The Critical Role of Penicillin in Syphilis Treatment and Emerging Resistance Challenges
Abstract
:1. Introduction
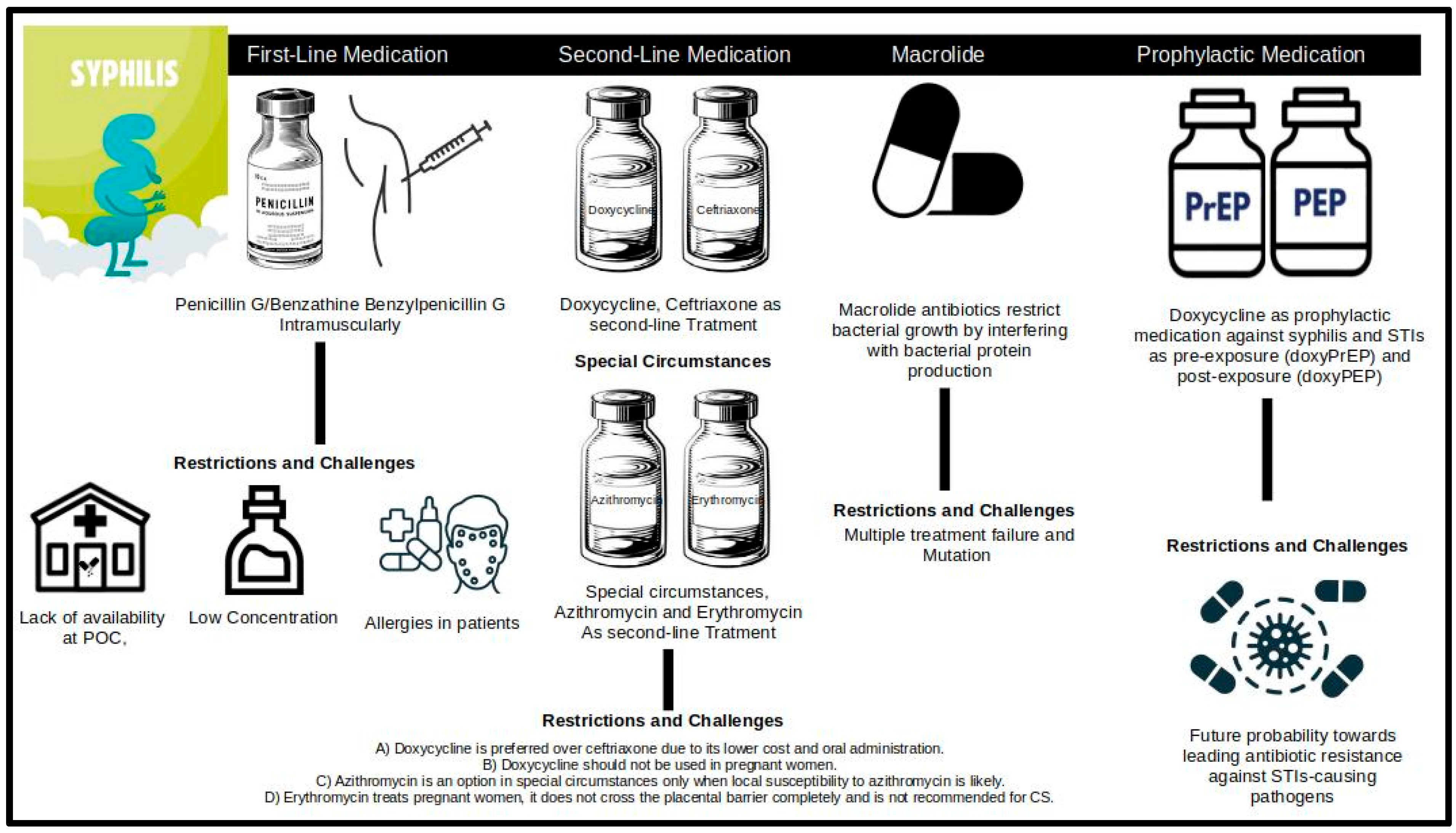
2. Treatment Regimen for Syphilis
3. Antimicrobial Resistance in T. pallidum
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ubals, M.; Nadal-Baron, P.; Arando, M.; Rivero, Á.; Mendoza, A.; Jorro, V.D.; Ouchi, D.; Pérez-Mañá, C.; Álvarez, M.; Alemany, A.; et al. Oral linezolid compared with benzathine penicillin G for treatment of early syphilis in adults (Trep-AB Study) in Spain: A prospective, open-label, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2024, 24, 404–416. [Google Scholar] [CrossRef]
- WHO. Implementing the Global Health Sector Strategies on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2022–2030. 2024. Available online: https://www.who.int/publications/i/item/9789240094925 (accessed on 26 July 2024).
- Stamm, L.V. Hope for new antibiotics for syphilis treatment. EBioMedicine 2021, 66, 103320. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, D.; Partridge, E.; Lakshminrusimha, S. Congenital Syphilis—An Illustrative Review. Children 2023, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Jamal, S.B.; Gomes, L.G.R.; Profeta, R.; Sales-Campos, H.; Oliveira, C.J.F.; Aburjaile, F.F.; Tiwari, S.; Barh, D.; da Silva, M.V.; et al. Neuroinformatics Insights towards Multiple Neurosyphilis Complications. Venereology 2022, 1, 135–160. [Google Scholar] [CrossRef]
- Taylor, M.M.; Zhang, X.; Nurse-Findlay, S.; Hedman, L.; Kiarie, J. The amount of penicillin needed to prevent mother-to-child transmission of syphilis. Bull. World Health Organ. 2016, 94, 559–559A. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for the Treatment of Treponema Pallidum (Syphilis); World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.who.int/publications/i/item/9789241549714 (accessed on 26 July 2024).
- Bungener, S.L.; Post, L.; Berends, I.; Steensma, T.D.; de Vries, A.L.; Popma, A. Talking About Sexuality With Youth: A Taboo in Psychiatry? J. Sex. Med. 2022, 19, 421–429. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Prim. 2017, 3, 17073. [Google Scholar] [CrossRef]
- Elendu, C.B.; Amaechi, D.C.M.; Elendu, I.D.B.; Elendu, T.C.B.; Amaechi, E.C.M.; Usoro, E.U.M.; Chima-Ogbuiyi, N.L.B.; Agbor, D.B.A.; Onwuegbule, C.J.M.; Afolayan, E.F.M.; et al. Global perspectives on the burden of sexually transmitted diseases: A narrative review. Medicine 2024, 103, e38199. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, Z.; Zhang, X.; Huang, S.; Ding, X.; Zhou, J.; Yao, J.; Li, W.; Liu, S.; Zhao, F. Resurgence of syphilis: Focusing on emerging clinical strategies and preclinical models. J. Transl. Med. 2023, 21, 917. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.M.; Kasal, N.; Montag, C.; Dawdani, A.; Almirol, E.; Montgomery, J.M.C.; Zimmer, D.; Ridgway, J.; A Schneider, J. Factors Related to the Rise of Congenital Syphilis From the Perspectives of Prenatal Providers and Birthing Parents in Chicago, IL, USA. Open Forum Infect. Dis. 2024, 11, ofae595. [Google Scholar] [CrossRef]
- Salomè, S.; Cambriglia, M.D.; Montesano, G.; Capasso, L.; Raimondi, F. Congenital Syphilis: A Re-Emerging but Preventable Infection. Pathogens 2024, 13, 481. [Google Scholar] [CrossRef]
- Cao, W.; Thorpe, P.G.; O’callaghan, K.; Kersh, E.N. Advantages and limitations of current diagnostic laboratory approaches in syphilis and congenital syphilis. Expert Rev. Anti-Infect. Ther. 2023, 21, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Seghers, F.; Taylor, M.M.; Storey, A.; Dong, J.; Wi, T.C.; Wyber, R.; Ralston, K.; Nguimfack, B.D. Securing the supply of benzathine penicillin: A global perspective on risks and mitigation strategies to prevent future shortages. Int. Health 2023, 16, 279–282. [Google Scholar] [CrossRef]
- WHO. Technical Consultation on Preventing and Managing Global Stock Outs of Medicines; WHO: Geneva, Switzerland, 2015. Available online: https://www.who.int/publications/m/item/technical-consultation-on-preventing-and-managing-global-stock-outs-of-medicines (accessed on 26 July 2024).
- Nurse-Findlay, S.; Taylor, M.M.; Savage, M.; Mello, M.B.; Saliyou, S.; Lavayen, M.; Seghers, F.; Campbell, M.L.; Birgirimana, F.; Ouedraogo, L.; et al. Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews. PLoS Med. 2017, 14, e1002473. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.S.; de Souza, A.S.S.; Braga, J.U. A quem afetou o desabastecimento de penicilina para sífilis no Rio de Janeiro, 2013–2017? Rev. Saude Publica 2020, 54, 109. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guideline on Syphilis Screening and Treatment for Pregnant Women; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/publications/i/item/9789241550093 (accessed on 26 July 2024).
- Ikeuchi, K.; Fukushima, K.; Tanaka, M.; Yajima, K.; Imamura, A. Clinical efficacy and tolerability of 1.5 g/day oral amoxicillin therapy without probenecid for the treatment of syphilis. Sex. Transm. Infect. 2021, 98, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.S.; Stafylis, C.; Celum, C.; Grennan, T.; Haire, B.; Kaldor, J.; Luetkemeyer, A.F.; Saunders, J.M.; Molina, J.-M.; Klausner, J.D. Doxycycline Prophylaxis for Bacterial Sexually Transmitted Infections. Clin. Infect. Dis. 2019, 70, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Cornelisse, V.J.; Riley, B.; A Medland, N. Australian consensus statement on doxycycline post-exposure prophylaxis (doxy-PEP) for the prevention of syphilis, chlamydia and gonorrhoea among gay, bisexual and other men who have sex with men. Med. J. Aust. 2024, 220, 381–386. [Google Scholar] [CrossRef]
- Luetkemeyer, A. Taking Antibiotic after Sex Cuts STIs by Two-Thirds, ‘DoxyPEP’ Study Finds—The 24th International AIDS Conference—Doxycycline Study Was Stopped Early Due to Its High Efficacy, International AIDS Conference Hears. August 2022. Available online: https://www.aidsmap.com/news/jul-2022/taking-antibiotic-after-sex-cuts-stis-two-thirds-doxypep-study-finds (accessed on 26 July 2024).
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Stamm, L.V. Global Challenge of Antibiotic-Resistant Treponema pallidum. Antimicrob. Agents Chemother. 2010, 54, 583–589. [Google Scholar] [CrossRef]
- Tantalo, L.C.; Lieberman, N.A.P.; Pérez-Mañá, C.; Suñer, C.; Mayans, M.V.; Ubals, M.; González-Beiras, C.; Rodríguez-Gascón, A.; Canut, A.; González-Candelas, F.; et al. Antimicrobial susceptibility of Treponema pallidum subspecies pallidum: An in-vitro study. Lancet Microbe 2023, 4, e994–e1004. [Google Scholar] [CrossRef] [PubMed]
- Elbiss, H.; Osman, N. Placental transport of Erythromycin and its effect on placental inflammatory factors. Pak. J. Med. Sci. 2022, 39, 75–79. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chi, K.-H.; Pillay, A.; Nachamkin, E.; Su, J.R.; Ballard, R.C. Detection of the A2058G and A2059G 23S rRNA Gene Point Mutations Associated with Azithromycin Resistance in Treponema pallidum by Use of a TaqMan Real-Time Multiplex PCR Assay. J. Clin. Microbiol. 2013, 51, 908–913. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Engelman, J.; Kent, C.K.; Lukehart, S.A.; Godornes, C.; Klausner, J.D. Azithromycin-Resistant Syphilis Infection: San Francisco, California, 2000–2004. Clin. Infect. Dis. 2006, 42, 337–345. [Google Scholar] [CrossRef]
- Martin, I.E.; Tsang, R.S.W.; Sutherland, K.; Tilley, P.; Read, R.; Anderson, B.; Roy, C.; Singh, A.E. Molecular Characterization of Syphilis in Patients in Canada: Azithromycin Resistance and Detection of Treponema pallidum DNA in Whole-Blood Samples versus Ulcerative Swabs. J. Clin. Microbiol. 2009, 47, 1668–1673. [Google Scholar] [CrossRef]
- Gultom, D.A.; Rosana, Y.; Efendi, I.; Indriatmi, W.; Yasmon, A. Detection and identification of azithromycin resistance mutations on Treponema pallidum 23S rRNA gene by nested multiplex polymerase chain reaction. Med. J. Indones. 2017, 26, 90–96. [Google Scholar] [CrossRef]
- Matějková, P.; Flasarová, M.; Zákoucká, H.; Bořek, M.; Křemenová, S.; Arenberger, P.; Woznicová, V.; Weinstock, G.M.; Šmajs, D. Macrolide treatment failure in a case of secondary syphilis: A novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J. Med Microbiol. 2009, 58, 832–836. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Hu, W.; Luo, H.; Zhou, J.; Li, C.; Chen, C. Gene subtype analysis of Treponema pallidum for drug resistance to azithromycin. Exp. Ther. Med. 2018, 16, 1009–1013. [Google Scholar] [CrossRef]
- Wang, X.; Abliz, P.; Deng, S. Molecular Characteristics of Macrolide Resistance in Treponema pallidum from Patients with Latent Syphilis in Xinjiang, China. Infect. Drug Resist. 2023, 16, 1231–1236. [Google Scholar] [CrossRef]
- Alsallamin, I.; Alsallamin, A.; Greene, S.; Hammad, F.; Bawwab, A. A Case of Neurosyphilis With Penicillin Failure. Cureus 2022, 14, e21456. [Google Scholar] [CrossRef]
- Liu, D.; He, S.-M.; Zhu, X.-Z.; Liu, L.-L.; Lin, L.-R.; Niu, J.-J.; Yang, T.-C. Molecular Characterization Based on MLST and ECDC Typing Schemes and Antibiotic Resistance Analyses of Treponema pallidum subsp. pallidum in Xiamen, China. Front. Cell. Infect. Microbiol. 2021, 10, 618747. [Google Scholar] [CrossRef]
- Mora-Ochomogo, M.; Lohans, C.T. β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med. Chem. 2021, 12, 1623–1639. [Google Scholar] [CrossRef]
- Mi, H.-F.; Shen, X.; Chen, X.-Q.; Zhang, X.-L.; Ke, W.-J.; Xiao, Y. Association between treatment failure in patients with early syphilis and penicillin resistance-related gene mutations of Treponema pallidum: Protocol for a multicentre nested case–control study. Front. Med. 2023, 10, 1131921. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.T.; Manzoli, I.R.; Soares, D.B.; Reis, G.P. Treponema pallidum: Mecanismos de resistência aos antibióticos. Rev. Multidiscip. Saúde 2021, 2, 11. [Google Scholar] [CrossRef]
- Lopes, M.D.d.S.; Carvalho, M.S.; Silva, C.d.A.; Martins, T.L.S.; Silva, G.R.d.C.e. Resistência do treponema pallidum frente ao tratamento convencional da sífilis. Rev. Eletrônica Acervo Enferm. 2024, 24, e19369. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Liu, Z.; Xie, Y. Treponema pallidum outer membrane proteins: Current status and prospects. Pathog. Dis. 2022, 80, ftac023. [Google Scholar] [CrossRef]
- Tao, Y.-T.; Gao, T.-Y.; Li, H.-Y.; Ma, Y.-T.; Li, H.-J.; Xian-Yu, C.-Y.; Deng, N.-J.; Zhang, C. Global, regional, and national trends of syphilis from 1990 to 2019: The 2019 global burden of disease study. BMC Public Health 2023, 23, 754. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. 18 July 2022. Available online: https://www.who.int/publications/i/item/9789240053779 (accessed on 26 July 2024).
- Barros, G.M.C.; Carvalho, D.D.A.; Cruz, A.S.; Morais, E.K.L.; Sales-Moioli, A.I.L.; Pinto, T.K.B.; Almeida, M.C.D.; Sanchez-Gendriz, I.; Fernandes, F.; Barbalho, I.M.P.; et al. Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis. Int. J. Environ. Res. Public Health 2022, 19, 16206. [Google Scholar] [CrossRef] [PubMed]
- Silva, Â.A.O.; Lima, A.A.; Vasconcelos, L.C.M.; Almeida, R.A.; Freitas, N.E.M.; Oliva, T.A.; Silva, M.F.d.C.R.d.; Marchini, F.K.; Zanchin, N.I.T.; de Siqueira, I.C.; et al. Evaluating the diagnostic accuracy of TpN17 and TmpA recombinant proteins in syphilis detection: A phase II study. Front. Microbiol. 2024, 15, 1348437. [Google Scholar] [CrossRef]
- Taylor, M.M.; Kara, E.O.; Araujo, M.A.L.; Silveira, M.F.; Miranda, A.E.; Coelho, I.C.B.; Bazzo, M.L.; Pereira, G.F.M.; Giozza, S.P.; Bermudez, X.P.D.; et al. Phase II trial evaluating the clinical efficacy of cefixime for treatment of active syphilis in non-pregnant women in Brazil (CeBra). BMC Infect. Dis. 2020, 20, 405. [Google Scholar] [CrossRef] [PubMed]
| Acquired Resistance of T. pallidum to Recommended Treatment Drugs | ||
|---|---|---|
| Treatment | Type of Resistance or Hypotheses | Year |
| MACROLIDES | First clinical failure. | 1964 [25] |
| First case of congenital syphilis. | 1976 [27] | |
| Erythromycin | Identified 23S rRNA gene mutation in the SS14 strain of T. pallidum. | 1977 [25] |
| Azithromycin | A2058G mutation identified, a cross-mutation of the 23S rRNA gene from 1977 | 2000–2004 [28] |
| Spiramycin | A new 23S rRNA gene mutation, A2059G, was discovered. | 2009 [32] |
| BETA-LACTAMS | First clinical failure was initially not linked to resistance. | 2022 [36] |
| Benzathine Penicillin G (Example: Methicillin) | First penicillin failure. | 2023 [39] |
| Non-synonymous single nucleotide polymorphism (SNP) mutation, encoding penicillin regulatory proteins, found in Chinese strains. | ||
| Penicillin-based drugs | Essential M proteins: strengthening of the bacterial capsule; increased adhesion capacity; immune system evasion | 2021–2022 [40,41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, A.K.; Rodrigues Gomes, L.G.; Ferreira Maciel de Oliveira, A.; de Castro Soares, S.; Azevedo, V. The Critical Role of Penicillin in Syphilis Treatment and Emerging Resistance Challenges. Diseases 2025, 13, 41. https://doi.org/10.3390/diseases13020041
Jaiswal AK, Rodrigues Gomes LG, Ferreira Maciel de Oliveira A, de Castro Soares S, Azevedo V. The Critical Role of Penicillin in Syphilis Treatment and Emerging Resistance Challenges. Diseases. 2025; 13(2):41. https://doi.org/10.3390/diseases13020041
Chicago/Turabian StyleJaiswal, Arun Kumar, Lucas Gabriel Rodrigues Gomes, Aline Ferreira Maciel de Oliveira, Siomar de Castro Soares, and Vasco Azevedo. 2025. "The Critical Role of Penicillin in Syphilis Treatment and Emerging Resistance Challenges" Diseases 13, no. 2: 41. https://doi.org/10.3390/diseases13020041
APA StyleJaiswal, A. K., Rodrigues Gomes, L. G., Ferreira Maciel de Oliveira, A., de Castro Soares, S., & Azevedo, V. (2025). The Critical Role of Penicillin in Syphilis Treatment and Emerging Resistance Challenges. Diseases, 13(2), 41. https://doi.org/10.3390/diseases13020041









