Abstract
Malaria poses a significant threat to pregnant women in sub-Saharan Africa, necessitating effective interventions like the intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine (IPTp-SP). However, challenges persist in the uptake and effectiveness of this intervention. This scoping review aims to explore IPTp-SP uptake in African countries, identify influencing factors, and assess its effectiveness in preventing malaria and adverse outcomes in pregnancy. This scoping review follows Arksey and O’Malley’s framework, employing the PRISMA-ScR guidelines for reporting. Searches were conducted in PubMed, Embase, Scopus, JSTOR, Web of Science, Google Scholar, and ProQuest, focusing on studies post-2000 published in the English language. The search produced 15,153 records, of which 104 full-text records were eligible and 101 papers were included in this review. The findings suggest varying IPTp-SP uptake rates, spanning from 5.3% to 98.9%, with their effectiveness supported by longitudinal studies, randomised controlled-trials (RCTs), cross-sectional surveys, and mixed-method studies. IPTp-SP demonstrates efficacy in reducing malaria during pregnancy, placental parasitaemia, and anaemia episodes, alongside improved birth outcomes. Common adverse effects of IPTp-SP include prematurity and low birth weight. Facilitators of IPTp-SP uptake include education and ANC attendance, while commonly reported barriers included inadequate knowledge and healthcare system challenges. The findings also suggest adverse effects such as prematurity, low birth weight, and maternal and perinatal mortality associated with IPTp-SP uptake. It is vital to strengthen antenatal care services by integrating comprehensive counselling on IPTp-SP and address healthcare system challenges. Community engagement, women’s empowerment, and context-specific interventions are necessary for promoting IPTp-SP uptake and improving maternal and neonatal health outcomes in Africa.
1. Introduction
Malaria, a formidable global health challenge, disproportionately affects economically disadvantaged regions, especially in Africa. In 2021 alone, Africa accounted for 95% of the 247 million reported cases and nearly 96% of the associated deaths [1,2]. Vulnerable populations, notably pregnant women and young children in endemic regions, face heightened risks, evidenced by an annual incidence of approximately 25 million cases among pregnant women in Africa, significantly amplifying the risk of maternal mortality [3,4,5,6]. This pressing issue requires urgent attention and innovative, equitable solutions to alleviate the impact on the most vulnerable members of our global community.
Pregnancy exacerbates women’s susceptibility to severe malaria by compromising their immune system [7,8]. This heightened vulnerability, which can lead to severe anaemia and gestational hypertension, results in a high mortality rate approaching 50% among pregnant women in malaria-endemic regions [4,6,9,10]. Moreover, the impact of malaria during pregnancy extends to significant risks for the developing foetus, elevating the likelihood of preterm birth and a low birth weight due to intrauterine growth restriction [7], with an estimated 900,000 low-birth-weight deliveries globally attributed to malaria, of which about 100,000 resulted in newborn mortality [11]. Surviving infants with a low birth weight face an increased risk of impaired intellectual and social development in later years, emphasizing the urgent need for targeted interventions in malaria-endemic regions [12,13,14]. The intricate interplay of these factors underscores the necessity for comprehensive strategies to safeguard maternal and neonatal well-being.
The World Health Organization (WHO) implemented a range of measures in 2000 to assess the effects of malaria during pregnancy. One of the main interventions was the use of intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP), which is highly recommended [15,16,17,18]. IPTp-SP is a malaria-prevention strategy where pregnant women take doses of SP at scheduled intervals to reduce the risk of malaria infection and its associated complications [17,18]. Despite these efforts to protect pregnant women in Africa against malaria, recent analyses suggest that Africa is falling short of its malaria morbidity and mortality targets, achieving only 45% and 47% of the indicators, respectively [17]. This shortfall raises concerns about the United Nations’ Sustainable Development Goal 3, which aims to reduce the maternal mortality ratio to less than 70 per 100,000 live births by 2030.
Recognizing the potential of IPTp-SP to enhance maternal and neonatal health, as emphasized by the WHO in 2012, it is essential to acknowledge persistent challenges in its uptake, accessibility, and effectiveness, as highlighted in a recent study by Nana et al. [19]. Despite over two decades of research on IPTp-SP in Africa, comprehensive reviews that aim to synthesize and map these studies remain scarce. The existing reviews have primarily focused on different aspects of IPTp. For instance, van Eijk et al. [20] examined the resistance of IPTp-SP, Egbujor et al. [21] discussed interventions aimed at improving its uptake, and Kayentao et al. [22] evaluated the effectiveness of two versus three or more doses of SP on the risk of low birth weight in Africa. However, these reviews did not address the prevalence of and factors influencing the uptake of IPTp-SP in Africa. Moreover, these reviews included few studies, probably due to the focus on experimental designs, interventional studies, and, also, stringent eligibility. Additionally, Hill et al. [23] explored barriers to the uptake of both IPTp-SP and insecticide-treated nets (ITNs) without a sub-group analysis specifically focusing on barriers to IPTp uptake. Another review by Sylla et al. [24] attempted to address this issue but did exclusively consider health system barriers affecting the uptake of IPTp in Africa. There is a clear need for a review methodology that allows a comprehensive mapping of the evidence on the prevalence of, facilitators of, barriers to, and effectiveness of IPTp-SP, the common type of IPT treatment for the prevention of malaria in pregnancy in sub-Saharan Africa (SSA). This approach will provide a more holistic understanding of the factors influencing the uptake of IPTp-SP and offer insights into how to improve its implementation and effectiveness.
Therefore, a scoping review is crucial to thoroughly investigate the uptake of IPTp-SP in Africa, discern the factors that influence access to this intervention, and consolidate the existing body of evidence regarding its effectiveness in preventing malaria and mitigating associated adverse outcomes. The evidence from this review will emerge as a valuable resource for healthcare service providers, policymakers, and stakeholders, delivering valuable insights into utilization levels, barriers to access, and outcomes associated with IPTp-SP in the African context. Furthermore, evidence from this review will help develop context-specific interventions that have a high potential for improving the accessibility and uptake of IPTp-SP in Africa. This review will inform future studies and systematic reviews by highlighting the gap and kind of evidence that exist on this subject. Thus, the insights into the factors influencing access to IPTp-SP, its effectiveness, and barriers to its utilization in Africa may help in conducting a robust systematic review and meta-analysis.
The socio-ecological framework [25] was used as a framework in organising the factors (facilitators and barriers) that influence the uptake of IPTp-SP. The socio-ecological framework, proposed by Bronfenbrenner in 1994, offers a comprehensive approach to understanding human behaviour within the context of various environmental systems. This model identifies multiple levels of influence, including the individual, interpersonal, community, organizational, and societal levels, and emphasizes the dynamic interactions between these levels. In the context of IPTp-SP uptake, the socio-ecological model allows for the exploration of factors at each of these levels that either facilitate or impede the adoption of the intervention. The strength of the socio-ecological model lies in its holistic approach to understanding health behaviours by considering the complex interplay between individual, interpersonal, and environmental factors. By examining factors across multiple levels of influence, the model allows for a more comprehensive understanding of the determinants of IPTp-SP uptake. This facilitates the development of multi-level interventions that address barriers at various levels, leading to more effective strategies for improving IPTp-SP coverage and ultimately reducing the burden of malaria in pregnancy.
2. Methods
2.1. Research Design
This review followed the framework proposed by Arksey and O’Malley, [26], which consists of six consecutive stages: (1) formulating the research question, (2) identifying relevant studies, (3) selecting studies, (4) organising data, (5) compiling, summarising, and reporting results, and (6) seeking consultation. The reporting of this review followed the PRISMA-ScR checklist by Tricco et al. [27]. Utilisation of PRISMA-ScR ensured a structured and comprehensive approach to reporting that aligns with best practices in scoping review methodologies. This review utilised primary studies that had ethical clearance and followed appropriate ethical standards in conducting their studies. Furthermore, this scoping review protocol has been registered in the Open Science Framework with the number doi:10.17605/OSF.IO/UNC7R.
2.2. Research Questions
The following research questions guided this review:
- What is the level of IPTp-SP uptake during pregnancy in Africa?
- What are the facilitators and barriers to IPTp-SP access among pregnant women in Africa?
- What is the effectiveness of IPTp-SP among pregnant women in Africa?
- What are the adverse effects of IPTp-SP on maternal and neonatal outcomes in Africa?
2.3. Search Strategy
A comprehensive search was performed in five databases, namely PubMed, Embase, Scopus, JSTOR, and Web of Science. Researchers [28] have stated that these specific databases contain a diverse array of the relevant literature on health. The authors created a search strategy by employing a combination of regulated terminologies like medical subject headings (MeSH) and specific keywords for conducting searches in the designated databases. A preliminary search was conducted in PubMed (See Table 1), and the MeSH terms were further refined for searching in the remaining databases. Moreover, an extra hand search was conducted on Google, ProQuest, OSF Registries, the WHO repository, and Google Scholar to collect additional relevant literature. Lastly, the reference lists of eligible studies were manually checked for additional eligible records. The last search was conducted on 13 May 2024.

Table 1.
Search strategy conducted in PubMed and examples of searches conducted in main databases.
This table presents our comprehensive search strategy to identify studies on the uptake and effectiveness of intermittent preventive treatment in pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) in Africa. The first part of the table shows the planned search strategy which contains the lists of search terms for various concepts, including IPTp-SP and its uptake and effectiveness, pregnancy, and a geographical focus on Africa. The second part of the table details the actual search strategies used in specific databases like PubMed, Embase, Scopus, Web of Science, and JSTOR. Each entry describes the precise search strings and keywords employed to identify relevant studies on the uptake and effectiveness of IPTp-SP, ensuring a thorough and systematic review of the literature. It is worth noting that not all planned search terms were used together in every database search. Instead, the search was adjusted to fit each database’s system, which sometimes required a more focused approach to get the best results. For example, during a manual search for specific papers, we used the names of individual countries instead of “Africa” to find relevant studies that might have been missed using a broader term.
2.4. Inclusion Criteria
This review included all studies conducted on IPTp-SP in African individuals. Studies published in English were utilized to ensure easier comprehension by the authors. The proposed review included studies published in 2000 and later because the WHO introduced the use of IPTp-SP as a prophylactic treatment for malaria in 2000 [17,29]. The authors incorporated both peer-reviewed and grey studies from the literature to guarantee comprehensive retrieval of all pertinent papers applicable to this review.
2.5. Exclusion Criteria
The authors excluded studies that specifically examined IPTp-SP with people from continents other than Africa. Preprints, abstracts, letters to editors, commentaries, and reviews were excluded from this review.
2.6. Study Selection
The authors uploaded the identified articles into the Mendeley software (Version 1.19.3) and eliminated duplicates. Titles and abstracts were screened by 25 trained postgraduate students. Three of the authors (SAA, TDA, MA) oversaw and guided this process. The screening of titles and abstracts was done to get only full-text eligible records. Reference lists of full-text eligible studies were checked for additional papers. Subsequently, the authors divided themselves into two groups, namely (GOB, TDA, ROD, JA, JK, GO) and (PFD, CO, DFA, FN, NKY, CMB), and independently evaluated the full-text studies based on the specified criteria for inclusion. The groups addressed and resolved their disagreements in weekly sessions. The screening procedure was overseen by MA and SAA. The use of two groups for the evaluation of full-text studies and the resolution of disagreements in weekly sessions further strengthened the reliability of the review. The PRISMA flow diagram was utilized to report the search results from each database, screening process, and screening results.
2.7. Data Extraction
The authors created a framework for extracting relevant information. The data that were extracted included information such as the authors, publication year, study location, study objective, study design, demographic, sample size, uptake level, barriers and facilitators, effectiveness, and adverse effects. This form was tested to collect data from four studies. Following that, two sets of authors (GOB, TDA, ROD, JA, JK, GO) and (PFD, CO, DFA, FN, NKY) independently extracted the data from the included studies. Other authors (AAD, MA, and SAA) reviewed the extraction. To guarantee the quality and accuracy of the retrieved data, the authors and reviewers scheduled weekly meetings to address any inconsistencies and other challenges.
2.8. Collating, Summarising and Reporting the Results
The characteristics of included studies were subjected to a thorough analysis using Microsoft Excel, resulting in the generation of tables, charts, and maps to visually depict important information. Thematic analysis and synthesis of findings from included studies were done. The authors thoroughly engaged with the retrieved data through extensive reading. This enabled them to formulate the first codes that effectively capture the core information. The initial codes were categorized into overarching themes and subthemes, each carefully defined and labelled, creating an organized structure that directed the subsequent reporting process. Narrative reporting of the findings was done.
2.9. Consultation
As outlined in the framework of Arksey and O’Malley [26], consultation occurred at every stage of the review procedure. A certified librarian was consulted during the search and screening of relevant studies. The proposed evaluation sought inputs from the directors of the Malaria Control Programme regarding any pertinent material and information. Also, mothers who have ever taken the IPTp-SP were consulted for their input during the conceptualisation of the review and discussion of the findings. Consultations were undertaken to ensure a comprehensive review, gather diverse perspectives, and incorporate valuable insights from stakeholders to enhance the quality and relevance of the evaluation process. These consultations aimed to enrich the review by accessing a wide range of source from the literature, expert opinions, and lived experiences, thereby strengthening the validity and applicability of the findings and recommendations.
3. Results
3.1. Search Results
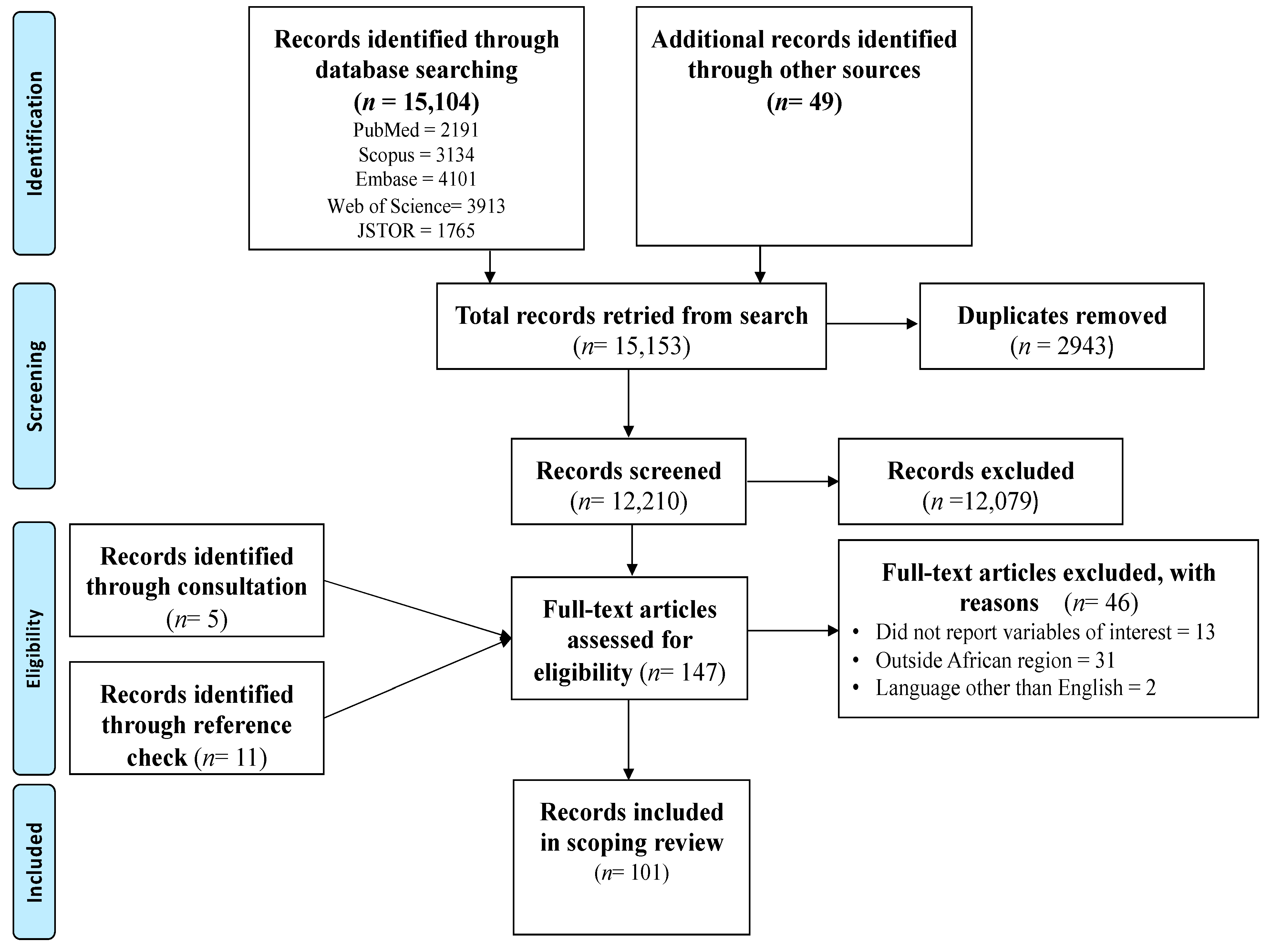
The search conducted in the five main databases produced 15,104 records. An additional 49 records were retrieved from searches conducted in other databases. The use of the Mendeley software allowed the deletion of 2943 duplicate records. The screening of the remaining 12,210 titles and abstracts led to the exclusion of 12,079 records. These records were not eligible and were not related to the variables of interest, were outside the geographical area and population, or did not focus on pregnant women. After screening of the titles and abstracts, 131 full texts were eligible for further screening. Checking the reference list resulted in an additional 11 full-text eligible records while consultations with a digital librarian resulted in the retrieval of 5 additional records. Hence, 147 full-text eligible records were further screened for inclusion. Finally, 46 records were excluded with reasons and 101 records were included in this scoping review. See Figure 1 for the PRISMA flow chart.
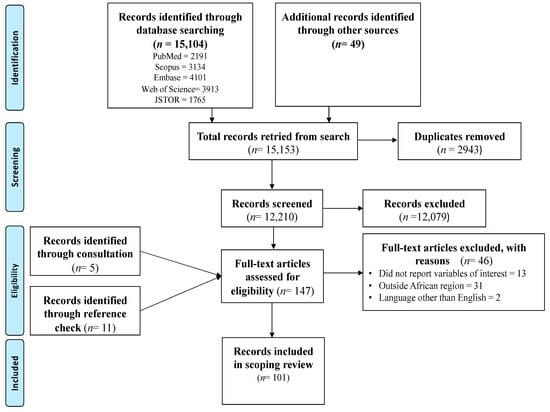
Figure 1.
PRISMA flow chart of the search results and screening process.
3.2. Characteristics of Included Studies
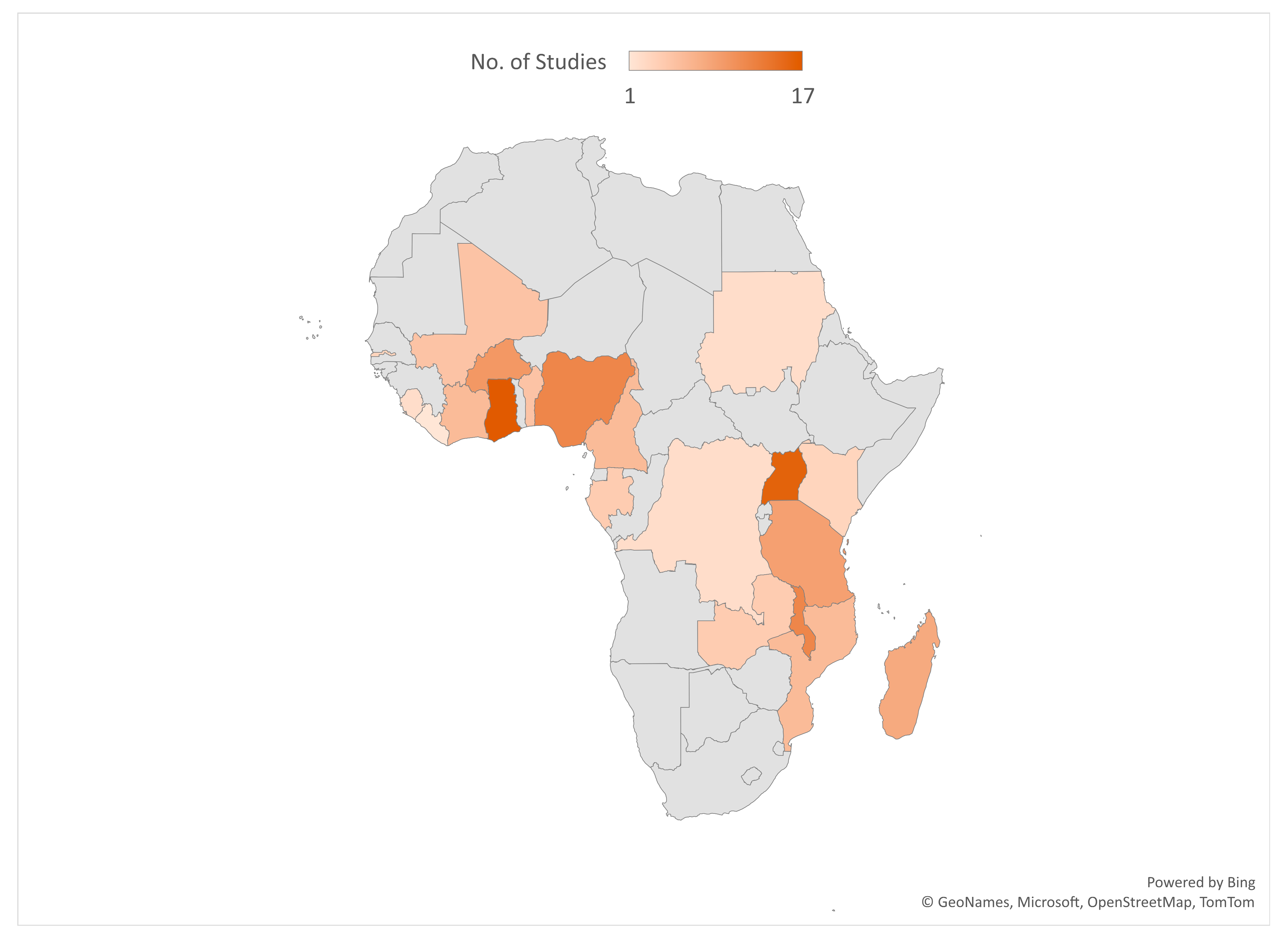
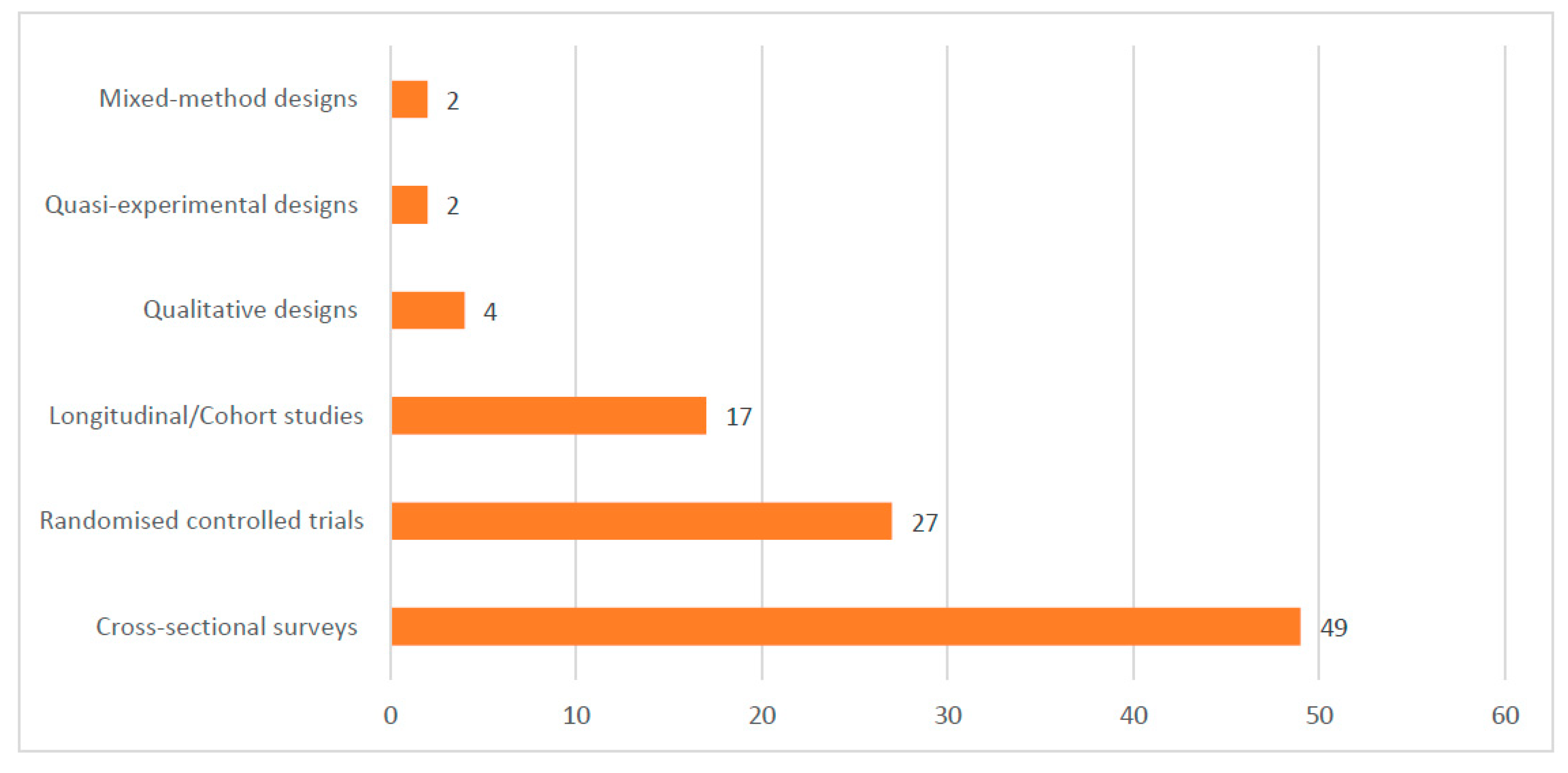
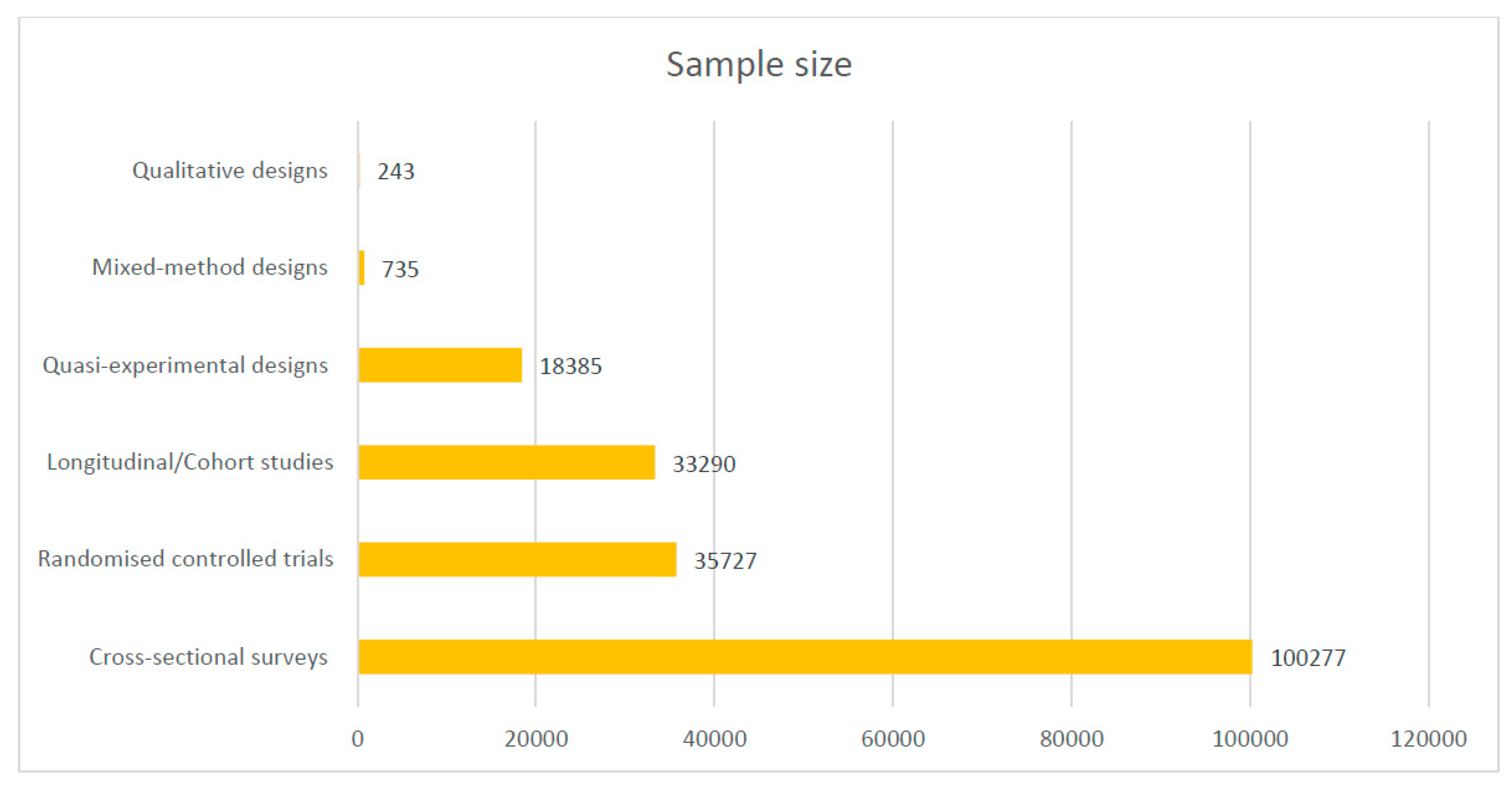
Most of the included studies were conducted in Ghana (17), Uganda (16), Malawi (12), Nigeria (12), and Burkina Faso (10). See the details of where the studies were conducted in Figure 2. Out of the 101 included studies, 49 were cross-sectional surveys. See Figure 3 for details on the study types. A total of 188,657 samples were used in the included studies. Details on the sample size according to the different study designs are presented in Figure 4.
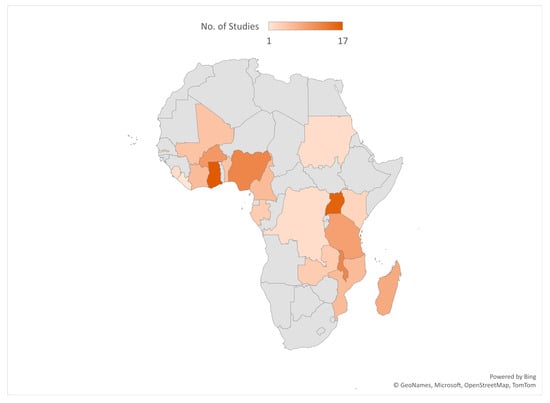
Figure 2.
Countries where included studies were conducted.
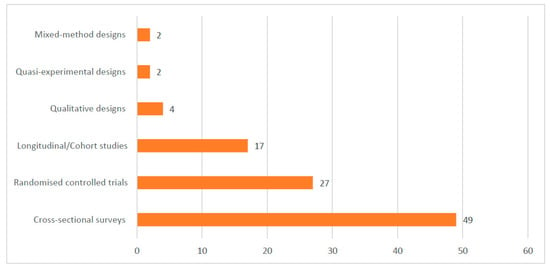
Figure 3.
Designs used in the included studies.
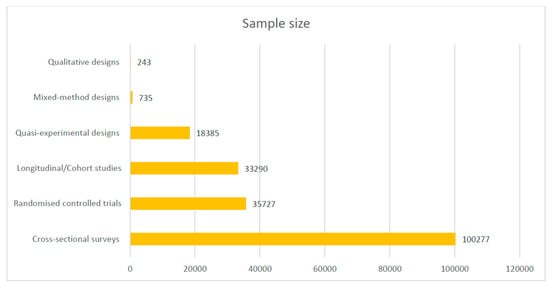
Figure 4.
Sample size of included studies according to designs.
3.3. Rate of Uptake of IPTp-SP Based on Reported Doses
Thirty-seven (37) studies reported on the rate of IPTp-SP uptake based on the number of doses. For at least one dose of IPTp-SP, the uptake rates ranged from 5.3% [30] to 98.9% [31]. For at least two doses of IPTp-SP, the uptake rates ranged from 15.0% [32] to 95.5% [33]. At least three doses of IPTp-SP had reported uptake rates ranging from 6.0% [34] to 93.2% [35]. Additionally, for at least four doses or more, the uptake rates ranged from 7.5% [36] to 42.3% [30]. Finally, the uptake rate for five doses or more was reported as 17.1% by Vandy et al. [31]. The uptake rates were lower for studies that reported on at least three doses or more compared to studies that focused one or two doses. Thus, the IPTp-SP uptake rate decreases with the number of doses. Studies that sampled participants in healthcare settings like ANC facilities [37,38,39] and delivery units [40] generally reported higher IPTp-SP uptakes compared to studies that relied on populations from community settings or the general population. See details in Table 2.

Table 2.
Rate of uptake of IPTp-SP.
3.4. Factors Affecting the Uptake of IPTp-SP
The factors affecting the influence of IPTp-SP were put into two main categories, facilitators and barriers. Each category was put into the socio-ecological model.
3.4.1. Facilitators of IPTp-SP Uptake
Forty-two (42) studies reported on factors that facilitate the uptake of IPTp-SP in SSA [31,34,35,36,37,45,46,48,50,51,52,53,54,55,56,57,59,61,62,63,64,65,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. These factors were organised according to the socio-ecological framework.
Individual Factors
For the facilitation of IPTp-SP uptake, several factors have been identified as influential. Regarding individuals’ educational levels, any level of education can play a role [59,71,72]. However, Mushi et al. [61] specifies that primary education may enhance IPTp-SP uptake. Similarly, while some studies highlight the positive impact of secondary or higher education on IPTp-SP utilization [34,35,73,74], others indicate that individuals with no education have higher chances of utilizing IPTp-SP [53]. This demonstrates that evidence on the influence of education has been inconsistent across studies. Although age has been reported as a significant predictor of IPTp-SP, this has been inconsistent across studies. For instance, the uptake is higher among women aged 15–29 years [49,54,75,76]. Other studies reported a higher uptake among women aged 30–39 years [72,75,77]. Additionally, being married or living with a partner is stressed as significant in facilitating IPTp-SP usage [75].
Comprehensive knowledge about IPTp-SP, as highlighted by [31,48,49], is associated with a better uptake. Understanding prophylaxis for malaria, as demonstrated in a study by [78], helps pregnant women make informed choices. Sangho et al. [59] emphasizes the importance of knowing the recommended dose of IPTp-SP, which improves its effectiveness. Awareness of malaria risks during pregnancy, as underscored by Azizi et al. [51], is crucial for prevention. The promotion of awareness about malaria [75] and IPTp-SP [55] encourages uptake. Furthermore, exposure to media messages, as demonstrated by [36,37,71], Barrow et al. [53], and [56], spreads information and encourages IPTp-SP use.
Knowledge about IPTp-SP, including its prophylaxis and recommended dose, contributes to its uptake [31,48,49,59,78]. Studies also suggest that awareness of malaria in pregnancy and its dangers [51], as well as awareness about malaria-prevention programs and IPTp-SP [55,75], positively influence IPTp-SP uptake. ANC attendance in the first trimester [61] or the second trimester [60,84] improves the uptake of IPTp-SP. Thus, the early initiation of ANC [59] in a government health facility [61] is crucial for IPTp-SP uptake. Having three [59] or four or more [51,52,56,61,63] ANC visits is positively associated with IPTp-SP uptake. Other facilitators include having three or more children [49], being a woman with advanced pregnancy (24–40 weeks) [77], and being a woman who tested positive for malaria [77]. Anaemic pregnant women [77] and those who use insecticide-treated nets (ITNs) [68] also show an improved IPTp-SP uptake.
Interpersonal Factors
Higher levels of education among husbands [74] and being part of a richer household [34,65,71] are significant predictors of IPTp-SP uptake. Included studies also indicate that educating men about the dangers of malaria in pregnancy [70] and providing education and counselling on malaria during antenatal care (ANC) attendance [79] improve the uptake of IPTp-SP.
Institutional Factors
The institutional facilitators include the proximity of health facilities [51], trust in the healthcare system [80], the continuous training of healthcare providers [70], high stock levels of IPT-SP [63,79], and the distribution of IPT-SP during ANC [69,74]. Additionally, utilizing the directly observed treatments (DOTS) strategy [81], the wider coverage of ANC [80], and increased ANC attendance [35,45,57] have been identified as predictors of IPTp-SP uptake. Exposure to media messages also plays a significant role in promoting awareness and the uptake of IPTp-SP [36,37,53,56,71].
Community/Societal Factors
The community/societal facilitators include factors such as rural dwelling [50], residence in urban areas [82], the encouragement of social networks [80], favourable social norms for IPTp-SP [83], and community sensitization efforts [84]. Additionally, the community delivery/distribution of IPTp-SP [79,87], the presence of health centres [59], and proximity to the nearest health centre [84] also play a role in facilitating IPTp-SP uptake. See details in Table 3.

Table 3.
Facilitators of IPTp-SP uptake.
3.4.2. Barriers to IPTp-SP Uptake
Thrity-two (32) studies reported on barriers to the uptake of IPTp-SP in SSA [32,34,35,36,48,50,51,54,55,57,63,65,66,76,77,80,81,82,83,84,88,89,90,91,92,93,94,95]. These factors were organised according to the socio-ecological framework.
Individual
The barriers to IPTp-SP uptake encompass various individual factors which contribute to poor knowledge and awareness. Studies indicate that women with inadequate knowledge about IPTp-SP [94,95] are less likely to complete the recommended dose of IPTp-SP [36]. Factors such as fear of side effects [81], the perception of adverse effects on pregnant women [93], and refusal to take SP [80] also hinder uptake. Late initiation and irregular attendance of ANC appointments contribute to suboptimal IPTp-SP use [80,85,94], alongside women who receive the first dose in the third trimester [63] and multigravida women being less likely to complete the recommended dose [43]. A higher parity [35,65], HIV-positive status [81], history of child death [42], and lower educational attainment [42,50,55] also serve as barriers. Additionally, being single [42] and belonging to poor socioeconomic backgrounds [54] are associated with reduced IPTp-SP uptake.
Interpersonal
Interpersonal barriers to IPTp-SP uptake often revolve around household economic and social factors. Studies indicate that households facing economic challenges, such as the poorest households [79,92] and those with a lower socioeconomic status [65], may encounter difficulties in accessing IPTp-SP. Additionally, issues related to autonomy and decision-making power within households can impede IPTp-SP uptake, particularly among women who lack the freedom to make health-related choices [66].
Institutional Factors
The institutional barriers to the uptake of IPTp-SP are multifaceted and encompass various challenges within healthcare systems and provider practices. One significant obstacle is the lack of adequate training and supervision for healthcare workers, leading to inconsistencies in IPTp-SP administration [91]. Moreover, inadequate information being provided to women about IPTp-SP during antenatal care visits further hampers its uptake [48,80,85]. Another issue is the inadequacy of routine training for healthcare providers, which contributes to gaps in knowledge and practice regarding IPTp-SP [48]. Healthcare provider’s refusal to provide services [91] and high staff turnover rates also pose significant challenges [90]. Additionally, uncertainty among healthcare providers about the safety and efficacy of IPTp-SP can influence their recommendations to pregnant women, further impacting its uptake [91]. The inconsistent provision of antenatal care services [93] and perceptions that IPTp-SP has adverse effects on pregnant women also contribute to a low uptake [91]. Moreover, inadequate counselling or support for pregnant women during ANC visits exacerbates the problem [92]. Financial barriers, including ANC costs and health financing challenges, further limit access to IPTp-SP for vulnerable populations [77,80]. Frequent stockouts of SP and other essential supplies at healthcare facilities are additional barriers to IPTp-SP uptake, resulting in missed opportunities for administration [57,66,82,89,92].
Insufficient information provided to women about IPTp-SP during antenatal care visits is a significant issue [48,80,85]. Furthermore, misinformation about the standard doses of IPTp-SP persists, hindering effective administration [93]. A lack of comprehensive knowledge of IPTP policy among healthcare providers further complicates the situation [77], as does the inadequate supply of SP to the health sector, leading to stockouts and missed opportunities for administration [91]. Demand and supply bottlenecks exacerbate the problem, creating challenges in accessing IPTp-SP [77]. Moreover, inadequate supply for the private sector limits access to SP in certain settings [91]. Limited healthcare infrastructure, including long waiting times at ANC clinics, presents additional barriers to IPTp-SP uptake [89,92]. The poor implementation of directly observed therapy strategies further hinders effective administration [66]. Non-institutional births, inconsistent guidelines for IPTp, and inadequate guidelines for healthcare providers also contribute to low uptake rates [91]. The geographical challenge of having a working distance of more than 30 min from an ANC clinic further exacerbates the issue [50].
Community/Societal Factors
In remote or disadvantaged regions, particularly rural areas, accessing IPTp-SP presents significant challenges [36,51,54,65,77,89]. Long distances to health facilities exacerbate this issue, making it difficult for pregnant women to access antenatal care services and receive IPTp-SP [51,88,92]. Additionally, a lack of reliable transportation will further impede access [51]. Cultural beliefs can hinder IPTp-SP uptake, as some communities may hold views that conflict with modern medical practices, leading to hesitation in adopting new interventions [92]. Details of barriers to IPTp-SP uptake is presented in Table 4.

Table 4.
Barriers to IPTp-SP uptake.
3.5. Effectiveness of IPTp-SP during Pregnancy
The effectiveness of IPTp is organised into two categories, one based on pregnancy and maternal health outcomes and effectives, and the second based on the number of doses administered.
3.5.1. Effectiveness of IPTp-SP on Pregnancy and Maternal Health Outcomes
Thirteen longitudinal designs [37,40,44,46,47,58,68,96,97,98,99,100,101], 10 randomised controlled trials [69,70,102,103,104,105,106,107,108,109], 12 cross-sectional surveys [30,33,34,38,56,63,64,110,111,112,113,114], and 2 mixed-method studies [32,115] reported on the effectiveness of IPTp-SP. Across various study designs, IPTp-SP consistently reduces malaria during pregnancy [40], placental parasitemia [37,47,56,58,63,64,70,96,97,98,103,105,110], and peripheral parasitemia [106]. It is also effective in improving birth outcomes, such as increasing the birth weight in newborns of primigravida [44], reducing the number of low birth weight cases [46,47,56,63,64,68,97,103,104,112,113], and enhancing overall birth weights, particularly with the administration of three or more doses [33]. Additionally, IPTp-SP improves hemoglobin levels in primigravida [44], reduces the number of episodes of anemia and severe anemia [46,47,97,104,107], and lowers the risk of anemia in primigravida [44].
A randommised trial reported that the use of insecticide-treated nets (ITNs) further enhances IPTp-SP’s efficacy [101]. ITNs provide an additional layer of protection against mosquito bites, thereby reducing malaria transmission and complementing the preventive effects of IPTp-SP. Notably, monthly doses of IPTp-SP are more efficacious than two doses, especially among HIV-positive pregnant women [69]. Despite some contrary evidence from randomised controlled trials (RCTs) suggesting an increased risk of placental parasitemia and clinical malaria [99,108], the overall synthesis indicates that IPTp-SP is highly effective. IPTp-SP is also effective at reducing the number of cases of asymptomatic malaria during pregnancy and low birth weight [109]. Mixed-method studies and cross-sectional surveys align with these findings, highlighting reductions in maternal anemia [32,112,115], maternal parasitemia [34,111,115], and infections in newborns [64]. These studies collectively shows IPTp-SP’s crucial role in enhancing maternal and neonatal health by reducing malaria-related complications during pregnancy. See details in Table 5.

Table 5.
Effectiveness of IPTp based on study design.
3.5.2. Effectiveness of IPTp-SP Based on Doses
Nineteen (19) studies reported on the effectiveness of IPTp-SP based on the number of doses administered. For instance, a study has shown that HIV-positive mothers receiving a single dose of SP had lower levels of peripheral antibodies against apical membrane antigen-1 and variant surface antigens, as well as lower levels of cord antibodies against erythrocyte-binding antigen-175 and the parasite lysate, than HIV-positive placebo recipients [121]. Additionally, receiving at least one or more doses of IPTp-SP correlates with a decrease in placental parasitemia, indicating its efficacy in preventing malaria transmission to the fetus [37]. Moreover, receiving at least two doses has shown benefits, with a low prevalence of placental parasitemia observed among HIV-negative women [69]. The completion of at least two or three doses of IPTp-SP has also been linked to reduced placental malaria, as well as protection against low birth weights and preterm births [103]. Pregnant women receiving at least two or more doses of IPTp-SP exhibit reduced placental malaria parasitemia [56], improved birth outcomes [38], and lower odds of a low birth weight [34,56]. However, taking monthly doses of IPTp has demonstrated superiority over two doses, particularly among HIV-positive pregnant women [69]. Other evidence suggests no superiority of only two doses of IPTp-SP in HIV-positive women concerning placental malaria, anaemia, or birth outcomes [122].
Receiving at least three doses of IPTp has been associated with a decreased risk of infant low birth weight [63] and placental malaria infection [63,110]. Furthermore, pregnant women who received at least three doses showed a lower prevalence of peripheral malaria [110] and peripheral parasitemia [106]. Furthermore, receiving at least three or more doses of IPTp has shown promising results, including a reduction in malaria during pregnancy [40,104], a lower prevalence of malaria [118], and improved birth weights [33,104]. Thus, receiving at least three or more doses has been associated with an average birth weight of more than 360 g [60] and an 83% decrease in the risk of a low birth weight [117]. For individuals receiving at least four or more doses of IPTp, the benefits include lower placental infection rates [30], decreased episodes of malaria at delivery [46], and a reduced prevalence of anaemia in pregnant women [46], with no reported cases of jaundice [46]. See details in Table 6.

Table 6.
Effectiveness of IPTp based on doses.
3.6. Adverse Effects of IPTp-SP
Thirteen studies reported on adverse effects of IPTp-SP [30,32,57,81,92,93,101,106,119,121,123,124,125]. Studies have linked IPTp-SP usage with increased rates of prematurity [30,57,106,123], low birth weights [30,57,106,123], and small-for-gestational-age infants [123]. In terms of intrauterine, IPTp-SP has been associated with an increased risk of miscarriages [30], congenital abnormalities [123], and abortions [119]. Furthermore, infants exposed to IPTp-SP may face extrauterine complications such as death [119], developmental delays, chronic health conditions, impaired immune function, and a heightened risk for malaria [92]. Additionally, stillbirths have been reported [119,123], along with symptoms like fever, cough, and conjunctivitis [122]. Others include dizziness [81], nausea [32,81,101], vomiting [32,101,124], skin reactions [93,124], general body weakness [32,93,101], headache [101], palpitations [32], and itching [32]. Also, studies have reported severe side effects including the emergence of PfdhFr C59R mutation [123], maternal anaemia [106], maternal death, and perinatal death [123]. Details of extracted data for this review is presented in Supplementary Materials.
4. Discussion
4.1. Summary of Findings
The findings from this review highlight the effectiveness and uptake of IPTp-SP as well as factors influencing IPTp-SP uptake. In terms of uptake, studies reported varying rates ranging from as low as 5.3% to as high as 98.9%, depending on the number of doses received. The effectiveness of IPTp-SP is supported by evidence from longitudinal studies, RCTs, cross-sectional surveys, and mixed-method studies. Longitudinal studies demonstrate its impact on reducing the number of cases of malaria during pregnancy, protecting against placental parasitemia, improving birth outcomes, and enhancing haemoglobin levels. RCTs indicate that IPTp-SP reduces the prevalence of malaria, placental malaria, and episodes of anaemia. Cross-sectional surveys reveal reductions in placental parasitemia, maternal parasitemia, and infections in newborns associated with IPTp-SP usage. Mixed-method studies further support the effectiveness of IPTp-SP by highlighting reductions in maternal anaemia and parasitemia. However, some studies suggest challenges and adverse effects associated with IPTp-SP uptake, including prematurity, low birth weights, small-for-gestational-age infants, miscarriages, congenital abnormalities, abortions, and extrauterine complications such as developmental delays, chronic health conditions, impaired immune function, and a heightened risk for malaria. Severe side effects include the emergence of specific mutations, maternal anaemia, maternal death, and perinatal death. Facilitators of IPTp-SP uptake include individual factors like education and awareness, ANC attendance, and community support, while barriers include factors such as inadequate knowledge, fear of side effects, late ANC initiation, healthcare system challenges, and cultural beliefs.
4.2. Uptake of IPTp-SP
The reported rates of IPTp-SP uptake based on the number of doses received demonstrate considerable variability across different studies, indicating varying levels of adherence to the recommended regimen. The findings suggest that, while some pregnant women receive the minimum required dose, a substantial proportion still do not achieve optimal uptake levels. The low uptake rates for one dose or more, ranging from 5.3% to 98.9%, indicate significant disparities in IPTp-SP administration among pregnant women in different settings. This variability could be attributed to several factors, including differences in healthcare access, awareness about IPTp-SP, cultural beliefs, and healthcare provider practices [30,31]. For instance, higher uptake rates might be observed in areas with better healthcare infrastructure, greater community awareness programs, and effective ANC services [54].
The wide variability in uptake rates of IPTp-SP underscores the complex interplay of factors influencing pregnant women’s adherence to the recommended dosage regimen. Factors such as ANC attendance, the quality of healthcare services, patient education, and socio-economic status are pivotal in shaping IPTp-SP uptake [32,34,35]. Disparities in ANC attendance could affect the opportunity for healthcare providers to administer IPTp-SP, thereby influencing the uptake rates [32]. Additionally, variations in the quality of ANC services, including counselling on IPTp-SP, may impact pregnant women’s understanding and acceptance of the intervention [34]. Patient education initiatives that emphasize the importance of IPTp-SP in preventing malaria during pregnancy could enhance awareness and the uptake [35]. Moreover, socio-economic factors such as the income level and access to healthcare resources may influence women’s ability to attend ANC visits regularly and afford IPTp-SP, thus affecting the uptake rates [35].
The relatively low uptake rates for at least four doses or more suggest that many pregnant women do not complete the full course of IPTp-SP as recommended by the guidelines. This incomplete adherence to the recommended regimen could compromise the effectiveness of IPTp-SP in preventing malaria during pregnancy and improving maternal and neonatal health outcomes. Possible reasons for suboptimal uptake rates across different dose categories may include challenges such as stockouts of SP, inadequate ANC attendance, lack of awareness about the benefits of IPTp-SP, fear of side effects, and cultural beliefs surrounding pregnancy and medication use [30,36].
4.3. Facilitators and Barriers to IPTp-SP Uptake in Africa
4.3.1. Facilitators
Facilitators such as education, awareness, ANC attendance, and community sensitization efforts significantly enhance IPTp-SP uptake. Higher levels of education among pregnant women and their partners correlate with better knowledge and understanding of malaria-prevention strategies, including IPTp-SP [71,73,84]. This can be attributed to the fact that education often correlates with better access to information and healthcare services, empowering individuals to make informed decisions about their health. Comprehensive knowledge about IPTp-SP and awareness of malaria risks during pregnancy promote informed decision-making and encourage pregnant women to utilize IPTp-SP [31,59]. When women understand the benefits of IPTp-SP and its role in preventing malaria-related complications during pregnancy, they are more likely to seek and adhere to the recommended doses [73].
The early initiation of ANC and completion of ANC visits are crucial facilitators of IPTp-SP uptake, emphasizing the importance of timely and consistent access to healthcare services during pregnancy [59,61]. ANC visits provide opportunities for healthcare providers to educate women about IPTp-SP and ensure its timely administration, reinforcing the importance of integrating malaria prevention into routine antenatal care [59]. Community sensitization efforts play a vital role in disseminating information and fostering a supportive environment for IPTp-SP uptake [84]. By raising awareness about the importance of malaria prevention and promoting IPTp-SP uptake at the community level, these efforts help to overcome cultural barriers and encourage positive health-seeking behaviours among pregnant women.
4.3.2. Barriers
Barriers to IPTp-SP uptake stem from various individual, interpersonal, institutional, and community-level factors. Inadequate knowledge about IPTp-SP, fear of side effects, and the late initiation of ANC appointments hinder its uptake by limiting pregnant women’s understanding and acceptance of the intervention [63,94]. These issues may stem from gaps in healthcare education and counselling, coupled with misinformation about IPTp-SP’s safety and efficacy [81]. Additionally, barriers like fear of side effects could be exacerbated by cultural beliefs and previous negative experiences with healthcare interventions [81]. Individual and context-specific educational interventions and comprehensive counseling during ANC visits could help address misconceptions and alleviate concerns about IPTp-SP.
Household economic constraints, limited autonomy in decision-making, and cultural beliefs further impede access to healthcare services and influence healthcare-seeking behaviours [66,72,92]. These barriers may result from socioeconomic disparities, patriarchal norms, and traditional health practices prevalent in many African communities. Addressing these issues requires targeted interventions aimed at improving economic empowerment, promoting gender equality, and sensitizing communities about the importance of modern healthcare practices.
Healthcare system challenges, including inadequate training for healthcare providers, stockouts of SP, and long waiting times at ANC clinics, pose significant barriers to IPTp-SP uptake by limiting access to services and undermining the effectiveness of malaria-prevention programs [89,91]. These challenges could stem from resource constraints, weak healthcare infrastructure, and inefficient supply chain management systems in many healthcare settings in Africa [91]. Addressing these issues requires investments in healthcare workforce training, supply chain optimization, and infrastructure improvement to ensure the effective delivery of IPTp-SP and other essential healthcare services.
Geographical remoteness, unreliable transportation, and cultural beliefs also present substantial challenges, highlighting the need for targeted interventions to address structural and systemic barriers [51,92]. Mobile health clinics, community health workers, and community engagement initiatives can help bridge the gap and ensure equitable access to IPTp-SP for women living in remote or disadvantaged areas.
4.4. Effectiveness of IPTp-SP
The findings from various studies underscore the dual nature of IPTp-SP (preventive and therapeutic effects), its significant effectiveness in reducing malaria during pregnancy and improving maternal and neonatal health outcomes, alongside some notable challenges that demand deeper exploration. Longitudinal studies consistently demonstrate the positive impact of IPTp-SP in reducing malaria prevalence and protecting against placental parasitemia, ultimately leading to improved birth outcomes. These findings are crucial as they provide robust evidence supporting the implementation of IPTp-SP programs in malaria-endemic regions [37,40,96,97].
The findings reported by Umeh et al. [99] and González et al. [108] necessitate a deeper understanding of the factors influencing the effectiveness of IPTp-SP. For instance, Umeh et al. [99] found no significant reduction in malaria parasitemia among pregnant women receiving IPTp-SP, suggesting potential challenges in program implementation in the African setting or the drug’s efficacy. This indicates the importance of investigating factors such as drug-resistance patterns, patient compliance, and variations in healthcare delivery that may differently affect the effectiveness of IPTp-SP across diverse settings.
Similarly, the study by González et al. [108], indicating an increased risk of placental parasitemia and clinical malaria among pregnant women receiving IPTp-SP, raises questions about the contextual factors influencing malaria transmission dynamics and the drug’s efficacy. Possible explanations for this discrepancy include differences in malaria transmission intensity, variations in drug-resistance patterns, or limitations in study design [96]. Addressing these discrepancies requires a comprehensive understanding of local epidemiological factors, drug-resistance patterns, and healthcare infrastructure to effectively tailor malaria-prevention strategies.
Furthermore, the comparative effectiveness of different dosing regimens highlighted by studies such as Mlugu et al. [109] shows the need for personalized approaches to IPTp-SP delivery based on individual patient characteristics and regional epidemiology. While monthly doses may be more effective in certain populations, such as HIV-positive pregnant women, the lack of superiority over two-dose regimens in other contexts suggests the importance of considering specific risk factors and the local malaria epidemiology when determining optimal IPTp-SP protocols [126]. These findings emphasize the need for adaptive and context-specific strategies to optimize the impact of IPTp-SP in malaria prevention during pregnancy.
4.5. Adverse Effects of IPTp-SP
The adverse effects associated with IPTp-SP usage, as highlighted in various studies, underscore the complexity and potential risks involved in its administration during pregnancy. The increased rates of prematurity, low birth weights, and small-for-gestational-age infants suggest potential implications for fetal growth and development [30,57,123]. These adverse outcomes may be attributed to various factors, including drug toxicity, maternal health status, and genetic predispositions [123]. Additionally, the association of IPTp-SP with miscarriages, congenital abnormalities, and abortions raises concerns about its safety and potential teratogenic effects [30,107,123]. These findings emphasize the need for careful consideration and the monitoring of pregnant women receiving IPTp-SP to mitigate the risk of adverse pregnancy outcomes.
Furthermore, the reported extrauterine complications, such as developmental delays, chronic health conditions, impaired immune function, and increased susceptibility to malaria, suggest broader health implications for both mothers and infants [92]. These complications may result from direct drug effects, drug interactions, or underlying health conditions exacerbated by IPTp-SP usage [30]. Additionally, the occurrence of stillbirths and symptoms like fever, cough, and conjunctivitis shows the importance of vigilant surveillance and the reporting of adverse events associated with IPTp-SP [122].
The spectrum of the reported side effects, including dizziness, nausea, vomiting, skin reactions, weakness, headache, palpitations, and itching, reflects the diverse physiological responses to IPTp-SP administration [32,81,93,101,124]. While some of these side effects may be transient and mild, others, such as severe anaemia and perinatal death, pose significant health risks and warrant immediate medical attention [106,123]. These adverse reactions may be influenced by individual susceptibility, drug metabolism, and underlying health conditions, highlighting the need for personalized risk assessment and monitoring during IPTp-SP administration.
Moreover, the emergence of specific mutations, such as the PfdhFr C59R mutation, associated with IPTp-SP usage raises concerns about drug resistance and the long-term efficacy of malaria-prevention strategies [125]. It is important to note that mutations in the dhps gene, which are also prevalent, are of particular concern as they further impact the effectiveness of IPTp-SP. This suggests the importance of ongoing surveillance and research to monitor drug-resistance patterns and optimize treatment regimens to combat malaria effectively.
4.6. Limitations
While this scoping review followed a structured framework and employed a comprehensive search strategy across multiple databases to investigate the uptake and effectiveness of IPTp-SP in African settings, several limitations are acknowledged. The review included only studies published in the English language, potentially introducing language bias and overlooking relevant literature in other languages. Additionally, there might be a publication bias, as studies with statistically significant findings or positive outcomes are more likely to be published, potentially skewing the overall conclusions. This review did not appraise the quality of the included studies, indicating a need for caution when interpreting the findings and drawing conclusions. Furthermore, the time frame of the review and the focus on African countries might have excluded valuable insights from other regions and historical contexts.
4.7. Implications for Policy and Practice
Based on the findings of this study, several practical implications and recommendations for policy and practice in Africa emerge. Strengthening ANC services is paramount. This involves integrating comprehensive counselling on IPTp-SP into routine antenatal care. Pregnant women should be educated about the benefits of IPTp-SP, their recommended dosing regimen, and any potential misconceptions that should be addressed. Healthcare providers need to be trained to ensure consistent and accurate information dissemination during ANC visits. Community engagement and sensitization are crucial strategies to promote IPTp-SP uptake. Implementing community-based awareness campaigns and sensitization programs can address cultural beliefs and encourage positive health-seeking behaviours among pregnant women. Engaging community leaders, traditional birth attendants, and community health workers can help effectively disseminate information. Addressing healthcare system challenges is essential. Measures should be taken to ensure the consistent availability of SP and other essential commodities at healthcare facilities. Training and capacity-building initiatives for healthcare providers can improve their knowledge and skills in IPTp-SP administration and counselling. Streamlining ANC services to reduce waiting times and enhance their overall quality of care is imperative.
Empowering women and enhancing access to ANC services are critical steps. Economic empowerment programs targeting vulnerable populations can improve their ability to afford ANC services and essential medications like SP. Promoting gender equality and women’s empowerment initiatives can enhance women’s decision-making power regarding their health and healthcare utilization. Expanding access to ANC services through mobile health clinics, community outreach programs, and innovative service-delivery models can reach women in remote or underserved areas. Tailoring interventions to local contexts is essential. Context-specific research should be conducted to understand the unique socio-cultural, economic, and healthcare system factors influencing IPTp-SP utilization in specific regions or communities. Targeted interventions should be designed and implemented to address the specific needs and challenges faced by pregnant women and healthcare providers in each context, ensuring cultural appropriateness, contextual relevance, and long-term sustainability.
4.8. Recommendations for Future Studies
Future reviews should consider appraising the quality of included studies to enhance the rigour and reliability of their findings, thereby enabling more robust conclusions and recommendations. Furthermore, there is a need for longitudinal studies that follow pregnant women throughout their antenatal care to assess the uptake and effectiveness of IPTp-SP over time, allowing for a better understanding of adherence patterns and outcomes. Moreover, future research should explore the impact of socio-cultural factors, healthcare system dynamics, and geographical variations on the uptake and effectiveness of IPTp-SP to inform tailored interventions and policies. Additionally, comparative effectiveness studies evaluating different dosing regimens, delivery approaches, and adjunct interventions can provide valuable insights into optimizing IPTp-SP strategies for diverse populations and settings. Finally, interdisciplinary collaborations between researchers, healthcare providers, policymakers, and community stakeholders are essential to ensure the translation of research findings into evidence-based practices and policies that effectively address the burden of malaria during pregnancy in Africa.
5. Conclusions
The findings of this review provide valuable insights into the uptake and effectiveness of IPTp-SP in African settings, as well as the factors influencing its utilization. The varying rates of uptake across different studies highlight the need for targeted interventions to improve adherence to the recommended dosage regimen. While IPTp-SP has demonstrated significant effectiveness in reducing malaria during pregnancy and improving maternal and neonatal health outcomes, challenges such as suboptimal uptake rates and adverse effects underscore the complexity of its implementation. Facilitators of IPTp-SP uptake, including education, awareness, ANC attendance, and community sensitization efforts, play a crucial role in promoting its uptake and should be integrated into routine antenatal care. Conversely, barriers such as inadequate knowledge, fear of side effects, healthcare system challenges, and cultural beliefs need to be addressed through targeted interventions and policy initiatives. Interdisciplinary collaborations between researchers, healthcare providers, policymakers, and community stakeholders are essential to translate research findings into evidence-based practices and policies that effectively address the burden of malaria during pregnancy in Africa. By addressing these challenges and implementing tailored interventions, we can enhance the uptake and effectiveness of IPTp-SP, ultimately improving maternal and neonatal health outcomes and reducing the burden of malaria in African communities.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diseases12090203/s1, Extracted Data [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,127,128,129,130,131].
Author Contributions
Conceptualization: G.O.B., M.A., S.A.A. and J.E.H.J.; screening and extraction of data: T.D.A., A.A., R.O.-D., J.A., J.E.K., G.O., P.F.D., D.F.A., N.K.Y., C.M.B., B.A., B.N. and G.O.O.; Introduction and methodology: F.O.O., I.K.A., M.A., J.A., N.K.Y. and G.O.; descriptive and thematic analysis: S.A.A., G.O.B., T.D.A., C.M.B., G.O.O. and D.F.A.; results and discussion: P.F.D., S.A.A., M.A., F.O.O., D.F.A. and J.E.H.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study received no external funding. However, the authors sincerely thank Bielefeld University, Germany, for providing financial support through the Institutional Open Access Publication Fund for the article processing charge (APC).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The raw and analysed data are available with the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Odwe, G.; Matanda, D.J.; Zulu, T.; Kizito, S.; Okoth, O.; Kangwana, B. Women’s empowerment and uptake of sulfadoxine–pyrimethamine for intermittent preventive treatment of malaria during pregnancy: Results from a cross-sectional baseline survey in the Lake endemic region, Kenya. Malar. J. 2023, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Cibulskis, R.E.; Alonso, P.; Aponte, J.; Aregawi, M.; Barrette, A.; Bergeron, L.; Fergus, C.A.; Knox, T.; Lynch, M.; Patouillard, E.; et al. Malaria: Global progress 2000–2015 and future challenges. Infect. Dis. Poverty 2016, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Chaponda, E.B.; Chandramohan, D.; Michelo, C.; Mharakurwa, S.; Chipeta, J.; Chico, R.M. High burden of malaria infection in pregnant women in a rural district of Zambia: A cross-sectional study. Malar. J. 2015, 14, 380. [Google Scholar] [CrossRef]
- Lufele, E.; Umbers, A.; Ordi, J.; Ome-Kaius, M.; Wangnapi, R.; Unger, H.; Tarongka, N.; Siba, P.; Mueller, I.; Robinson, L.; et al. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malar. J. 2017, 16, 427. [Google Scholar] [CrossRef]
- Schantz-Dunn, J.; Nour, N.M. Malaria and pregnancy: A global health perspective. Rev. Obstet. Gynecol. 2009, 2, 186–192. [Google Scholar]
- Mruma, H.A.; McQuillan, R.; Norrie, J. The association of malaria infection and gestational hypertension in Africa: Systematic review and meta-analysis. J. Glob. Health 2020, 10, 20417. [Google Scholar] [CrossRef] [PubMed]
- Centres for Disease Control and Prevention. Intermittent Preventive Treatment of Malaria in Pregnant Women; CDC: Atlanta, GA, USA, 2018.
- Radeva-Petrova, D.; Kayentao, K.; ter Kuile, F.O.; Sinclair, D.; Garner, P. Drugs for preventing malaria in pregnant women in endemic areas: Any drug regimen versus placebo or no treatment. Cochrane Database Syst. Rev. 2014, 2014, CD000169. [Google Scholar] [CrossRef]
- Oyerogba, O.P.; Adedapo, A.; Awokson, T.; Odukogbe, A.-T.; Aderinto, N. Prevalence of malaria parasitaemia among pregnant women at booking in Nigeria. Health Sci. Rep. 2023, 6, e1337. [Google Scholar] [CrossRef]
- Sutarto, S.; Wardani, D.; Oktarlina, R.; Aryanti, S.; Indriyani, R. Risk factors for malaria in pregnant women. J. Kesehat. Masy. 2019, 14, 332–339. [Google Scholar] [CrossRef]
- Chua, C.L.L.; Hasang, W.; Rogerson, S.J.; Teo, A. Poor birth outcomes in malaria in pregnancy: Recent insights into mechanisms and prevention approaches. Front. Immunol. 2021, 12, 621382. [Google Scholar] [CrossRef]
- Weckman, A.M.; Conroy, A.L.; Madanitsa, M.; Gnaneswaran, B.; McDonald, C.R.; Kalilani-Phiri, L.; Chandna, J.; Ali, D.; Mwapasa, V.; Khairallah, C.; et al. Neurocognitive outcomes in Malawian children exposed to malaria during pregnancy: An observational birth cohort study. PLoS Med. 2021, 18, e1003701. [Google Scholar] [CrossRef]
- Na, J.Y.; Jung, D.; Cha, J.H.; Kim, D.; Son, J.; Hwang, J.K.; Kim, T.H.; Park, H.-K. Learning-Based Longitudinal Prediction Models for Mortality Risk in Very-Low-Birth-Weight Infants: A Nationwide Cohort Study. Neonatology 2023, 120, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Landes, J.K.; Reid, C.L.; Campbell, C.; French, N.; Anderson, M. Cognitive outcomes in children and adolescents born very preterm: A meta-analysis. Dev. Med. Child. Neurol. 2018, 60, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E.; Selesho, M.I.; van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of malaria. J. Ethnopharmacol. 2019, 245, 112176. [Google Scholar] [CrossRef] [PubMed]
- Kabanywanyi, A.M.; MacArthur, J.R.; A Stolk, W.; Habbema, J.D.F.; Mshinda, H.; Bloland, P.B.; Abdulla, S.; Kachur, S.P. Malaria in pregnant women in an area with sustained high coverage of insecticide-treated bed nets. Malar. J. 2008, 7, 133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organisation. World Malaria Report 2022; World Health Organisation: Geneva, Switzerland, 2022; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 19 March 2024).
- World Health Organization. Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP); World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/bitstream/handle/10665/337990/WHO-HTM-GMP-2012.05-eng.pdf?sequence=1&isAllowed=y (accessed on 19 March 2024).
- Nana, R.R.D.; Hawadak, J.; Foko, L.P.K.; Kumar, A.; Chaudhry, S.; Arya, A.; Singh, V. Intermittent preventive treatment with Sulfadoxine pyrimethamine for malaria: A global overview and challenges affecting optimal drug uptake in pregnant women. Pathog. Glob. Health 2023, 117, 462–475. [Google Scholar] [CrossRef]
- van Eijk, A.M.; A Larsen, D.; Kayentao, K.; Koshy, G.; Slaughter, D.E.C.; Roper, C.; Okell, L.C.; Desai, M.; Gutman, J.; Khairallah, C.; et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of in-termittent preventive therapy for malaria in pregnancy in Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 546–556. [Google Scholar] [CrossRef]
- Ernest, E.C.; Chinaecherem, I.D.; Madukaku, C.U.; Godswill, E.U.; Chigozie, E.J.; Winnie, D.U.; Ogazirilem, E.C.; Vivian, C.I.; Nanush, D.; Chimdimma, D.I.; et al. The effectiveness of interventions for improving the uptake of Intermittent Pre-ventive Treatment (IPT) for malaria control among pregnant women in sub-saharan Africa: A systematic literature review. Arch. Prev. Med. 2023, 8, 008–019. [Google Scholar] [CrossRef]
- Kayentao, K.; Garner, P.; van Eijk, A.M.; Naidoo, I.; Roper, C.; Mulokozi, A.; MacArthur, J.R.; Luntamo, M.; Ashorn, P.; Doumbo, O.K.; et al. Intermittent Preventive Therapy for Malaria during Pregnancy Using 2 vs. 3 or More Doses of Sul-fadoxine-Pyrimethamine and Risk of Low Birth Weight in Africa: Systematic Review and Meta-analysis. JAMA 2013, 309, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Hoyt, J.; van Eijk, A.M.; D’Mello-Guyett, L.; ter Kuile, F.O.; Steketee, R.; Smith, H.; Webster, J. Factors Affecting the Delivery, Access, and Use of Interventions to Prevent Malaria in Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001488. [Google Scholar] [CrossRef]
- Thiam, S.; Kimotho, V.; Gatonga, P. Why are IPTp coverage targets so elusive in sub-Saharan Africa? A systematic review of health system barriers. Malar. J. 2013, 12, 353. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Ecological models of human development. Int. Encycl. Educ. 1994, 3, 37–43. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Aveyard, H. Doing a Literature Review in Health and Social Care: A Practical Guide; McGraw-Hill Publishing Company: New York, NY, USA, 2023. [Google Scholar]
- Mutakatala, M.; Makukula, M.K.; Sianchapa, B. Experiences and Coping Strategies of School Going Breastfeeding Adolescents from Katima-Mulilo and Sesheke Secondary Schools in Sesheke district, Western Province of Zambia. J. Matern. Child. Health 2023, 8, 370–381. [Google Scholar] [CrossRef]
- Dosoo, D.K.; Malm, K.; Oppong, F.B.; Gyasi, R.; Oduro, A.; Williams, J.; Atibilla, D.; Peprah, N.Y.; Twumasi, M.; Owusu-Agyei, S.; et al. Effectiveness of intermittent preventive treatment in pregnancy with sulphadoxine-pyrimethamine (IPTp-SP) in Ghana. BMJ Glob. Health 2021, 6, e005877. [Google Scholar] [CrossRef] [PubMed]
- Vandy, A.O.; Peprah, N.Y.; Jerela, J.Y.; Titiati, P.; Manu, A.; Akamah, J.; Maya, E.T.; Torpey, K. Factors influencing adherence to the new intermittent preventive treatment of malaria in pregnancy policy in Keta District of the Volta region, Ghana. BMC Pregnancy Childbirth 2019, 19, 424. [Google Scholar] [CrossRef]
- Osei Tutu, E. Intermittent Preventive Treatment of Malaria in Pregnancy: Its Effects on Maternal Morbidity and Neonatal Birthweight in Offinso District of Ashanti Region, Ghana. Ph.D. Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2009. [Google Scholar]
- Quakyi, I.; Tornyigah, B.; Houze, P.; Kusi, K.A.; Coleman, N.; Escriou, G.; Laar, A.; Cot, M.; Fobil, J.; Asare, G.Q.; et al. High uptake of Intermittent Preventive Treatment of malaria in pregnancy is associated with improved birth weight among pregnant women in Ghana. Sci. Rep. 2019, 9, 5–12. [Google Scholar] [CrossRef]
- Arinaitwe, E.; Ades, V.; Walakira, A.; Ninsiima, B.; Mugagga, O.; Patil, T.S.; Schwartz, A.; Kamya, M.R.; Nasr, S.; Chang, M.; et al. Intermittent Preventive Therapy with Sulfadoxine-Pyrimethamine for Malaria in Pregnancy: A Cross-Sectional Study from Tororo, Uganda. PLoS ONE 2013, 8, 6–11. [Google Scholar] [CrossRef]
- Buh, A.; Kota, K.; Bishwajit, G.; Yaya, S. Prevalence and associated factors of taking intermittent preventive treatment in pregnancy in Sierra Leone. Trop. Med. Infect. Dis. 2019, 4, 32. [Google Scholar] [CrossRef]
- Sinyange, D.; Mukumbuta, N.; Mutale, L.S.; Mumbole, H.; Hamainza, B.; Sialubanje, C. Uptake of four or more doses of sulfadoxine pyrimethamine for intermittent preventive treatment of malaria during pregnancy in Zambia: Findings from the 2018 malaria in pregnancy survey. BMJ Open 2023, 13, e073287. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Konaté, A.; Bougouma, E.C.; Asamoa, K.; Cotte, A.H.; Ouédraogo, A.; Newman, R.D.; Moran, A.C.; Diarra, A.; Parise, M.E. Malaria prevention during pregnancy: Assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupéla District, Burkina Faso. Am. J. Trop. Med. Hyg. 2006, 75, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mace, K.E.; Chalwe, V.; Katalenich, B.L.; Nambozi, M.; Mubikayi, L.; Mulele, C.K.; E Wiegand, R.; Filler, S.J.; Kamuliwo, M.; Craig, A.S.; et al. Evaluation of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in preg-nancy: A retrospective birth outcomes study in Mansa, Zambia. Malar. J. 2015, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Nyunt, M.M.; Adam, I.; Kayentao, K.; van Dijk, J.; Thuma, P.; Mauff, K.; Little, F.; Cassam, Y.; Guirou, E.; Traore, B.; et al. Pharmacokinetics of Sulfadoxine and Pyrimethamine in Intermittent Preventive Treatment of Malaria in Pregnancy. Clin. Pharmacol. Ther. 2009, 87, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Mpogoro, F.J.; Matovelo, D.; Dosani, A.; Ngallaba, S.; Mugono, M.; Mazigo, H.D. Attitudes to town and country planning. Malar. J. 2014, 13, 455. [Google Scholar] [CrossRef]
- Tonga, C.; Kimbi, H.K.; Anchang-Kimbi, J.K.; Nyabeyeu, H.N.; Bissemou, Z.B.; Lehman, L.G. Malaria Risk Factors in Women on Intermittent Preventive Treatment at Delivery and Their Effects on Pregnancy Outcome in Sanaga-Maritime, Cameroon. PLoS ONE 2013, 8, e65876. [Google Scholar] [CrossRef]
- Ouma, P.O.; Van Eijk, A.M.; Hamel, M.J.; Sikuku, E.; Odhiambo, F.; Munguti, K.; Ayisi, J.G.; Kager, P.A.; Slutsker, L. The effect of health care worker training on the use of intermittent preventive treatment for malaria in pregnancy in rural western Kenya. Trop. Med. Int. Health 2007, 12, 953–961. [Google Scholar] [CrossRef]
- Holtz, T.H.; Kachur, S.P.; Roberts, J.M.; Marum, L.H.; Mkandala, C.; Chizani, N.; Macheso, A.; Parise, M.E. Use of antenatal care services and intermittent preventive treatment for malaria among pregnant women in Blantyre District, Malawi. Trop. Med. Int. Health 2004, 9, 77–82. [Google Scholar] [CrossRef]
- Oduro, A.R.; Fryauff, D.J.; Koram, K.A.; Rogers, W.O.; Anto, F.; Atuguba, F.; Anyorigiya, T.; Adjuik, M.; Ansah, P.; Hodgson, A.; et al. Sulfadoxine-pyrimethamine-based intermittent preventive treatment, bed net use, and antenatal care during pregnancy: Demographic trends and impact on the health of newborns in the Kassena Nankana District, North-eastern Ghana. Am. J. Trop. Med. Hyg. 2010, 83, 79–89. [Google Scholar] [CrossRef]
- Bouyou-Akotet, M.K.; Mawili-Mboumba, D.P.; Kombila, M. Antenatal care visit attendance, intermittent preventive treatment and bed net use during pregnancy in Gabon. BMC Pregnancy Childbirth 2013, 13, 52. [Google Scholar] [CrossRef]
- Mbonye, A.K.; Bygbjerg, I.; Magnussen, P. Intermittent preventive treatment of malaria in pregnancy: A community-based delivery system and its effect on parasitemia, anemia and low birth weight in Uganda. Int. J. Infect. Dis. 2008, 12, 22–29. [Google Scholar] [CrossRef][Green Version]
- Feng, G.; Simpson, J.A.; Chaluluka, E.; Molyneux, M.E.; Rogerson, S.J. Decreasing burden of malaria in pregnancy in malawian women and its relationship to use of intermittent preventive therapy or bed nets. PLoS ONE 2010, 5, e12012. [Google Scholar] [CrossRef] [PubMed]
- Diengou, N.H.; Cumber, S.N.; Nkfusai, C.N.; Mbinyui, M.S.; Viyoff, V.Z.; Bede, F.; Akwah, L.; Tsoka-Gwegweni, J.M.; Anchang-Kimbi, J.K. Factors associated with the uptake of intermittent preventive treatment of malaria in pregnancy in the Bamenda health districts, Cameroon. Pan Afr. Med. J. 2020, 35, 42. [Google Scholar] [CrossRef]
- Wanzira, H.; Katamba, H.; Okullo, A.E.; Rubahika, D. The challenge of using intermittent preventive therapy with sulfadoxine/pyrimethamine among pregnant women in Uganda. Malar. J. 2016, 15, 401. [Google Scholar] [CrossRef] [PubMed]
- Sangaré, L.R.; Stergachis, A.; Brentlinger, P.E.; Richardson, B.A.; Staedke, S.G.; Kiwuwa, M.S.; Weiss, N.S. Determinants of use of intermittent preventive treatment of malaria in pregnancy: Jinja, Uganda. PLoS ONE 2010, 5, e15066. [Google Scholar] [CrossRef]
- Azizi, S.C.; Chongwe, G.; Chipukuma, H.; Jacobs, C.; Zgambo, J.; Michelo, C. Uptake of intermittent preventive treatment for malaria during pregnancy with Sulphadoxine-Pyrimethamine (IPTp-SP) among postpartum women in Zomba District, Malawi: A cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 108. [Google Scholar] [CrossRef]
- Okethwangu, D.; Opigo, J.; Atugonza, S.; Kizza, C.T.; Nabatanzi, M.; Biribawa, C.; Kyabayinze, D.; Ario, A.R. Factors associated with uptake of optimal doses of intermittent preventive treatment for malaria among pregnant women in Uganda: Analysis of data from the Uganda Demographic and Health Survey, 2016. Malar. J. 2019, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.; Barrow, S.; Jobe, A. Differentials in prevalence and correlates on uptake of tetanus toxoid and intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy: A community-based cross-sectional study in The Gambia. SAGE Open Med. 2022, 10, 20503121211065908. [Google Scholar] [CrossRef] [PubMed]
- Ameyaw, E.K. Uptake of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP) in Uganda: A national survey. Malar. J. 2022, 21, 285. [Google Scholar] [CrossRef]
- Arnaldo, P.; Rovira-Vallbona, E.; Langa, J.S.; Salvador, C.; Guetens, P.; Chiheb, D.; Xavier, B.; Kestens, L.; Enosse, S.M.; Rosanas-Urgell, A. Uptake of intermittent preventive treatment and pregnancy outcomes: Health facilities and community surveys in Chókwè district, southern Mozambique. Malar. J. 2018, 17, 109. [Google Scholar] [CrossRef]
- Anchang-Kimbi, J.K.; Kalaji, L.N.; Mbacham, H.F.; Wepnje, G.B.; Apinjoh, T.O.; Sumbele, I.U.N.; Dionne-Odom, J.; Tita, A.T.N.; Achidi, E.A. Coverage and effectiveness of intermittent preventive treatment in pregnancy with sulfadox-ine-pyrimethamine (IPTp-SP) on adverse pregnancy outcomes in the Mount Cameroon area, South West Cameroon. Malar. J. 2020, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Biteghe-Bi-Essone, J.C.; Imboumy-Limoukou, R.K.; Ekogha-Ovono, J.J.; Maghendji-Nzondo, S.; Sir-Ondo-Enguier, P.N.; Oyegue, L.S.; Lekana-Douki, J.B. Intermittent preventive treatment and malaria amongst pregnant women who give birth at the Centre Hospitalier Régional Paul Moukambi de Koula-Moutou in southeastern Gabon. Malar. J. 2022, 21, 315. [Google Scholar] [CrossRef]
- Mlugu, E.M.; Minzi, O.; Kamuhabwa, A.A.R.; Aklillu, E. Effectiveness of Intermittent Preventive Treatment with Dihydroartemisinin-Piperaqunine Against Malaria in Pregnancy in Tanzania: A Randomized Controlled Trial. Clin. Pharmacol. Ther. 2021, 110, 1478–1489. [Google Scholar] [CrossRef]
- Sangho, O.; Tounkara, M.; Whiting-Collins, L.J.; Beebe, M.; Winch, P.J.; Doumbia, S. Determinants of intermittent preventive treatment with sulfadoxine–pyrimethamine in pregnant women (IPTp-SP) in Mali, a household survey. Malar. J. 2021, 20, 231. [Google Scholar] [CrossRef]
- Mama, A.; Ahiabor, C.; Tornyigah, B.; Frempong, N.A.; Kusi, K.A.; Adu, B.; Courtin, D.; Houzé, S.; Deloron, P.; Ofori, M.F.; et al. Intermittent preventive treatment in pregnancy with sulfadoxine–pyrimethamine and parasite resistance: Cross-sectional surveys from antenatal care visit and delivery in rural Ghana. Malar. J. 2022, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Mushi, V.; Mbotwa, C.H.; Zacharia, A.; Ambrose, T.; Moshi, F.V. Predictors for the uptake of optimal doses of sulfadoxine-pyrimethamine for intermittent preventive treatment of malaria during pregnancy in Tanzania: Further analysis of the data of the 2015–2016 Tanzania demographic and health survey and malaria indicat. Malar J 2021, 20, 75. [Google Scholar] [CrossRef]
- Owusu-Boateng, I.; Anto, F. Intermittent preventive treatment of malaria in pregnancy: A cross-sectional survey to assess uptake of the new sulfadoxine-pyrimethamine five dose policy in Ghana. Malar. J. 2017, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Briand, V.; Denoeud, L.; Massougbodji, A.; Cot, M. Efficacy of intermittent preventive treatment versus chloroquine prophylaxis to prevent malaria during pregnancy in Benin. J. Infect. Dis. 2008, 198, 594–601. [Google Scholar] [CrossRef]
- Moukoko, C.E.E.; Foko, L.P.K.; Ayina, A.; Tornyigah, B.; Epote, A.R.; Penda, I.C.; Eboumbou, P.E.; Ebong, S.B.; Texier, G.; Nsango, S.E.; et al. Effectiveness of Intermittent Preventive Treatment with Sulfadoxine-Pyrimethamine in regnancy: Low coverage and high prevalence of plasmodium falciparum dhfr-dhps quintuple mutants as major challenges in Douala, an Urban Setting in Cameroon. Pathogens 2023, 12, 844. [Google Scholar] [CrossRef]
- Yaya, S.; Uthman, O.A.; Amouzou, A.; Bishwajit, G. Use of intermittent preventive treatment among pregnant women in sub-Saharan Africa: Evidence from malaria indicator surveys. Trop. Med. Infect. Dis. 2018, 3, 18. [Google Scholar] [CrossRef]
- Ameh, S.; Owoaje, E.; Oyo-Ita, A.; Kabiru, C.W.; Akpet, O.E.O.; Etokidem, A.; Enembe, O.; Ekpenyong, N. Barriers to and determinants of the use of intermittent preventive treatment of malaria in pregnancy in Cross River State, Nigeria: A cross-sectional study. BMC Pregnancy Childbirth 2016, 16, 99. [Google Scholar] [CrossRef]
- Gosling, R.D.; Gesase, S.; Mosha, J.F.; Carneiro, I.; Hashim, R.; Lemnge, M.; Mosha, F.W.; Greenwood, B.; Chandramohan, D. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 374, 1521–1532. [Google Scholar] [CrossRef]
- Desai, M.; Gutman, J.; L’Lanziva, A.; Otieno, K.; Juma, E.; Kariuki, S.; Ouma, P.; Were, V.; Laserson, K.; Katana, A.; et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: An open-lab. Lancet 2015, 386, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Filler, S.J.; Kazembe, P.; Thigpen, M.; Macheso, A.; Parise, M.E.; Newman, R.D.; Steketee, R.W.; Hamel, M. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. J. Infect. Dis. 2006, 194, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Aziken, M.E.; Akubuo, K.K.; Gharoro, E.P. Efficacy of intermittent preventive treatment with sulfadoxine- pyrimethamine on placental parasitemia in pregnant women in midwestern Nigeria. Int. J. Gynecol. Obstet. 2011, 112, 30–33. [Google Scholar] [CrossRef]
- Chukwu, C.; Onuoha, H.; Katty Okorafor, K.A.; Ojomo, O.; Mokuolu, O.A.; Ekholuenetale, M. Geopolitical zones differentials in intermittent preventive treatment in pregnancy (IPTp) and long lasting insecticidal nets (LLIN) utilization in Nigeria. PLoS ONE 2021, 16, e0254475. [Google Scholar] [CrossRef]
- Gies, S.; Coulibaly, S.O.; Ouattara, F.T.; D’Alessandro, U. Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: Impact on parasitaemia, anaemia and birth weight. Trop. Med. Int. Health 2009, 14, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Darteh, E.K.M.; Dickson, K.S.; Ahinkorah, B.O.; Owusu, B.A.; Okyere, J.; Salihu, T.; Bediako, V.B.; Budu, E.; Agbemavi, W.; Edjah, J.O.; et al. Factors influencing the uptake of intermittent preventive treatment among pregnant women in sub-Saharan Africa: A multilevel analysis. Arch. Public Health 2021, 79, 182. [Google Scholar] [CrossRef] [PubMed]
- Akpa, C.O.; Akinyemi, J.O.; Umeokonkwo, C.D.; Bamgboye, E.A.; Dahiru, T.; Adebowale, A.S.; Ajayi, I.O. Uptake of intermittent preventive treatment for malaria in pregnancy among women in selected communities of Ebonyi State, Ni-geria. BMC Pregnancy Childbirth 2019, 19, 457. [Google Scholar] [CrossRef]
- Kibusi, S.M.; Kimunai, E.; Hines, C.S. Predictors for uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in Tanzania. BMC Public Health 2015, 15, 540. [Google Scholar] [CrossRef]
- Yaya, S.; Kota, K.; Buh, A.; Bishwajit, G. Antenatal visits are positively associated with uptake of tetanus toxoid and intermittent preventive treatment in pregnancy in Ivory Coast. BMC Public Health 2019, 19, 1467. [Google Scholar] [CrossRef]
- Klu, D.; Owusu, L. Factors affecting the uptake of optimal doses of intermittent preventive treatment of malaria in pregnancy using sulfadoxine pyrimethamine in Ghana: New evidence from the 2019 malaria indicator survey. J. Public Health 2023, 1–11. [Google Scholar] [CrossRef]
- Amoran, O.E.; Ariba, A.A.; Iyaniwura, C.A. Determinants of intermittent preventive treatment of malaria during pregnancy (IPTp) utilization in a rural town in Western Nigeria. Reprod. Health 2012, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Msyamboza, K.P.; Savage, E.J.; Kazembe, P.N.; Gies, S.; Kalanda, G.; D’alessandro, U.; Brabin, B.J. Community-based distribution of sulfadoxine-pyrimethamine for intermittent preventive treatment of malaria during pregnancy improved coverage but reduced antenatal attendance in southern Malawi. Trop. Med. Int. Health 2009, 14, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Aberese-Ako, M.; Magnussen, P.; Ampofo, G.D.; Gyapong, M.; Ansah, E.; Tagbor, H. An ethnographic study of how health system, socio-cultural and individual factors influence uptake of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine in a Ghanaian context. PLoS ONE 2021, 16, e0257666. [Google Scholar] [CrossRef]
- Yoder, P.S.; Nsabagasani, X.; Eckert, E.; Moran, A.; Yé, Y. Perspectives of health care providers on the provision of intermittent preventive treatment in pregnancy in health facilities in Malawi. BMC Health Serv. Res. 2015, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, T.C.D.A.; Agboton-Zoumenou, M.A.; Garcia, A.; Massougbodji, A.; Briand, V.; Imorou, Y.; Cottrell, G. Field evaluation of the intermittent preventive treatment of malaria during pregnancy (IPTp) in Benin: Evolution of the coverage rate since its implementation. Parasit. Vectors 2011, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Awantang, G.N.; Babalola, S.O.; Koenker, H.; Fox, K.A.; Toso, M.; Lewicky, N. Malaria-related ideational factors and other correlates associated with intermittent preventive treatment among pregnant women in Madagascar. Malar. J. 2018, 17, 176. [Google Scholar] [CrossRef]
- Gies, S.; Coulibaly, S.O.; Ky, C.; Ouattara, F.T.; Brabin, B.I.; D’Alessandro, U. Community-based promotional campaign to improve uptake of intermittent preventive antimalarial treatment in pregnancy in Burkina Faso. Am. J. Trop. Med. Hyg. 2009, 80, 460–469. [Google Scholar] [CrossRef]
- Ndyomugyenyi, R.; Katamanywa, J. Intermittent preventive treatment of malaria in pregnancy (IPTp): Do frequent antenatal care visits ensure access and compliance to IPTp in Ugandan rural communities? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 536–540. [Google Scholar] [CrossRef]
- Gutman, J.; Slutsker, L. Intermittent preventive treatment with sulfadoxine-pyrimethamine: More than just an Antimalarial? Am. J. Trop. Med. Hyg. 2017, 96, 9–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gutman, J.R.; Stephens, D.K.; Tiendrebeogo, J.; Badolo, O.; Dodo, M.; Burke, D.; Williamson, J.; Vibbert, K.; Youll, S.J.; Savadogo, Y.; et al. A cluster randomized trial of delivery of intermittent preventive treatment of malaria in pregnancy at the community level in Burkina Faso. Malar. J. 2020, 19, 282. [Google Scholar] [CrossRef]
- Mubyazi, G.M. Perceived and real costs of antenatal care seeking and their implications for women’s access to intermittent preventive treatment of malaria in pregnancy in rural Tanzanian districts. J. Public Health Epidemiol. 2015, 1, 1009. [Google Scholar]
- Diala, C.C.; Pennas, T.; Marin, C.; Belay, K.A. Perceptions of intermittent preventive treatment of malaria in pregnancy (IPTp) and barriers to adherence in Nasarawa and Cross River States in Nigeria. Malar. J. 2013, 12, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Brentlinger, P.E.; Dgedge, M.; Correia, M.A.C.; Rojas, A.J.B.; Saúte, F.; Gimbel-Sherr, K.H.; A Stubbs, B.; Mercer, M.A.; Gloyd, S. Intermittent preventive treatment of malaria during pregnancy in central Mozambique. Bull. World Health Organ. 2007, 85, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rassi, C.; Graham, K.; Mufubenga, P.; King, R.; Meier, J.; Gudoi, S.S. Assessing supply-side barriers to uptake of intermittent preventive treatment for malaria in pregnancy: A qualitative study and document and record review in two regions of Uganda. Malar. J. 2016, 15, 341. [Google Scholar] [CrossRef]
- Huynh, B.-T.; Fievet, N.; Briand, V.; Borgella, S.; Massougbodji, A.; Deloron, P.; Cot, M. Consequences of gestational malaria on birth weight: Finding the best timeframe for intermittent preventive treatment administration. PLoS ONE 2012, 7, e35342. [Google Scholar] [CrossRef]
- Mubyazi, G.; Bloch, P.; Kamugisha, M.; Kitua, A.; Ijumba, J. Intermittent preventive treatment of malaria during pregnancy: A qualitative study of knowledge, attitudes and practices of district health managers, antenatal care staff and pregnant women in Korogwe District, North-Eastern Tanzania. Malar. J. 2005, 4, 31. [Google Scholar] [CrossRef]
- Launiala, A.; Honkasalo, M.L. Ethnographic study of factors influencing compliance to intermittent preventive treatment of malaria during pregnancy among Yao women in rural Malawi. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 980–989. [Google Scholar] [CrossRef]
- Oyefabi, A.; Sambo, M.; Sabitu, K. Effect of primary health care workers training on the knowledge and utilization of intermittent preventive therapy for malaria in pregnancy in Zaria, Nigeria. J. Med. Trop. 2015, 17, 4. [Google Scholar] [CrossRef]
- Mosha, D.; Chilongola, J.; Ndeserua, R.; Mwingira, F.; Genton, B. Effectiveness of intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy on placental malaria, maternal anaemia and birthweight in areas with high and low malaria transmission intensity in Tanzania. Trop. Med. Int. Health 2014, 19, 1048–1056. [Google Scholar] [CrossRef]
- Falade, C.O.; Yusuf, B.O.; Fadero, F.F.; Mokuolu, O.A.; Hamer, D.H.; Salako, L.A. Intermittent preventive treatment with sulphadoxine-pyrimethamine is effective in preventing maternal and placental malaria in Ibadan, south-western Nigeria. Malar. J. 2007, 6, 88. [Google Scholar] [CrossRef]
- de Kock, M.; Tarning, J.; Workman, L.; Nyunt, M.; Adam, I.; Barnes, K.; Denti, P. Pharmacokinetics of sulfadoxine and pyrimethamine for intermittent preventive treatment of malaria during pregnancy and after delivery. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 430–438. [Google Scholar] [CrossRef]
- Umeh, U.A.; Obi, S.N.; E Onah, H.; Ugwu, E.O.V.; Ajah, L.O.; Umeh, C.R.; Okafor, I.I. The impact of intermittent preventive treatment with sulfadoxine- pyrimethamine on the prevalence of malaria parasitaemia in pregnancy. Trop. Doct 2012, 42, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Gutman, J.; Mwandama, D.; Wiegand, R.E.; Ali, D.; Mathanga, D.P.; Skarbinski, J. Effectiveness of intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy on maternal and birth outcomes in Machinga District, Malawi. J. Infect. Dis. 2013, 208, 907–916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Offianan, A.T.; Penali, L.K.; Coulibaly, M.A.; Tiacoh, N.L.; Ako, A.A.B.; Adji, E.G.; Coulibaly, B.; Koffi, D.; Sarr, D.; Jambou, R.; et al. Comparative efficacy of uncontrolled and controlled intermittent preventive treatment during pregnancy (IPTp) with combined use of LLTNs in high resistance area to sulfadoxinepyrimethamine in Côte d’ivoire. Infect. Drug Resist. 2012, 5, 53–63. [Google Scholar] [CrossRef]
- Awine, T.; Belko, M.M.; Oduro, A.R.; Oyakhirome, S.; Tagbor, H.; Chandramohan, D.; Milligan, P.; Cairns, M.; Greenwood, B.; Williams, J.E. The risk of malaria in Ghanaian infants born to women managed in pregnancy with in-termittent screening and treatment for malaria or intermittent preventive treatment with sulfadoxine/pyrimethamine. Malar. J. 2016, 15, 46. [Google Scholar] [CrossRef][Green Version]
- Maiga, O.M.; Kayentao, K.; Traore, B.T.; Djimde, A.; Diallo, M.; Ongoiba, A.; Doumtabé, D.; Doumbo, S.; Traoré, M.S.; Dara, A.; et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the pre-vention of malaria during pregnancy in mali: A randomized controlled trial. Clin. Infect. Dis. 2011, 53, 215–223. [Google Scholar] [CrossRef]
- Tagbor, H.; Bruce, J.; Agbo, M.; Greenwood, B.; Chandramohan, D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: A randomised controlled non-inferiority trial. PLoS ONE 2010, 5, e14425. [Google Scholar] [CrossRef] [PubMed]
- COSMIC Consortium. Community-based malaria screening and treatment for pregnant women receiving standard intermittent preventive treatment with sulfadoxine-pyrimethamine: A multicenter (the gambia, burkina faso, and benin) cluster-randomized controlled trial. Clin. Infect. Dis. 2019, 68, 586–596. [Google Scholar] [CrossRef]
- Gill, C.J.; MacLeod, W.B.; Mwanakasale, V.; Chalwe, V.; Mwananyanda, L.; Champo, D.; Mukwamataba, D.; Chilengi, R.; Thea, D.M.; Hamer, D.H. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. J. Infect. Dis. 2007, 196, 1577–1584. [Google Scholar] [CrossRef]
- Mbonye, A.K.; Bygbjerg, I.C.; Magnussen, P. Intermittent preventive treatment of malaria in pregnancy: A new delivery system and its effect on maternal health and pregnancy outcomes in Uganda. Bull. World Health Organ. 2008, 86, 93–100. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Mombo-Ngoma, G.; Ouédraogo, S.; Kakolwa, M.A.; Abdulla, S.; Accrombessi, M.; Aponte, J.J.; Akerey-Diop, D.; Basra, A.; Briand, V.; et al. Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Negative Women: A Multicentre Randomized Controlled Trial. PLoS Med. 2014, 11, e1001733. [Google Scholar] [CrossRef]
- Mlugu, E.M.; Minzi, O.; Asghar, M.; Färnert, A.; Kamuhabwa, A.A.R.; Aklillu, E. Effectiveness of sulfadoxine–pyrimethamine for intermittent preventive treatment of malaria and adverse birth outcomes in pregnant women. Pathogens 2020, 9, 207. [Google Scholar] [CrossRef]
- Bedia-Tanoh, A.V.; Konaté, A.; Gnagne, A.P.; Miezan, A.J.S.; Kiki-Barro, P.C.M.; Angora, K.E.; Kassi, K.F.; Vanga-Bosson, A.H.; Djohan, V.; Menan, E.I.H.; et al. Effectiveness of intermittent preventive treatment with Sulfadoxine-Pyrimethamine in pregnant women in San Pedro, Côte D’Ivoire. Pathog. Glob. Health 2021, 115, 325–330. [Google Scholar] [CrossRef]
- Builder, M.; Anzaku, S.; Joseph, S. Effectiveness of Intermittent Preventive Treatment in Pregnancy with Sulphadox-ine-pyrimethamine against malaria in Northern Nigeria. Int. J. Recent Sci. Res. 2019, 10, 30693–30695. [Google Scholar]
- Kayiba, N.K.; Yobi, D.M.; Tchakounang, V.R.K.; Mvumbi, D.M.; Kabututu, P.Z.; Devleesschauwer, B.; Mukomena, E.S.; DeMol, P.; Hayette, M.-P.; Mvumbi, G.L.; et al. Evaluation of the usefulness of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine in a context with increased resistance of Plasmodium falciparum in Kingasani Hospital, Kinshasa in the Democratic Republic of Congo. Infect. Genet. Evol. 2021, 94, 105009. [Google Scholar] [CrossRef]
- Likwela, J.L.; D’Alessandro, U.; Lokwa, B.L.; Meuris, S.; Dramaix, M.W. Sulfadoxine-pyrimethamine resistance and intermittent preventive treatment during pregnancy: A retrospective analysis of birth weight data in the Democratic Republic of Congo (DRC). Trop. Med. Int. Health 2012, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.O.; Ceesay, F.K.; Obed, S.A.; Adjei, A.A.; Gyasi, R.K.; Rodney, P.; Ndjakani, Y.; Anderson, W.A.; Lucchi, N.W.; Stiles, J.K. Intermittent preventive treatment with sulfadoxine-pyrimethamine against malaria and anemia in pregnant women. Am. J. Trop. Med. Hyg. 2011, 85, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.E.; Kuile, F.O.t.; Rerolle, F.; Glymour, M.M.; Shiboski, S.; Gosling, R.; Gutman, J.; Kakuru, A.; Desai, M.; Kajubi, R.; et al. Overall, anti-malarial, and non-malarial effect of intermittent preventive treatment during pregnancy with sulfa-doxine-pyrimethamine on birthweight: A mediation analysis. Lancet Glob. Health 2020, 8, e942–e953. [Google Scholar] [CrossRef]
- Diarra, S.S.; Konaté, D.; Diawara, S.I.; Tall, M.; Diakité, M.; Doumbia, S. Factors Associated With Intermittent Preventive Treatment of Malaria during Pregnancy in Mali. J. Parasitol. 2019, 105, 299–302. [Google Scholar] [CrossRef]
- Mikomangwa, W.P.; Oms, M.; Aklillu, E.; Kamuhabwa, A.A.R. Adverse birth outcomes among mothers who received intermittent preventive treatment with Sulphadoxine-Pyrimethamine in the low malaria transmission region. BMC Pregnancy Childbirth 2019, 19, 236. [Google Scholar] [CrossRef] [PubMed]
- Fokam, E.B.; Ngimuh, L.; Anchang-Kimbi, J.K.; Wanji, S. Assessment of the usage and effectiveness of intermittent preventive treatment and insecticide-treated nets on the indicators of malaria among pregnant women attending antenatal care in the Buea Health District, Cameroon. Malar. J. 2016, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Mbonye, A.K.; Hansen, K.S.; Bygbjerg, I.C.; Magnussen, P. Intermittent preventive treatment of malaria in pregnancy: The incremental cost-effectiveness of a new delivery system in Uganda. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kayentao, K.; Kodio, M.; Newman, R.D.; Maiga, H.; Doumtabe, D.; Ongoiba, A.; Coulibaly, D.; Keita, A.S.; Maiga, B.; Mungai, M.; et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J. Infect. Dis. 2005, 191, 109–116. [Google Scholar] [CrossRef][Green Version]
- Serra-Casas, E.; Menéndez, C.; Bardají, A.; Quintó, L.; Dobaño, C.; Sigauque, B.; Jiménez, A.; Mandomando, I.; Chauhan, V.S.; Chitnis, C.E.; et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J. Infect. Dis. 2010, 201, 123–131. [Google Scholar] [CrossRef]
- Hamer, D.H.; Mwanakasale, V.; MacLeod, W.B.; Chalwe, V.; Mukwamataba, D.; Champo, D.; Mwananyanda, L.; Chilengi, R.; Mubikayi, L.; Mulele, C.K.; et al. Two-dose versus monthly intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine in HIV-seropositive pregnant Zambian women. J. Infect. Dis. 2007, 196, 1585–1594. [Google Scholar] [CrossRef]
- Tagbor, H.; Cairns, M.; Bojang, K.; Coulibaly, S.O.; Kayentao, K.; Williams, J.; Abubakar, I.; Akor, F.; Mohammed, K.; Bationo, R.; et al. A non-inferiority, individually randomized trial of intermittent screening and treatment versus inter-mittent preventive treatment in the control of malaria in pregnancy. PLoS ONE 2015, 10, e0132247. [Google Scholar] [CrossRef]
- Menéndez, C.; Bardají, A.; Sigauque, B.; Romagosa, C.; Sanz, S.; Serra-Casas, E.; Macete, E.; Berenguera, A.; David, C.; Dobaño, C.; et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS ONE 2008, 3, e1934. [Google Scholar] [CrossRef]
- Cisse, M.; Awandare, G.A.; Soulama, A.; Tinto, H.; Hayette, M.P.; Guiguemdé, R.T. Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar. J. 2017, 16, 38. [Google Scholar] [CrossRef]
- Obase, B.N.; Bigoga, J.D.; Nsagha, D.S. Malaria and HIV co-infection among pregnant women in Africa: Prevalence, effect on immunity and clinical management: Review. Int. J. Transl. Med. 2023, 3, 187–202. [Google Scholar] [CrossRef]
- Tarimo, D.S. Influencing Uptake of Intermittent Preventive Therapy with Sulfadoxine. East Afr. J. Public Health 2007, 4, 80–83. [Google Scholar] [PubMed]
- Esu, E.; Berens-Riha, N.; Pritsch, M.; Nwachuku, N.; Loescher, T.; Meremikwu, M. Intermittent screening and treatment with artemether-lumefantrine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in pregnancy: A facility-based, open-label, non-inferiority trial in Nigeria. Malar. J. 2018, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, S.O.; Falade, C.O.; Ajayi, I.O. Knowledge and utilization of intermittent preventive treatment for malaria among pregnant women attending antenatal clinics in primary health care centers in rural southwest, Nigeria: A cross-sectional study. BMC Pregnancy Childbirth 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Manun’Ebo, M.F.; Meremikwu, M.; Rabeza, V.R.; Sacoor, C.; Figueroa-Romero, A.; Arikpo, I.; Macete, E.; Ndombe, D.M.; Ramananjato, R.; et al. The impact of community delivery of intermittent preventive treatment of malaria in pregnancy on its coverage in four sub-Saharan African countries (Democratic Republic of the Congo, Madagascar, Mozambique, and Nigeria): A quasi-experimental multicentre evaluation. Lancet Glob. Health 2023, 11, e566–e574. [Google Scholar] [CrossRef]
- Madanitsa, M.; Barsosio, H.C.; Minja, D.T.R.; Mtove, G.; A Kavishe, R.; Dodd, J.; Saidi, Q.; Onyango, E.D.; Otieno, K.; Wang, D.; et al. Effect of monthly intermittent preventive treatment with dihydroartemisinin–piperaquine with and without azithromycin versus monthly sulfadoxine–pyrimethamine on adverse pregnancy outcomes in Africa: A dou-ble-blind randomised, partly placebo-controlled trial. Lancet 2023, 401, 1020–1036. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).