,65]. The complete vaccination schedule was reported by 48.14% of subjects from our database. The time interval between the second dose of vaccine to infection with SARS-CoV-2 was 14–180 days. We observed that the age of the subjects (51.5 vs. 56.03 years old, p = 0.001) and the time interval from infection to an evaluation in the CEC-CD (3.17 vs. 3.90 months, p = 0.0001) were lower for these individuals compared to the values identified for unvaccinated subjects.
5. Conclusions
The post-COVID symptoms are heterogeneous and persistent. Age, vaccination status, and the severity of COVID-19 were associated with different characteristics of patients with post-COVID symptoms with unscheduled visits to the CEC-CD. The symptoms for which the patients presented to this medical center—fatigue, dyspnea, and coughing—have caused a long-term (months) quality of life impairment. This aspect can have an important social impact since many of these patients are active people who were absent from work or had a low yield of the activities carried out. The medical evaluation of these patients with long COVID-19 revealed the new onset of certain chronic pathologies after SARS-CoV-2 infection: hypertension, diabetes mellitus, and atopy. A significant percentage of vaccinated people presented a mild form ofda COVID-19. As a result, residual lung damages were identified in significantly lower numbers in vaccinated patients compared to unvaccinated ones.
Author Contributions
Conceptualization, S.N., R.I.N. and D.G.C.; methodology, H.A.N., M.A.A.K.A., O.M.S., I.C.N. and M.C.C.; software, D.M., C.S. and H.A.N.; validation, R.I.N., S.N., D.G.C., D.M. and M.C.C.; formal analysis, I.-I.S.-S., M.A.A.K.A., O.M.S. and I.C.N.; investigation, D.G.C. and D.M.; resources, R.I.N. and S.N.; data curation, I.C.N., D.G.C. and M.C.C.; writing—original draft preparation, D.G.C.; writing—review and editing, D.M., D.G.C., R.I.N., S.N. and M.C.C.; visualization, I.-I.S.-S., M.A.A.K.A., O.M.S., I.C.N. and C.S.; supervision, R.I.N., S.N. and D.M.; project administration, S.N., R.I.N. and D.G.C.; funding acquisition, D.M., H.A.N. and I.C.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Institutional Review Board Statement
The study was performed after obtaining the Agreement of the Ethics Committee of Carol Davila Military Emergency Hospital, No. 620/09.08.2023. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the reported results are available from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; David, P.; Arnheim, D.; Shoenfeld, Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun. Rev. 2022, 21, 103071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, R.; Ma, H.; Zhang, W. The Pathogenesis of COVID-19-Related Taste Disorder and Treatments. J. Dent. Res. 2023, 102, 1191–1198. [Google Scholar] [CrossRef]
- Morsali, S.; Rezazadeh-Gavgani, E.; Oladghaffari, M.; Bahramian, S.; Hamzehzadeh, S.; Samadifar, Z.; Enamzadeh, E.; Sheikhalipour, Z.; Moradi, H.; Pourmehr, H.S.; et al. Effects of underlying heart failure on outcomes of COVID-19; a systematic review and meta-analysis. Rom. J. Intern. Med. 2023, 61, 6–27. [Google Scholar] [CrossRef]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert. Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 1, C1–C11. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; May, C.A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol. Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef] [PubMed]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe COVID-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 Vaccination on the Risk of Developing Long-COVID and on Existing Long-COVID Symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19): Post COVID-19 Condition; World Health Organization: Geneva, Switzerland, 2023. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 20 February 2024).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. Case definition working group on post-COVID-19 condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1284437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Wesselingh, R. Prevalence, pathogenesis and spectrum of neurological symptoms in COVID-19 and post-COVID-19 syndrome: A narrative review. Med. J. Aust. 2023, 219, 230–236. [Google Scholar] [CrossRef]
- Borczuk, A.C. Pathogenesis of Pulmonary Long COVID-19. Mod. Pathol. 2024, 37, 100378. [Google Scholar] [CrossRef] [PubMed]
- Baimukhamedov, C. How long is long COVID. Int. J. Rheum. Dis. 2023, 26, 190–192. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Qasmieh, S.A.; Kulkarni, S.G.; Teasdale, C.A.; Jones, H.E.; McNairy, M.; Borrell, L.N.; Nash, D. The Epidemiology of long coronavirus disease in US adults. Clin. Infect. Dis. 2023, 76, 1636–1645. [Google Scholar] [CrossRef]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Meagher, T. Long COVID—Into the third year. J. Insur. Med. 2023, 50, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.K.; Yu, M.W.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An insight on its pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Al-Shamrani, A.; Al-Shamrani, K.; Al-Otaibi, M.; Alenazi, A.; Aldosaimani, H.; Aldhalaan, Z.; Alalkami, H.; Yousef, A.A.; Kobeisy, S.; Alharbi, S. Residual cough and asthma-like symptoms post-COVID-19 in children. Children 2023, 10, 031. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Caracterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Bucciarelli, V.; Nasi, M.; Bianco, F.; Seferovic, J.; Ivkovic, V.; Gallina, S.; Mattioli, A.V. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: Gender makes a difference. Trends Cardiovasc. Med. 2022, 32, 12–17. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Kathuria, A.; Al Mahmeed, W.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; Cosentino, F.; et al. Cardiometabolic panel of international experts on syndemic COVID-19 (CAPISCO). Post-COVID syndrome, inflammation, and diabetes. J. Diabetes Complicat. 2022, 36, 108336. [Google Scholar] [CrossRef]
- Dadras, O.; SeyedAlinaghi, S.; Karimi, A.; Shamsabadi, A.; Qaderi, K.; Ramezani, M.; Mirghaderi, S.P.; Mahdiabadi, S.; Vahedi, F.; Saeidi, S.; et al. COVID-19 mortality and its predictors in the elderly: A systematic review. Health Sci. Rep. 2022, 5, e657. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; Harris, A.M. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Nalbandian, A.; Desai, A.D.; Wan, E.Y. Post-COVID-19 Condition. Annu. Rev. Med. 2023, 74, 55–64. [Google Scholar] [CrossRef]
- Morioka, S.; Tsuzuki, S.; Maruki, T.; Terada, M.; Miyazato, Y.; Kutsuna, S.; Saito, S.; Shimanishi, Y.; Takahashi, K.; Sanada, M.; et al. Epidemiology of post-COVID conditions beyond 1 year: A cross-sectional study. Public Health 2023, 216, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Jason, L.A.; Unutmaz, D.; Bateman, L.; Vernon, S.D. Improvement of Long COVID symptoms over one year. Front. Med. 2023, 9, 1065620. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, S.; Kwee, A.K.A.L.; Houweling, L.; de Wit-van Wijck, Y.; Mohamed Hoesein, F.A.A.; Downward, G.S.; Nossent, E.J.; Maitland-van der Zee, A.H. A systematic review of chest imaging findings in long COVID patients. J. Pers. Med. 2023, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, M.Z.; Li, X.; Lau, E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: A systematic review and meta-analysis. Infection 2022, 50, 1067–1109. [Google Scholar] [CrossRef]
- Patel, U.K.; Mehta, N.; Patel, A.; Patel, N.; Ortiz, J.F.; Khurana, M.; Urhoghide, E.; Parulekar, A.; Bhriguvanshi, A.; Patel, N.; et al. Long-Term neurological sequelae among severe COVID-19 patients: A systematic review and meta-analysis. Cureus 2022, 14, e29694. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Weerahandi, H.; Hochman, K.A.; Simon, E.; Blaum, C.; Chodosh, J.; Duan, E.; Garry, K.; Kahan, T.; Karmen-Tuohy, S.L.; Karpel, H.C.; et al. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. J. Gen. Intern. Med. 2021, 36, 738–745. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Cioboata, R.; Nicolosu, D.; Streba, C.T.; Vasile, C.M.; Olteanu, M.; Nemes, A.; Gheorghe, A.; Calarasu, C.; Turcu, A.A. Post-COVID-19 syndrome based on disease form and sssociated comorbidities. Diagnostics 2022, 12, 2502. [Google Scholar] [CrossRef]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. eLife 2022, 11, e78200. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Misra, A. Diabetes and COVID19: A bidirectional relationship. Nutr. Diabetes 2021, 11, 21. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Misra, A. Post COVID-19 syndrome (“Long COVID”) and diabetes: Challenges in diagnosis and management. Diabetes Metab. Syndr. 2021, 15, 102235. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 29, 12422. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Vaccination and Long COVID after infections not requiring hospitalization in health care workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).