Quality Management Outweighs Pandemic: Retrospective Analysis Shows Improved Quality of Care for Staphylococcus aureus Bacteremia Despite SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Parameters
2.2. Quality Management Parameters
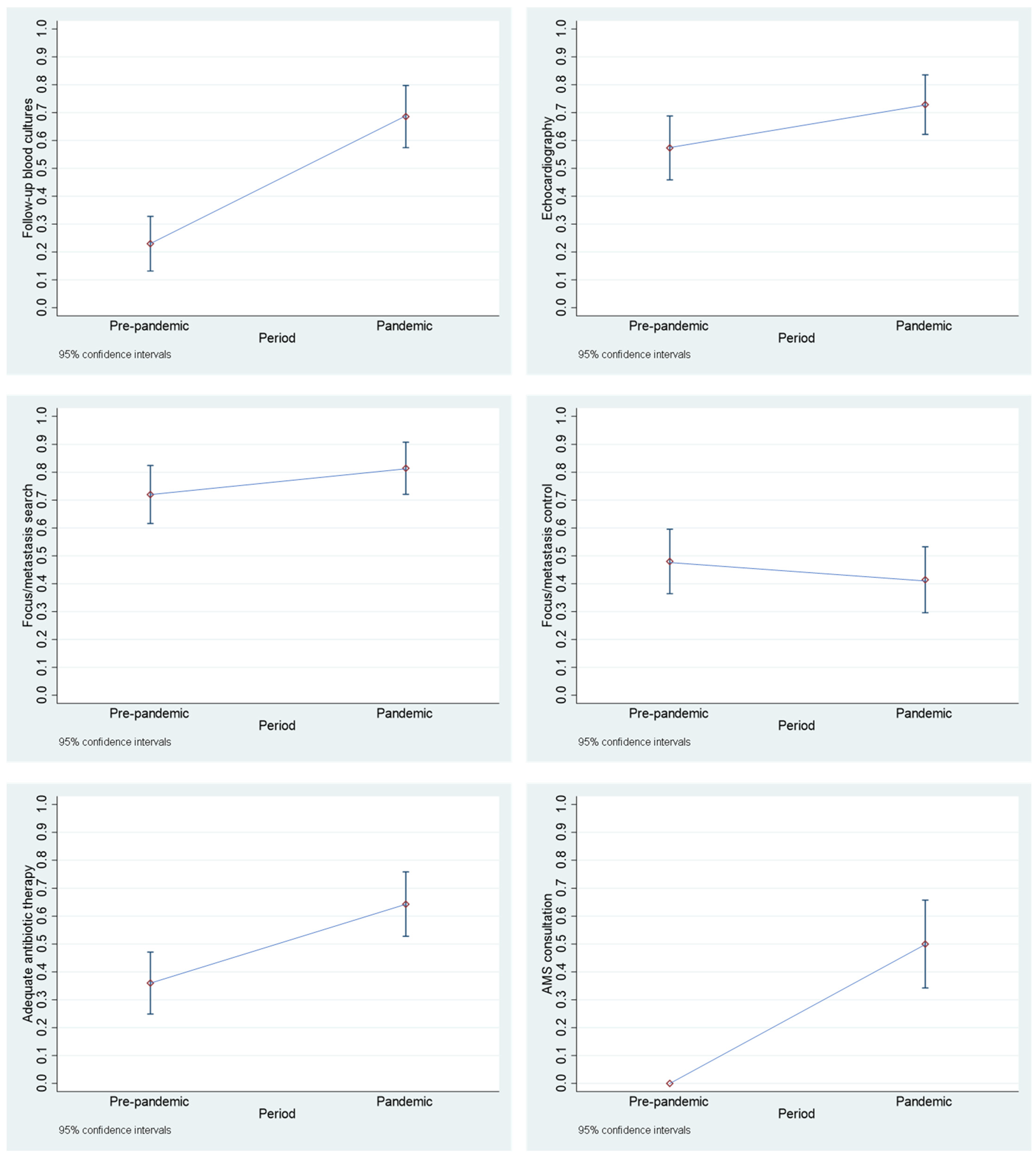
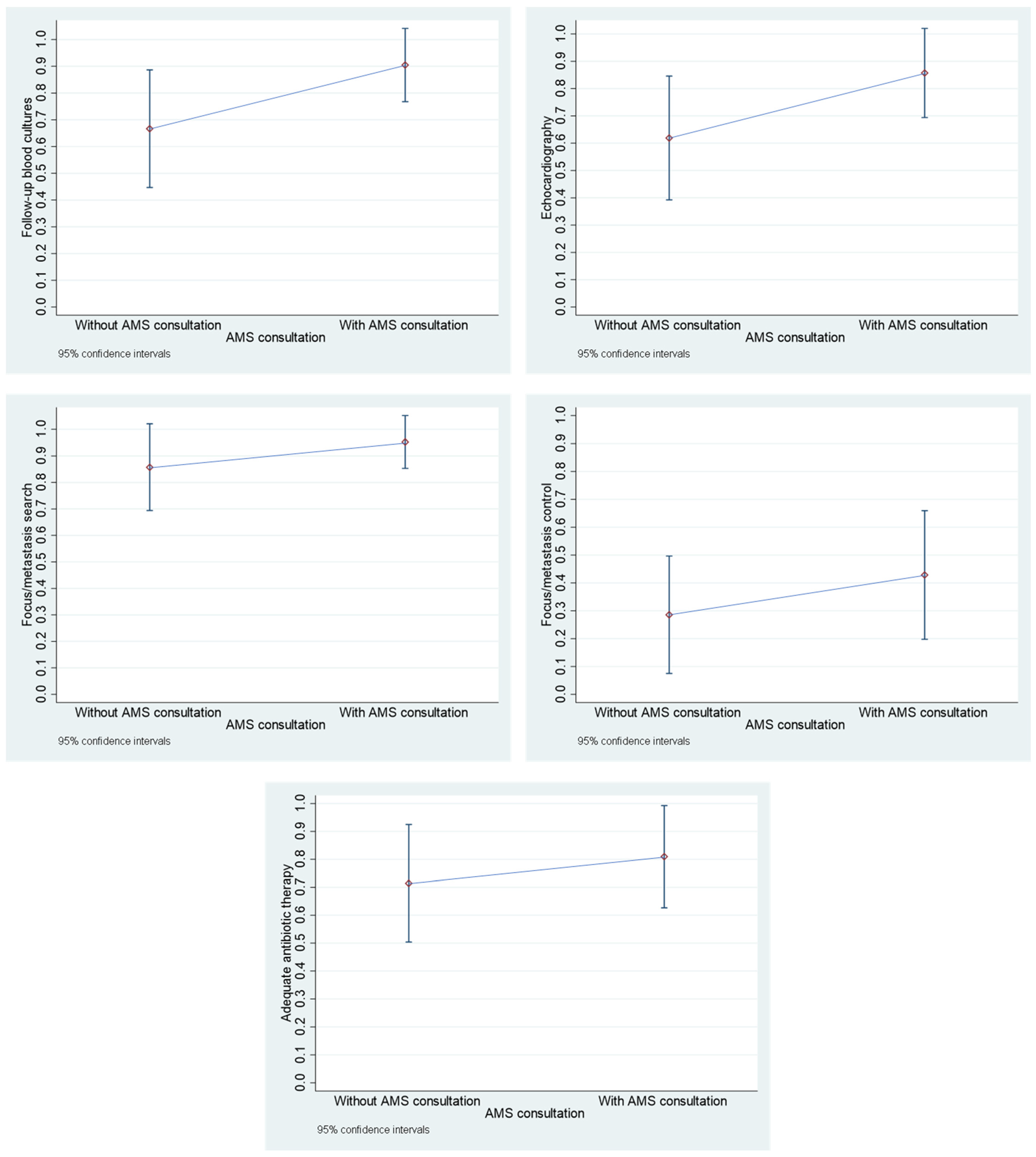
- Follow-up blood cultures: This parameter was considered to be met if follow-up blood cultures were taken every 48 to 96 h from the first positive blood culture until sterility was achieved.
- Echocardiography: This parameter was considered to be met if at least one transthoracic echocardiography (TTE) or transthoracic echocardiography (TEE) was performed. If only a TTE was performed, it was not determined whether an additional TEE would have been necessary or useful.
- Focus/metastasis search: This parameter was considered to be met if either a focus/metastasis was documented or, despite an unidentifiable focus at the clinic, further examinations (e.g., ultrasound, CT, MRI) were documented that were based on previous findings.
- Focus/metastasis control: This parameter was considered to be met if removal of catheters or devices or another focus/metastasis sanitation was documented.
- Adequate antibiotic therapy: This parameter was considered to be met if treatment was started early and with a suitable antibiotic (generally for MSSA: flucloxacillin or cefazolin, for MRSA: vancomycin or daptomycin, or if necessary other agents or combinations if effective and comprehensibly justified) and the duration of therapy was sufficiently long (uncomplicated SAB: at least 14 days after the first negative blood culture, complicated SAB: at least 4 weeks after the first negative blood culture, possibly longer for artificial valves or for other reasons, if comprehensibly justified).
- Antimicrobial Stewardship (AMS) team consultation: An AMS team consisting of an ID specialist, a microbiologist, a clinical pharmacist and a hygienist has been available since June 2021 [12]. From that point on, the parameter was taken into account and considered fulfilled if an AMS consultation was requested in the hospital information system.
2.3. Data Analysis
3. Results
3.1. Comparison of SARS-CoV-2-Negative and SARS-CoV-2-Positive Cases Within the “Pandemic Period”
3.2. Comparison of Cases Between the “Pre-Pandemic Period” and the “Pandemic Period”
3.3. Comparison of Cases with AMS Team Consultation and Cases Without AMS Team Consultation After June 2021 Within the “Pandemic Period”
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Pandemic’s Negative Impact on the Mortality
4.4. Implications for Policy, Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMS | Antimicrobial stewardship |
| CCI | Charlson Comorbidity Index |
| DH | Duration of hospital stay |
| ID | Infectious diseases |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| QCI | Quality of care index |
| SAB | Staphylococcus aureus bacteremia |
| SARS-CoV-2 | Severe acute respiratory syndrome-corona virus-2 |
| SOP | Standard operational procedure |
| TEE | Trans-esophageal echocardiography |
| TTE | Trans-thoracal echocardiography |
| WHO | World Health Organization |
References
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2013, 5, 4–11. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Kaasch, A.J.; Barlow, G.; Edgeworth, J.D.; Fowler, V.G.; Hellmich, M.; Hopkins, S.; Kern, W.V.; Llewelyn, M.J.; Rieg, S.; Rodriguez-Baño, J.; et al. Staphylococcus aureus bloodstream infection: A pooled analysis of five prospective, observational studies. J. Infect. 2014, 68, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hindy, J.-R.; A Quintero-Martinez, J.; Lee, A.T.; Scott, C.G.; Gerberi, D.J.; Mahmood, M.; DeSimone, D.C.; Baddour, L.M. Incidence Trends and Epidemiology of Staphylococcus aureus Bacteremia: A Systematic Review of Population-Based Studies. Cureus 2022, 14, e25460. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.; Stokes, W. The Golden Grapes of Wrath – Staphylococcus aureus Bacteremia: A Clinical Review. Am. J. Med. 2023, 136, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Timsit, J.-F. Management and Prevention of Central Venous Catheter-Related Infections in the ICU. Semin. Respir. Crit. Care Med. 2019, 40, 508–523. [Google Scholar] [CrossRef]
- AK ART. Staphylococcus aureus Bakteriämie (SAB). 2019. Available online: https://wmm.pic-mediaserver.de/z202002/pdf/Downloads/Handlungsempfehlung_SAB.pdf (accessed on 3 January 2025).
- Mathies, D.; Rauschning, D.; Vonderhecken, J.; Wagnera, U.; Müllera, F.; Schneidera, D.; Eckhardta, T.; Schüßlera, M.; Schmidt-Borkoa, K.; Bickelet, C.; et al. SARS-CoV-2-Pandemie in Deutschland—Erste Erfahrungen im BundeswehrZentralkrankenhaus Koblenz. Wehrmedizinische Monatsschrift 2020, 64, e16. [Google Scholar]
- Arientová, S.; Jícha, Z.; Beran, O.; Holub, M. Decreased quality of care for Staphylococcus aureus bacteremia during the COVID-19 pandemic. BMC Infect. Dis. 2022, 22, 1–5. [Google Scholar] [CrossRef]
- Böing, C.W.; Froböse, N.J.; Schaumburg, F.; Kampmeier, S. Impact of the COVID-19 Pandemic on the Management of Staphylococcus aureus Bloodstream Infections in a Tertiary Care Hospital. Pathogens 2023, 12, 611. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Zeidler, D.; Scheumann, G.; Bäßler, C.; Döhla, M.; Preuß, D.; Rauschning, D. The whole is greater than the sum of its parts: An interim summary on the ABS team at the Bundeswehr Central Hospital Koblenz. GMS Hyg. Infect. Control. 2025, 20, Doc11. [Google Scholar] [CrossRef]
- Fries, B.L.; Licitra, C.; Crespo, A.; Akhter, K.; Busowski, M.T.; Salazar, D.; Wallace, M.R. Infectious Diseases Consultation and the Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2013, 58, 598–599. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, L.E.; del Toro, M.D.; Gálvez-Acebal, J.; Bereciartua-Bastarrica, E.; Fariñas, M.C.; Sanz-Franco, M.; Natera, C.; Corzo, J.E.; Lomas, J.M.; Pasquau, J.; et al. Impact of an Evidence-Based Bundle Intervention in the Quality-of-Care Management and Outcome of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2013, 57, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Arientová, S.; Beran, O.; Štefan, M.; Čurdová, M.; Holub, M. Bakteriémie vyvolaná Staphylococcus aureus—význam správného přístupu k diagnostice a léčbě. Epidemiol. Mikrobiol. Imunol. 2018, 67, 88–91. [Google Scholar]
- Vogel, M.; Schmitz, R.P.; Hagel, S.; Pletz, M.W.; Gagelmann, N.; Scherag, A.; Schlattmann, P.; Brunkhorst, F.M. Infectious disease consultation for Staphylococcus aureus bacteremia—A systematic review and meta-analysis. J. Infect. 2016, 72, 19–28. [Google Scholar] [CrossRef]
- Bai, A.D.; Showler, A.; Burry, L.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Impact of Infectious Disease Consultation on Quality of Care, Mortality, and Length of Stay in Staphylococcus aureus Bacteremia: Results From a Large Multicenter Cohort Study. Clin. Infect. Dis. 2015, 60, 1451–1461. [Google Scholar] [CrossRef]
- Willekens, R.; Puig-Asensio, M.; Suanzes, P.; Fernández-Hidalgo, N.; Larrosa, M.N.; González-López, J.J.; Rodríguez-Pardo, D.; Pigrau, C.; Almirante, B. Mortality in Staphylococcus aureus bacteraemia remains high despite adherence to quality indicators: Secondary analysis of a prospective cohort study. J. Infect. 2021, 83, 656–663. [Google Scholar] [CrossRef]
- Lorenzo-Hernández, E.; Rivas-Ruiz, F.; Del Arco-Jiménez, A. Consequences of the COVID-19 Pandemic on the Incidence, Management and Outcomes of Staphylococcus aureus Bacteraemia: Experience in a Spanish Hospital. Pathogens 2024, 13, 847. [Google Scholar] [CrossRef]
- Falces-Romero, I.; Bloise, I.; García-Rodríguez, J.; Cendejas-Bueno, E.; Montero-Vega, M.D.; Romero, M.P.; García-Bujalance, S.; Toro-Rueda, C.; Ruiz-Carrascoso, G.; Quiles-Melero, I.; et al. Staphylococcus aureus bacteremia in patients with SARS-CoV-2 infection. Med. Clin. (Engl. Ed.) 2023, 160, 495–498. [Google Scholar] [CrossRef]
- Abdollahi, A.; Nojomi, M.; Karimi, Y.; Ranjbar, M. Mortality patterns in patients with Staphylococcus aureus bacteremia during the COVID-19 pandemic: Predictors and insights. Heliyon 2024, 10, e24511. [Google Scholar] [CrossRef]
- Budak, E.A.; Karahan, M.; Han, Ç.Ç. Evaluation of anxiety and fear of COVID-19 in patients admitted to an ophthalmology outpatient clinic. Int. J. Clin. Pr. 2021, 75, e14519. [Google Scholar] [CrossRef]
- Einav, S.; Tankel, J. The unseen pandemic: Treatment delays and loss to follow-up due to fear of COVID. J. Anesthesia, Analg. Crit. Care 2022, 2, 1–4. [Google Scholar] [CrossRef]
- Smolić, Š.; Fabijančić, M.; Blaževski, N. How did fear of COVID-19 affect access to healthcare in Central and Eastern Europe? Findings from populations aged 50 or older after the outbreak. East. Eur. Econ. 2022, 61, 571–590. [Google Scholar] [CrossRef]
- Boulos, M.; Bassal, T.; Layyous, A.; Basheer, M.; Assy, N. Inflammation in COVID-19: A Risk for Superinfections. COVID 2022, 2, 1609–1624. [Google Scholar] [CrossRef]
- Lubkin, A.; Bernard-Raichon, L.; DuMont, A.L.; Jimenez, A.M.V.; Putzel, G.G.; Gago, J.; Zwack, E.E.; Olusanya, O.; Boguslawski, K.M.; Dallari, S.; et al. SARS-CoV-2 infection predisposes patients to coinfection with Staphylococcus aureus. mBio 2024, 15, e0166724. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, L.; Schmitt, D. „Tarragona-Strategie“—Adäquate Antibiotikatherapie auf der Intensivstation. Med. Klin.—Intensiv. und Notfallmedizin 2014, 109, 156–161. [Google Scholar] [CrossRef]
- Integration von SARS-CoV-2 als Erreger von Infektionen in der endemischen Situation in die Empfehlungen der KRINKO „Infektionsprävention im Rahmen der Pflege und Behandlung von Patienten mit übertragbaren Krankheiten“. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2023, 66, 1279–1301. [CrossRef]
- UK Health Security Agency. COVID-19: Information and Advice for Health and Care Professionals: Background Information and Advice on Coronavirus (COVID-19) for Health and Care Professionals; Guidance; 2023. Available online: https://www.gov.uk/guidance/covid-19-information-and-advice-for-health-and-care-professionals (accessed on 4 January 2025).
- Hsiao, C.; Sun, J.; Chiang, Y.; Chen, H.; Liu, T. Experience of patients with COVID-19 in hospital isolation in Taiwan. Nurs. Heal. Sci. 2021, 23, 888–897. [Google Scholar] [CrossRef]
- Chang, W.-H. The influences of the COVID-19 pandemic on medical service behaviors. Taiwan. J. Obstet. Gynecol. 2020, 59, 821–827. [Google Scholar] [CrossRef]
- Tran, K.; Bell, C.; Stall, N.; Tomlinson, G.; McGeer, A.; Morris, A.; Gardam, M.; Abrams, H.B. The Effect of Hospital Isolation Precautions on Patient Outcomes and Cost of Care: A Multi-Site, Retrospective, Propensity Score-Matched Cohort Study. J. Gen. Intern. Med. 2016, 32, 262–268. [Google Scholar] [CrossRef]
- Lu, G.; Businger, M.; Dollfus, C.; Wozniak, T.; Fleck, M.; Heroth, T.; Lock, I.; Lipenkova, J. Agenda-Setting for COVID-19: A Study of Large-Scale Economic News Coverage Using Natural Language Processing. Int. J. Data Sci. Anal. 2022, 15, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Peña-Moreno, A.; Torres-Soblechero, L.; López-Blázquez, M.; Butragueño-Laiseca, L. Fatal Staphylococcus Aureus Endocarditis Misdiagnosed as Multisystem Inflammatory Syndrome in Children. Pediatr. Infect. Dis. J. 2021, 41, e58–e59. [Google Scholar] [CrossRef]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Leistner, B.; Rauschning, D.; Hagen, R.M.; Srečec, F.; Mutters, N.T.; Weppler, R.; Mutschnik, C.; Döhla, M. Logistic Stewardship: Supporting Antimicrobial Stewardship Programs Based on Antibiotics Goods Flow. Antibiotics 2025, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Jones, M.P.; Schweizer, M.L.; Livorsi, D.J.; Perencevich, E.N.; Richardson, K.; Beck, B.F.; Alexander, B.; Ohl, M.E. Association of Infectious Diseases Consultation With Long-term Postdischarge Outcomes Among Patients With Staphylococcus aureus Bacteremia. JAMA Netw. Open 2020, 3, e1921048. [Google Scholar] [CrossRef]
- Nagao, M.; Yamamoto, M.; Matsumura, Y.; Yokota, I.; Takakura, S.; Teramukai, S.; Ichiyama, S. Complete adherence to evidence-based quality-of-care indicators for Staphylococcus aureus bacteremia resulted in better prognosis. Infection 2016, 45, 83–91. [Google Scholar] [CrossRef]
| Discrimination Parameter | |||
|---|---|---|---|
| SARS-CoV-2 | Negative | Positive | |
| Population Parameters | p-Value | ||
| N | 59 | 11 | |
| Age (Median [IQR]) | 66 [58–79] | 77 [52–85] | 0.518 |
| Male (n [%]) | 36 [61.0%] | 7 [63.6%] | 1.000 |
| DH (Median [IQR]) | 20 [13–31] | 12 [7–21] | 0.088 |
| MRSA (n [%]) | 3 [5.1%] | 0 [0.0%] | 1.000 |
| CCI (Median [IQR]) | 5 [3–8] | 9 [4–11] | 0.026 * |
| Mortality (n/N [%]) | 16/56 [28.6%] | 6 [54.6%] | 0.157 |
| MSSA (n/N [%]) | 16/16 [100.0%] | 6/6 [100.0%] | |
| MRSA (n/N [%]) | 0/16 [0.0%] | 0/6 [0.0%] | |
| Source of infection (n [%]) | |||
| Catheter-related (n [%]) | 8 [13.6%] | 0 [0.0%] | 0.333 |
| Skin and soft tissue (n [%]) | 8 [13.6%] | 1 [9.1%] | |
| Lung (n [%]) | 12 [20.3%] | 3 [27.3%] | |
| Bone and joint (n [%]) | 11 [18.6%] | 1 [9.1%] | |
| Infectious endocarditis (n [%]) | 7 [11.9%] | 0 [0.0%] | |
| Urinary tract (n [%]) | 3 [5.1%] | 1 [9.1%] | |
| Meningitis (n [%]) | 0 [0.0%] | 0 [0.0%] | |
| Unidentified (n [%]) | 10 [17.0%] | 5 [45.4%] | |
| Quality management parameters | p-value | ||
| Follow-up blood cultures (n [%]) | 40 [67.8%] | 8 [72.7%] | 1.000 |
| Echocardiography (n [%]) | 42 [71.2%] | 9 [81.8%] | 0.715 |
| TTE (n [%]) | 26 [44.1%] | 3 [27.3%] | 0.342 |
| TEE (n [%]) | 33 [55.9%] | 8 [72.7%] | 0.342 |
| Focus/metastasis search (n [%]) | 48 [81.4%] | 9 [81.8%] | 1.000 |
| Focus/metastasis control (n [%]) | 27 [45.8%] | 2 [18.2%] | 0.108 |
| Adequate antibiotic therapy (n [%]) | 37 [62.7%] | 8 [72.7%] | 0.735 |
| AMS consultation (n [%]) | 19 [32.2%] | 2 [18.2%] | 0.485 |
| QCI (Median [IQR]) | 2 [2–3] | 3 [2–3] | 0.839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakoby, L.; Molitor, E.; Mutters, N.T.; Weppler, R.; Rauschning, D.; Döhla, M. Quality Management Outweighs Pandemic: Retrospective Analysis Shows Improved Quality of Care for Staphylococcus aureus Bacteremia Despite SARS-CoV-2. Diseases 2025, 13, 104. https://doi.org/10.3390/diseases13040104
Jakoby L, Molitor E, Mutters NT, Weppler R, Rauschning D, Döhla M. Quality Management Outweighs Pandemic: Retrospective Analysis Shows Improved Quality of Care for Staphylococcus aureus Bacteremia Despite SARS-CoV-2. Diseases. 2025; 13(4):104. https://doi.org/10.3390/diseases13040104
Chicago/Turabian StyleJakoby, Lena, Ernst Molitor, Nico T. Mutters, Ruth Weppler, Dominic Rauschning, and Manuel Döhla. 2025. "Quality Management Outweighs Pandemic: Retrospective Analysis Shows Improved Quality of Care for Staphylococcus aureus Bacteremia Despite SARS-CoV-2" Diseases 13, no. 4: 104. https://doi.org/10.3390/diseases13040104
APA StyleJakoby, L., Molitor, E., Mutters, N. T., Weppler, R., Rauschning, D., & Döhla, M. (2025). Quality Management Outweighs Pandemic: Retrospective Analysis Shows Improved Quality of Care for Staphylococcus aureus Bacteremia Despite SARS-CoV-2. Diseases, 13(4), 104. https://doi.org/10.3390/diseases13040104







