Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Search Terms and Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
3. Epidemiology, Anatomy and Inflammatory–Oxidative Mechanisms in BPH
3.1. Global Epidemiology and Clinical Symptomatology
3.2. Prostatic Zonal Anatomy and Cellular Interplay
3.3. Chronic Inflammation as a Contributor to BPH Pathogenesis
3.4. Emerging Perspectives on Oxidative Stress in BPH
4. Oxidative Stress Pathways in BPH
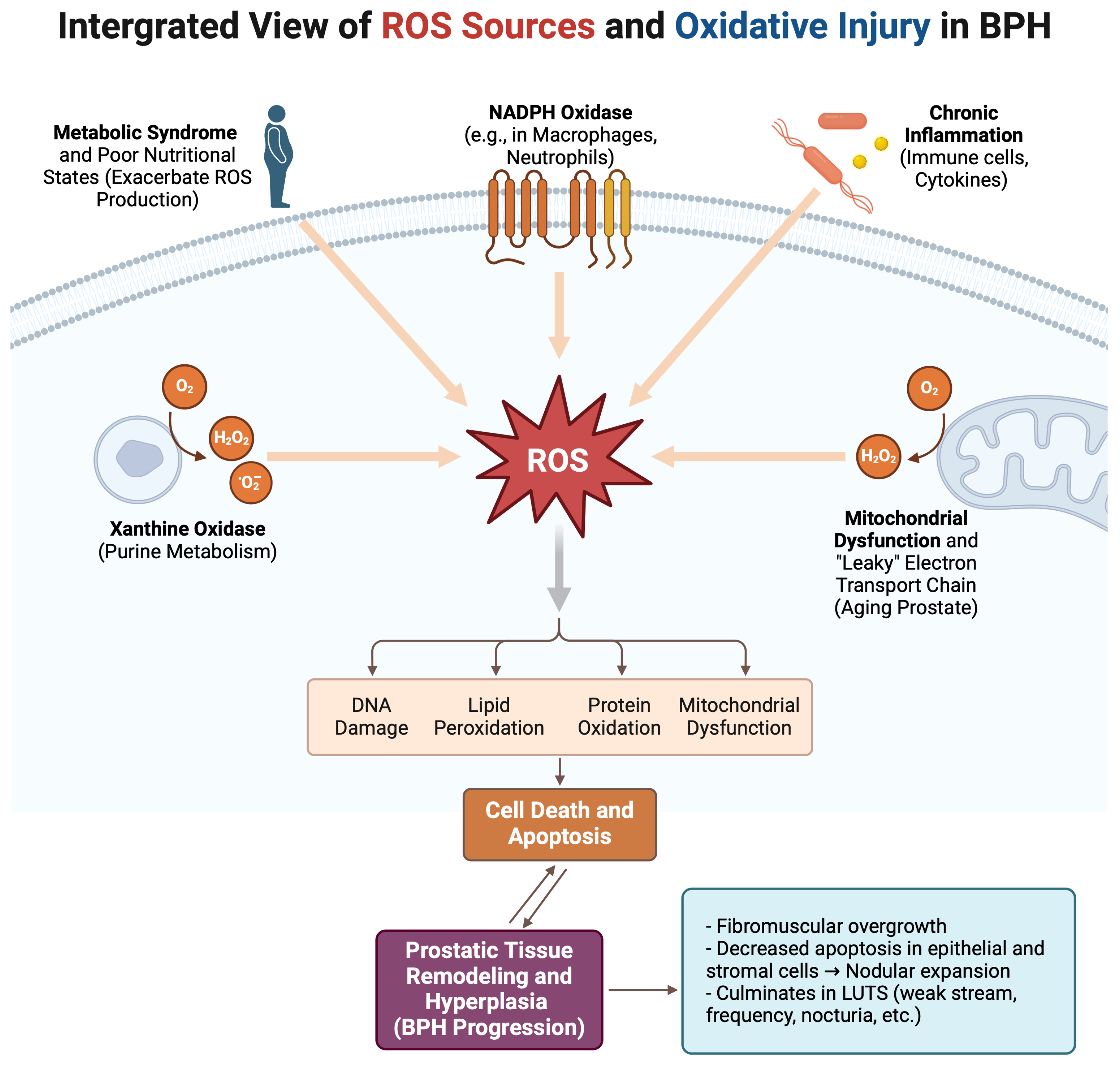
4.1. Defining Oxidative Stress and Its Primary Sources
4.2. Intracellular Signaling Roles of ROS
4.3. Antioxidant Deficiencies and Their Impact on BPH
5. The Interplay of Apoptosis and Oxidative Stress in BPH Progression
5.1. Regulation of Apoptosis in Prostate Homeostasis
5.2. OS-Mediated Pathways of Cell Death and Survival
5.3. Synergistic Interactions Between Apoptosis and Inflammation
6. Hormonal Interplay and Oxidative Stress in BPH
6.1. Androgenic Regulation and ROS Generation
6.2. Estrogenic Influencens and Prostate Pathobiology
6.3. Metabolic Syndrome and Hormonal–Inflammatory Interactions
7. Inflammation, Oxidative Stress, and Clinical Implications in BPH
7.1. Chronic Inflammation as a Catalyst for Prostatic Overgrowth
7.2. Inflammatory Cell Dynamics and Reactive Oxygen Species
7.3. Self-Perpetuating Cycles: The Inflammatory–Oxidative Pathway
7.4. Relationship with Symptom Severity
7.5. Potential Biomarkers for Prostatic Inflammation and OS
7.6. Oxidative Stress and Differentiating BPH from Prostate Cancer
8. Therapeutic and Preventive Strategies Targeting Oxidative Stress in BPH
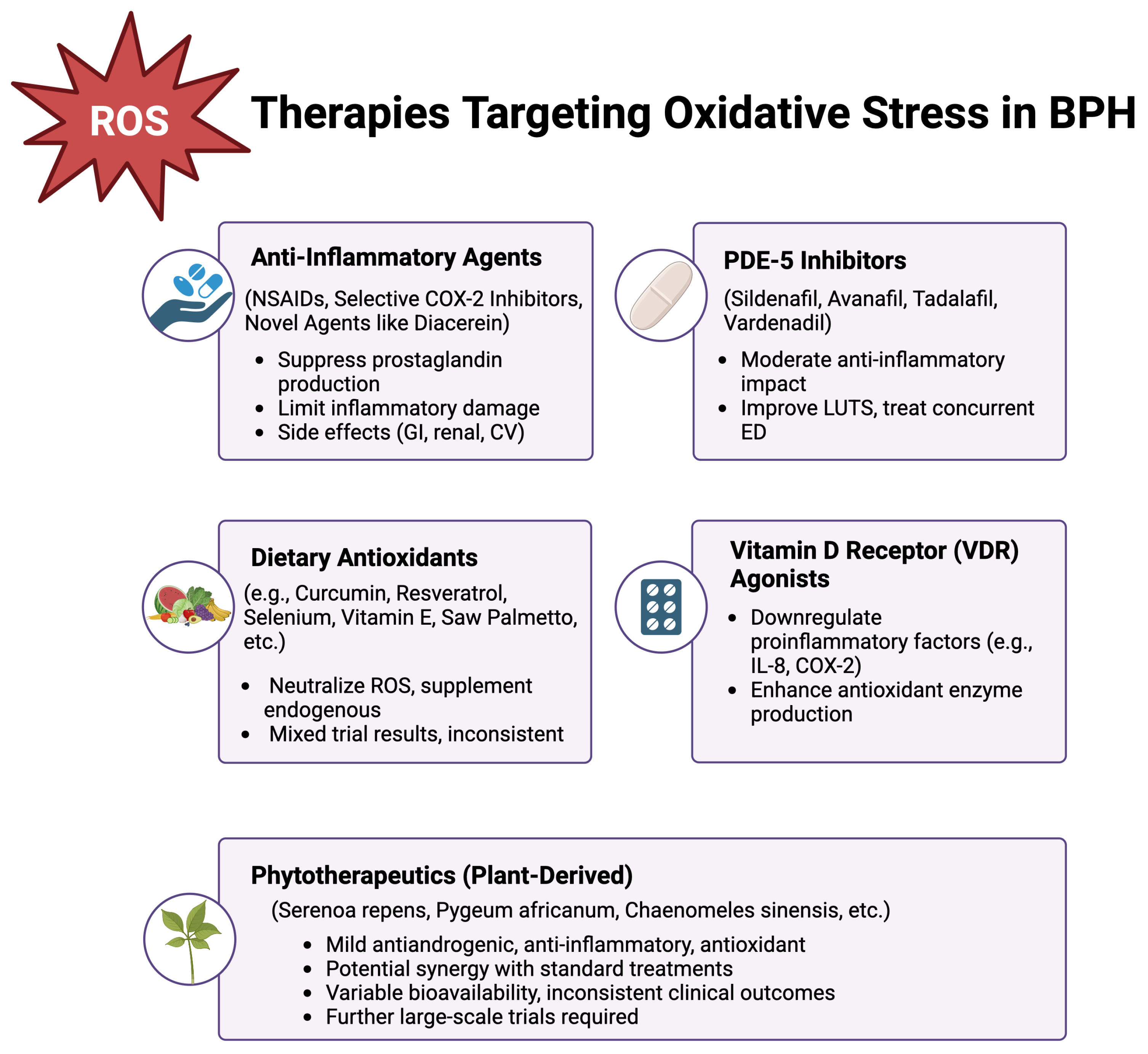
8.1. Conventional Pharmacological Treatments
8.2. Anti-Inflammatory Approaches
8.3. Dietary Antioxidants and Nutritional Supplements
8.4. Vitamin D Receptor Agonists
8.5. Phytotherapeutics
9. Future Directions
9.1. Remaining Gaps in Knowledge
9.2. Potential for Novel Biomarkers and Personalized Treatments
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, Z.; Wang, J.; Xiao, Y.; Luo, J.; Xu, L.; Chen, Z. Global burden of benign prostatic hyperplasia in males aged 60–90 years from 1990 to 2019: Results from the global burden of disease study 2019. BMC Urol. 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Garraway, W.M.; Collins, G.N.; Lee, R.J. High prevalence of benign prostatic hypertrophy in the community. Lancet 1991, 338, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Carson, C., 3rd; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, Y.; Kazzazi, A.; Momtahen, S.; Laze, J.; Djavan, B. Correlation between benign prostatic hyperplasia and inflammation. Curr. Opin. Urol. 2013, 23, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.E.; Stix, U.; Handisurya, A.; Willheim, M.; Haitel, A.; Reithmayr, F.; Paikl, D.; Ecker, R.C.; Hrachowitz, K.; Kramer, G.; et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab. Investig. 2003, 83, 1131–1146. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Peehl, D.M. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J. Urol. 2004, 172, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar]
- Inamura, S.; Terada, N. Chronic inflammation in benign prostatic hyperplasia: Pathophysiology and treatment options. Int. J. Urol. 2024, 31, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Zou, L.Q. Flavonoids as therapeutic agents for epilepsy: Unveiling anti-inflammatory and antioxidant pathways for novel treatments. Front. Pharmacol. 2024, 15, 1457284. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Benign Prostatic Hyperplasia Collaborators. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2022, 3, e754–e776. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.K. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Curr. Bladder Dysfunct. Rep. 2010, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef]
- Hammarsten, J.; Hogstedt, B.; Holthuis, N.; Mellstrom, D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998, 1, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 139. [Google Scholar] [CrossRef] [PubMed]
- Launer, B.M.; McVary, K.T.; Ricke, W.A.; Lloyd, G.L. The rising worldwide impact of benign prostatic hyperplasia. BJU Int. 2021, 127, 722–728. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E. Anatomy of the prostate and morphogenesis of BPH. Prog. Clin. Biol. Res. 1984, 145, 27–53. [Google Scholar]
- Lepor, H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev. Urol. 2005, 7 (Suppl. S4), S3–S12. [Google Scholar] [PubMed]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.R.; Yang, L.Y.; Liu, Z.T. Chronic inflammation in benign prostatic hyperplasia: Implications for therapy. Med. Hypotheses 2008, 70, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Ngai, H.Y.; Yuen, K.S.; Ng, C.M.; Cheng, C.H.; Chu, S.P. Metabolic syndrome and benign prostatic hyperplasia: An update. Asian J. Urol. 2017, 4, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 120–122. [Google Scholar] [CrossRef] [PubMed]
- Elkahwaji, J.E. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res. Rep. Urol. 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Z.; Liu, G.; Daneshgari, F.; MacLennan, G.T.; Gupta, S. Metabolic syndrome, inflammation and lower urinary tract symptoms: Possible translational links. Prostate Cancer Prostatic Dis. 2016, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Inferrera, A.; Navarra, M.; Calapai, G.; Magno, C.; Gangemi, S. Oxidative stress in benign prostatic hyperplasia: A systematic review. Urol. Int. 2015, 94, 249–254. [Google Scholar] [CrossRef]
- Oseni, S.O.; Naar, C.; Pavlovic, M.; Asghar, W.; Hartmann, J.X.; Fields, G.B.; Esiobu, N.; Kumi-Diaka, J. The Molecular Basis and Clinical Consequences of Chronic Inflammation in Prostatic Diseases: Prostatitis, Benign Prostatic Hyperplasia, and Prostate Cancer. Cancers 2023, 15, 3110. [Google Scholar] [CrossRef]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Begley, L.A.; Kasina, S.; MacDonald, J.; Macoska, J.A. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 2008, 43, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Mabed, D.; Lobaccaro, J.M.; Lahouel, M. Oxidative stress in benign prostate hyperplasia. Andrologia 2016, 48, 69–73. [Google Scholar] [CrossRef]
- Kyprianou, N.; Tu, H.; Jacobs, S.C. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol. 1996, 27, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Fu, X.; Yang, S.; Li, Y.; Liu, J.; DiSanto, M.E.; Chen, P.; Zhang, X. The Emerging Role of Cell Adhesion Molecules on Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2023, 24, 2870. [Google Scholar] [CrossRef]
- Saker, Z.; Tsintsadze, O.; Jiqia, I.; Managadze, L.; Chkhotua, A. Importance of apoptosis markers (MDM2, BCL-2 AND Bax) in benign prostatic hyperplasia and prostate cancer. Georgian Med. News 2015, 7–14. [Google Scholar]
- Iacopino, F.; Angelucci, C.; Lama, G.; Zelano, G.; La Torre, G.; D’Addessi, A.; Giovannini, C.; Bertaccini, A.; Macaluso, M.P.; Martorana, G.; et al. Apoptosis-related gene expression in benign prostatic hyperplasia and prostate carcinoma. Anticancer Res. 2006, 26, 1849–1854. [Google Scholar] [PubMed]
- Claus, S.; Wrenger, M.; Senge, T.; Schulze, H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol. Res. 1993, 21, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.G.; Choi, E.J. Apoptotic signaling pathways: Caspases and stress-activated protein kinases. J. Biochem. Mol. Biol. 2002, 35, 24–27. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal 2005, 7, 472–481. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, E.; Giorgio, M.; Pelicci, P.G. Apoptosis and aging: Role of p66Shc redox protein. Antioxid. Redox Signal 2006, 8, 600–608. [Google Scholar] [CrossRef]
- Migliaccio, E.; Giorgio, M.; Mele, S.; Pelicci, G.; Reboldi, P.; Pandolfi, P.P.; Lanfrancone, L.; Pelicci, P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999, 402, 309–313. [Google Scholar] [CrossRef]
- Wang, W.; Bergh, A.; Damber, J.E. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 2004, 61, 60–72. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Di Silverio, F.; Gentile, V.; De Matteis, A.; Mariotti, G.; Giuseppe, V.; Luigi, P.A.; Sciarra, A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: A retrospective analysis. Eur. Urol. 2003, 43, 164–175. [Google Scholar] [CrossRef]
- Wong, Y.C.; Wang, Y.Z. Growth factors and epithelial-stromal interactions in prostate cancer development. Int. Rev. Cytol. 2000, 199, 65–116. [Google Scholar] [CrossRef]
- Asirvatham, A.J.; Schmidt, M.; Gao, B.; Chaudhary, J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology 2006, 147, 257–271. [Google Scholar] [CrossRef]
- Pathak, S.; Singh, R.; Verschoyle, R.D.; Greaves, P.; Farmer, P.B.; Steward, W.P.; Mellon, J.K.; Gescher, A.J.; Sharma, R.A. Androgen manipulation alters oxidative DNA adduct levels in androgen-sensitive prostate cancer cells grown in vitro and in vivo. Cancer Lett. 2008, 261, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Hara, I.; Kamidono, S.; Eto, H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J. Urol. 2004, 171, 1533–1536. [Google Scholar] [CrossRef]
- Fernandez-Checa, J.C.; Kaplowitz, N.; Garcia-Ruiz, C.; Colell, A.; Miranda, M.; Mari, M.; Ardite, E.; Morales, A. GSH transport in mitochondria: Defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997, 273, G7–G17. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, T.; Aoki, D.; Kanetake, H.; Inoue, S.; Muramatsu, M.; Hishikawa, Y.; Koji, T. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 2003, 88, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Linja, M.J.; Savinainen, K.J.; Tammela, T.L.; Isola, J.J.; Visakorpi, T. Expression of ERalpha and ERbeta in prostate cancer. Prostate 2003, 55, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Kim, S.I.; Chun, D.C.; Cho, N.H.; Chung, B.C.; Park, B.W.; Hong, S.J. Development of rat prostatitis model by oral administration of isoflavone and its characteristics. Yonsei Med. J. 2001, 42, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Boileau, T.W.; Bray, T.M. Dietary influences on endocrine-inflammatory interactions in prostate cancer development. Arch. Biochem. Biophys. 2004, 428, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ricke, W.A.; McPherson, S.J.; Bianco, J.J.; Cunha, G.R.; Wang, Y.; Risbridger, G.P. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008, 22, 1512–1520. [Google Scholar] [CrossRef]
- Smith, M.R.; Morton, R.A.; Barnette, K.G.; Sieber, P.R.; Malkowicz, S.B.; Rodriguez, D.; Hancock, M.L.; Steiner, M.S. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J. Urol. 2010, 184, 1316–1321. [Google Scholar] [CrossRef]
- Thompson, I.M.; Coltman, C.A.; Brawley, O.W.; Ryan, A. Chemoprevention of prostate cancer. Semin. Urol. 1995, 13, 122–129. [Google Scholar] [PubMed]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Akaihata, H.; Hata, J.; Aikawa, K.; Yanagida, T.; Matsuoka, K.; Koguchi, T.; Hoshi, S.; Ogawa, S.; Kataoka, M.; et al. The association between local atherosclerosis of the prostatic artery and benign prostatic enlargement in humans: Putative mechanism of chronic ischemia for prostatic enlargement. Prostate 2018, 78, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Akaihata, H.; Hata, J.; Hiraki, H.; Honda, R.; Tanji, R.; Onagi, A.; Koguchi, T.; Hoshi, S.; Ogawa, S.; et al. The association between local arteriosclerosis of the prostatic arteries and chronic inflammation in human benign prostatic enlargement. Prostate 2019, 79, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tsounapi, P.; Oikawa, R.; Shimizu, S.; Honda, M.; Sejima, T.; Kinoshita, Y.; Tomita, S. Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci. Rep. 2014, 4, 3822. [Google Scholar] [CrossRef] [PubMed]
- Konig, J.E.; Senge, T.; Allhoff, E.P.; Konig, W. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate 2004, 58, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Mitteregger, D.; Marberger, M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur. Urol. 2007, 51, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Weidner, W. Chronic prostatitis-an infectious disease? J. Antimicrob. Chemother. 2000, 46, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef]
- Roehrborn, C.G. Definition of at-risk patients: Baseline variables. BJU Int. 2006, 97 (Suppl. S2), 7–11; discussion 12–21. [Google Scholar] [CrossRef]
- Baltaci, S.; Orhan, D.; Gogus, C.; Turkolmez, K.; Tulunay, O.; Gogus, O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001, 88, 100–103. [Google Scholar] [CrossRef]
- Gradini, R.; Realacci, M.; Ginepri, A.; Naso, G.; Santangelo, C.; Cela, O.; Sale, P.; Berardi, A.; Petrangeli, E.; Gallucci, M.; et al. Nitric oxide synthases in normal and benign hyperplastic human prostate: Immunohistochemistry and molecular biology. J. Pathol. 1999, 189, 224–229. [Google Scholar] [CrossRef]
- Handisurya, A.; Steiner, G.E.; Stix, U.; Ecker, R.C.; Pfaffeneder-Mantai, S.; Langer, D.; Kramer, G.; Memaran-Dadgar, N.; Marberger, M. Differential expression of interleukin-15, a pro-inflammatory cytokine and T-cell growth factor, and its receptor in human prostate. Prostate 2001, 49, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vykhovanets, E.V.; Shukla, S.; MacLennan, G.T.; Vykhovanets, O.V.; Bodner, D.R.; Gupta, S. Il-1 beta-induced post-transition effect of NF-kappaB provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate 2009, 69, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Laverny, G.; Gacci, M.; Crescioli, C.; Maggi, M.; Adorini, L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J. Immunol. 2009, 182, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Kogan-Sakin, I.; Cohen, M.; Paland, N.; Madar, S.; Solomon, H.; Molchadsky, A.; Brosh, R.; Buganim, Y.; Goldfinger, N.; Klocker, H.; et al. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis 2009, 30, 698–705. [Google Scholar] [CrossRef]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Corsiero, E.; Laverny, G.; Silvestrini, E.; Chavalmane, A.; Morelli, A.; Sarchielli, E.; Vannelli, G.B.; et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate 2009, 69, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Adsule, S.; Li, Y.; Padhye, S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev. Med. Chem. 2007, 7, 599–608. [Google Scholar] [CrossRef]
- Pathak, S.K.; Sharma, R.A.; Steward, W.P.; Mellon, J.K.; Griffiths, T.R.; Gescher, A.J. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: Targets for chemopreventive strategies. Eur. J. Cancer 2005, 41, 61–70. [Google Scholar] [CrossRef]
- Slawin, K.M. The medical therapy of prostatic symptoms study: What will we learn? Rev. Urol. 2003, 5 (Suppl. S4), S42–S47. [Google Scholar] [PubMed]
- Bautista, O.M.; Kusek, J.W.; Nyberg, L.M.; McConnell, J.D.; Bain, R.P.; Miller, G.; Crawford, E.D.; Kaplan, S.A.; Sihelnik, S.A.; Brawer, M.K.; et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin. Trials 2003, 24, 224–243. [Google Scholar] [CrossRef]
- Merendino, R.A.; Salvo, F.; Saija, A.; Di Pasquale, G.; Tomaino, A.; Minciullo, P.L.; Fraccica, G.; Gangemi, S. Malondialdehyde in benign prostate hypertrophy: A useful marker? Mediat. Inflamm. 2003, 12, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Mariotti, G.; Salciccia, S.; Autran Gomez, A.; Monti, S.; Toscano, V.; Di Silverio, F. Prostate growth and inflammation. J. Steroid Biochem. Mol. Biol. 2008, 108, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Nargi, D.; Horton, L.; Reddy, B.S.; Bosland, M.C.; Narayanan, B.A. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinogenesis: Preventive effects of celecoxib. Prostate 2009, 69, 133–141. [Google Scholar] [CrossRef]
- Di Silverio, F.; Bosman, C.; Salvatori, M.; Albanesi, L.; Proietti Pannunzi, L.; Ciccariello, M.; Cardi, A.; Salvatori, G.; Sciarra, A. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Eur. Urol. 2005, 47, 72–78; discussion 78–79. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; De Marzo, A.M.; Smit, E.; Giovannucci, E.; Platz, E.A. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III). Prostate 2005, 62, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Mondaini, N.; Amuchastegui, S.; Degli Innocenti, S.; Carini, M.; Giubilei, G.; Fibbi, B.; Colli, E.; Maggi, M.; Adorini, L. Seminal plasma cytokines and chemokines in prostate inflammation: Interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 2007, 51, 524–533; discussion 533. [Google Scholar] [CrossRef] [PubMed]
- Mechergui, Y.B.; Ben Jemaa, A.; Mezigh, C.; Fraile, B.; Ben Rais, N.; Paniagua, R.; Royuela, M.; Oueslati, R. The profile of prostate epithelial cytokines and its impact on sera prostate specific antigen levels. Inflammation 2009, 32, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Xia, C.; Gomez, L.; Lamb, D.J.; Ittmann, M. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate 2004, 60, 153–159. [Google Scholar] [CrossRef]
- Vezyraki, P.; Vlachaki, A.; Baltogiannis, D.; Batistatou, A.; Tsampalas, S.; Simos, Y.V.; Kaltsas, A.; Pappas, P.; Dounousi, E.; Ragos, V.; et al. Impact of total PSA and percent free PSA in the differentiation of prostate disease: A retrospective comparative study implicating neoplastic and non-neoplastic entities. J. BUON 2019, 24, 2107–2113. [Google Scholar] [PubMed]
- Sandhu, J.S. Prostate cancer and chronic prostatitis. Curr. Urol. Rep. 2008, 9, 328–332. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Gronberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Silverman, R. Inflammation, infection, and prostate cancer. Curr. Opin. Urol. 2008, 18, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; De Marzo, A.M. Epidemiology of inflammation and prostate cancer. J. Urol. 2004, 171, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.; Groome, P.A.; Siemens, D.R. Inflammation and prostate cancer: A future target for prevention and therapy? Urol. Clin. N. Am. 2008, 35, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: An overview for the practising clinician. BJU Int. 2004, 94, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Araki, I.; Kamiyama, M.; Takihana, Y.; Komuro, M.; Furuya, Y. Diagnosis and treatment of voiding symptoms. Urology 2003, 62, 11–19. [Google Scholar] [CrossRef]
- Webber, R. Benign prostatic hyperplasia. Clin. Evid. 2004, 70, 1325–1326. [Google Scholar]
- Sakalis, V.; Sfiggas, V.; Vouros, I.; Salpiggidis, G.; Papathanasiou, A.; Apostolidis, A. Combination of solifenacin with tamsulosin reduces prostate volume and vascularity as opposed to tamsulosin monotherapy in patients with benign prostate enlargement and overactive bladder symptoms: Results from a randomized pilot study. Int. J. Urol. 2018, 25, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Inamura, S.; Fukiage, Y.; Kobayashi, H.; Tsutsumiuchi, M.; Seki, M.; Taga, M.; Fukushima, M.; Kobayashi, M.; Yokoyama, O.; Terada, N. Dutasteride, a 5 alpha reductase inhibitor, could be associated with the exacerbation of inflammation in patients with benign prostatic hyperplasia. Int. J. Urol. 2024, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Shimizu, T.; Tsounapi, P.; Higashi, Y.; Martin, D.T.; Nakamura, K.; Honda, M.; Inoue, K.; Saito, M. Effect of Silodosin, an Alpha1A-Adrenoceptor Antagonist, on Ventral Prostatic Hyperplasia in the Spontaneously Hypertensive Rat. PLoS ONE 2015, 10, e0133798. [Google Scholar] [CrossRef]
- Mahmud, S.M.; Tanguay, S.; Begin, L.R.; Franco, E.L.; Aprikian, A.G. Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. Eur. J. Cancer Prev. 2006, 15, 158–164. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Rodriguez, C.; Mondul, A.M.; Connell, C.J.; Henley, S.J.; Calle, E.E.; Thun, M.J. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J. Natl. Cancer Inst. 2005, 97, 975–980. [Google Scholar] [CrossRef]
- Roberts, R.O.; Jacobson, D.J.; Girman, C.J.; Rhodes, T.; Lieber, M.M.; Jacobsen, S.J. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin. Proc. 2002, 77, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; de Beer, P.M.; van der Merwe, L.; Heyns, C.F. COX-2 promoter polymorphisms and the association with prostate cancer risk in South African men. Carcinogenesis 2008, 29, 2347–2350. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Ching, T.T.; Wang, D.S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397–11403. [Google Scholar] [CrossRef]
- Rasheed, R.A.; Sadek, A.S.; Khattab, R.T.; Elkhamisy, F.A.A.; Abdelfattah, H.A.; Elshaer, M.M.A.; Almutairi, S.M.; Hussein, D.S.; Embaby, A.S.; Almoatasem, M.A.M. Diacerein provokes apoptosis, improves redox balance, and downregulates PCNA and TNF-alpha in a rat model of testosterone-induced benign prostatic hyperplasia: A new non-invasive approach. PLoS ONE 2023, 18, e0293682. [Google Scholar] [CrossRef]
- Sofikitis, N.; Kaltsas, A.; Dimitriadis, F.; Rassweiler, J.; Grivas, N.; Zachariou, A.; Kaponis, A.; Tsounapi, P.; Paterakis, N.; Karagiannis, A.; et al. The Effect of PDE5 Inhibitors on the Male Reproductive Tract. Curr. Pharm. Des. 2021, 27, 2697–2713. [Google Scholar] [CrossRef]
- Fleshner, N.E.; Kucuk, O. Antioxidant dietary supplements: Rationale and current status as chemopreventive agents for prostate cancer. Urology 2001, 57, 90–94. [Google Scholar] [CrossRef]
- Sikka, S.C. Role of oxidative stress response elements and antioxidants in prostate cancer pathobiology and chemoprevention--a mechanistic approach. Curr. Med. Chem. 2003, 10, 2679–2692. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef] [PubMed]
- Carraro, J.C.; Raynaud, J.P.; Koch, G.; Chisholm, G.D.; Di Silverio, F.; Teillac, P.; Da Silva, F.C.; Cauquil, J.; Chopin, D.K.; Hamdy, F.C.; et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1098 patients. Prostate 1996, 29, 231–240; discussion 232–241. [Google Scholar] [CrossRef]
- Levin, R.M.; Das, A.K. A scientific basis for the therapeutic effects of Pygeum africanum and Serenoa repens. Urol. Res. 2000, 28, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.C. Phytotherapy for the prostate. Br. J. Urol. 1996, 78, 325–336. [Google Scholar] [CrossRef]
- Buck, A.C. Is there a scientific basis for the therapeutic effects of serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J. Urol. 2004, 172, 1792–1799. [Google Scholar] [CrossRef]
- Wertz, K.; Siler, U.; Goralczyk, R. Lycopene: Modes of action to promote prostate health. Arch. Biochem. Biophys. 2004, 430, 127–134. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Moreno, J.; Nonn, L.; Swami, S.; Peehl, D.M.; Feldman, D. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: Role of anti-inflammatory activity. J. Bone Miner. Res. 2007, 22 (Suppl. S2), V74–V80. [Google Scholar] [CrossRef]
- Lee, G.H.; Shin, Y.S.; Kim, J.H.; Shim, D.H.; Lee, H.Y.; Zhao, L.; Rashid, M.M.U.; Cho, H.E.; Lee, J.J.; Kim, M.K.; et al. The Combination of Curcumae Radix and Syzygium Aromaticum Extracts Mitigates Benign Prostatic Hyperplasia through Anti-Proliferative and Anti-Inflammatory Effects. World J. Men’s Health 2024, 42, e94. [Google Scholar] [CrossRef]
- Kang, J.S.; Zhao, X.Y.; Lee, J.H.; Lee, J.S.; Keum, Y.S. Ethanol Extract of Chaenomeles sinensis Inhibits the Development of Benign Prostatic Hyperplasia by Exhibiting Anti-oxidant and Anti-inflammatory Effects. J. Cancer Prev. 2022, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Obermuller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J. Nutr. 2008, 138, 49–53. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Ba, W.; Xu, W.; Deng, Z.; Zhang, B.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of the Main Carotenoids from Tomatoes via Nrf2 and NF-kappaB Signaling Pathways. Nutrients 2023, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 2006, 16, 25–29. [Google Scholar] [CrossRef]
- Bechis, S.K.; Otsetov, A.G.; Ge, R.; Olumi, A.F. Personalized medicine for the management of benign prostatic hyperplasia. J. Urol. 2014, 192, 16–23. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Giannakas, T.; Stavropoulos, M.; Kratiras, Z.; Chrisofos, M. Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives. Diseases 2025, 13, 53. https://doi.org/10.3390/diseases13020053
Kaltsas A, Giannakas T, Stavropoulos M, Kratiras Z, Chrisofos M. Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives. Diseases. 2025; 13(2):53. https://doi.org/10.3390/diseases13020053
Chicago/Turabian StyleKaltsas, Aris, Timoleon Giannakas, Marios Stavropoulos, Zisis Kratiras, and Michael Chrisofos. 2025. "Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives" Diseases 13, no. 2: 53. https://doi.org/10.3390/diseases13020053
APA StyleKaltsas, A., Giannakas, T., Stavropoulos, M., Kratiras, Z., & Chrisofos, M. (2025). Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives. Diseases, 13(2), 53. https://doi.org/10.3390/diseases13020053






