Relationships of Isolated Nocturnal Hypertension with Glomerular Filtration Rate and Albuminuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Twenty-Four-Hour Ambulatory Blood Pressure Monitoring
2.2. Statistical Analysis
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Staessen, J.A.; Lu, L.; Li, L.H.; Wang, G.L.; Wang, J.G. Is isolated nocturnal hypertension a novel clinical entity? Findings from Chinese population study. Hypertension 2007, 50, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.Y.; Kim, S.W.; Choi, E.H.; Kim, J.H.; Nah, D.Y.; Shin, S.J.; Gu, N. Prevalence of masked hypertension: A population-based survey in a large city by using 24-hour ambulatory blood pressure monitoring. Korean Circ. J. 2016, 46, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Bakris, G.L. Recognition and management of masked hypertension: A review and novel approach. J. Am. Soc. Hypertens. 2013, 7, 244–252. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.G. Isolated nocturnal hypertension: A disease masked in the dark. Hypertension 2013, 61, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.R.; Espeche, W.G.; Balbín, E.; Leiva Sisnieguez, C.E.; Minetto, J.; Leiva Sisnieguez, B.C.; Maciel, P.M.; Stavile, R.N.; Carbajal, H.A. Prevalence of isolated nocturnal hypertension according to 2018 European Society of Cardiology and European Society of Hypertension office blood pressure categories. J. Hypertens. 2020, 38, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.Y.; Kim, J.S.; Kim, C.H.; Kim, J.H.; Lee, J.H.; Kim, S.W.; Nah, D.Y.; Gu, N.; Cho, E.J.; Sung, K.C.; et al. Prevalence and characteristics of isolated nocturnal hypertension in the general population. Korean J. Intern. Med. 2021, 36, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.H.; Shin, C.; Kim, S.; Kim, J.S.; Lim, S.Y.; Seo, H.S.; Lim, H.E.; Sung, K.C.; Cho, G.Y.; Lee, S.K.; et al. Prevalence of Isolated Nocturnal Hypertension and Development of Arterial Stiffness, Left Ventricular Hypertrophy, and Silent Cerebrovascular Lesions: The KoGES (Korean Genome and Epidemiology Study). J. Am. Heart Assoc. 2022, 11, e025641. [Google Scholar] [CrossRef]
- Kario, K.; Williams, B. Nocturnal Hypertension and Heart Failure: Mechanisms, Evidence, and New Treatments. Hypertension 2021, 78, 564–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, H.Q.; Li, Y.; Thijs, L.; Hansen, T.W.; Boggia, J.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Jeppesen, J.; et al. International Database on Ambulatory Blood Pressure In Relation to Cardiovascular Outcomes Investigators. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J. Hypertens. 2010, 28, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ren, H.; Xie, J.; Wang, W.; Li, Y.; Gao, P.; Chen, N. Association of Nighttime Masked Uncontrolled Hypertension With Left Ventricular Hypertrophy and Kidney Function Among Patients with Chronic Kidney Disease Not Receiving Dialysis. JAMA Netw. Open. 2022, 5, e2214460. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhang, J.; Ye, Z.; Zhang, Q.; Ma, X.; Peng, H.; Lou, T. Prognostic Effect of Isolated Nocturnal Hypertension in Chinese Patients With Nondialysis Chronic Kidney Disease. J. Am. Heart Assoc. 2016, 5, e004198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshide, S.; Suzuki, D.; Kario, K. Circadian Variation and Arterial Stiffness in Chronic Kidney Disease and Their Treatment. Am. J. Hypertens. 2021, 34, 456–458. [Google Scholar] [CrossRef]
- Borrelli, S.; Garofalo, C.; Gabbai, F.B.; Chiodini, P.; Signoriello, S.; Paoletti, E.; Ravera, M.; Bussalino, E.; Bellizzi, V.; Liberti, M.E.; et al. Dipping Status, Ambulatory Blood Pressure Control, Cardiovascular Disease, and Kidney Disease Progression: A Multicenter Cohort Study of CKD. Am. J. Kidney Dis. 2023, 81, 15–24e1. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Cuspidi, C.; Facchetti, R.; Bombelli, M.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Is night-time hypertension worse than daytime hypertension? A study on cardiac damage in a general population: The PAMELA study. J. Hypertens. 2017, 35, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Spruill, T.M.; Sarpong, D.F.; Agyemang, C.; Chaplin, W.; Pastva, A.; Martins, D.; Ravenell, J.; Pickering, T.G. Correlates of isolated nocturnal hypertension and target organ damage in a population-based cohort of African Americans: The Jackson Heart Study. Am. J. Hypertens. 2013, 26, 1011–1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukuda, M.; Munemura, M.; Usami, T.; Nakao, N.; Takeuchi, O.; Kamiya, Y.; Yoshida, A.; Kimura, G. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004, 65, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Kimura, G. Kidney and circadian blood pressure rhythm. Hypertension 2008, 51, 827–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohandas, R.; Douma, L.G.; Scindia, Y.; Gumz, M.L. Circadian rhythms and renal pathophysiology. J. Clin. Investig. 2022, 132, e148277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGlothan, K.R.; Wyatt, R.J.; Ault, B.H.; Hastings, M.C.; Rogers, T.; DiSessa, T.; Jones, D.P. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr. Transplant. 2006, 10, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Dello Strologo, L.; Iyengar, A.; Velusamy, V.; Greenbaum, L.A. Nocturnal hypertension and left ventricular hypertrophy in pediatric renal transplant recipients in South India. Pediatr. Transplant. 2020, 24, e13710. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, W.J.; Gong, W.Y.; Zhang, J.; Tang, H.; Peng, H.; Zhang, Q.Z.; Ye, Z.C.; Lou, T. High prevalence of isolated nocturnal hypertension in Chinese patients with chronic kidney disease. J. Am. Heart Assoc. 2015, 4, e002025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujita, H.; Matsuoka, S.; Awazu, M. Masked Isolated Nocturnal Hypertension in Children and Young Adults. Pediatr. Cardiol. 2018, 39, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, U.; Anarat, A.; Bayazit, A.K.; Bakkaloglu, A.S.; Bilginer, Y.; Caliskan, S.; Civilibal, M.; Doyon, A.; Duzova, A.; Kracht, D.; et al. The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study: Objectives, design, and methodology. Clin. J. Am. Soc. Nephrol. 2010, 5, 1642–1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Düzova, A.; Karabay Bayazit, A.; Canpolat, N.; Niemirska, A.; Kaplan Bulut, I.; Azukaitis, K.; Karagoz, T.; Oguz, B.; Erdem, S.; Anarat, A.; et al. Isolated nocturnal and isolated daytime hypertension associate with altered cardiovascular morphology and function in children with chronic kidney disease: Findings from the Cardiovascular Comorbidity in Children with Chronic Kidney Disease study. J. Hypertens. 2019, 37, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Rump, L.C.; Amann, K.; Orth, S.; Ritz, E. Sympathetic overactivity in renal disease: A window to understand progression and cardiovascular complications of uraemia? Nephrol. Dial. Transplant. 2000, 15, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Joles, J.A.; Koomans, H.A. Causes and consequences of increased sympathetic activity in renal disease. Hypertension 2004, 43, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Ligtenberg, G.; Klein, I.I.; Koomans, H.A.; Blankestijn, P.J. Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney Int. 2004, 65, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Pereira Redondo, J.C. Hipertensión arterial nocturna. Rev. Argent. Cardiol. 2024, 92, 267–268. [Google Scholar] [CrossRef]
- Briasoulis, A.; Silver, A.; Yano, Y.; Bakris, G.L. Orthostatic hypotension associated with baroreceptor dysfunction: Treatment approaches. J. Clin. Hypertens. 2014, 16, 141–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pisano, A.; Zoccali, C.; Bolignano, D.; D’Arrigo, G.; Mallamaci, F. Sleep apnoea syndrome prevalence in chronic kidney disease and end-stage kidney disease patients: A systematic review and meta-analysis. Clin. Kidney J. 2023, 17, sfad179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maqsood, M.H.; Messerli, F.H.; Skolnick, A.H.; Newman, J.D.; Berger, J.S.; Bangalore, S. Timing of Antihypertensive Drug Therapy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Hypertension 2023, 80, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
| Ambulatory Blood Pressure Phenotypes | |||||
|---|---|---|---|---|---|
| (A) Normal Daytime and Nocturnal BP (n = 273) | (B) Isolated Daytime HTN (n = 94) | (C) Isolated Nocturnal HTN (n = 152) | (D) Daytime and Nocturnal HTN (n = 820) | ANOVA or X2 Test (p) | |
| Gender (Men) (%) | 45.1 | 56.8 | 61.2 ^ | 62.6 ^^ | <0.0001 |
| Age (years) | 46.4 ± 14.1 | 46 ± 13.3 | 47.9 ± 14.9 | 47.1 ±11.9 | 0.59 |
| Smokers (%) | 23 | 31.5 | 23.5 | 31.2 * | 0.045 |
| Body mass index (Kg/m2) | 28.6 ± 5.3 | 28.8 ± 4.4 | 28.3 ± 4.8 | 28 ± 4.1 | 0.64 |
| Waist circumference (cm) | 97.3 ± 12.4 | 100.2 ± 10 | 94.7 ± 14.6 | 96.2 ± 12.1 | 0.83 |
| Total cholesterol (mg/dL) | 206.4 ± 41.3 | 204.2 ± 38.1 | 211.3 ± 44.7 | 206.1 ± 41.1 | 0.56 |
| HDL cholesterol (mg/dL) | 47.4 ± 11.1 | 46.5 ± 9.1 | 44.7 ± 11.6 | 45.5 ± 10.2 | 0.11 |
| Triglycerides (mg/dL) | 125 (84–170) | 108 (84–162) | 125 (81–169) | 127 (89–182) | 0.06 |
| LDL cholesterol (mg/dL) | 131.8 ± 39.1 | 130.9 ± 35.9 | 135.9 ± 42.2 | 134.4 ± 37.8 | 0.7 |
| Serum glucose (mg/dL) | 95.8 ± 22.1 | 95.2 ± 18.2 | 95.6 ± 20.1 | 96.2 ± 19.8 | 0.83 |
| Diabetes (%) | 6.3 | 9 | 9 | 6.7 | 0.68 |
| Metabolic syndrome (%) | 34.8 | 38.9 | 47.4 | 51.7 | <0.0001 |
| Ambulatory Blood Pressure Phenotypes | |||||
|---|---|---|---|---|---|
| (A) Normal Daytime and Nocturnal BP (n = 273) | (B) Isolated Daytime HTN (n = 94) | (C) Isolated Nocturnal HTN (n = 152) | (D) Daytime and Nocturnal HTN (n = 820) | ANOVA or X2 Test (p) | |
| Duration of hypertension (months) | 12 (7–24) | 12 (6–36) | 12 (8–24) | 24 (11–48) * | <0.001 |
| Drug therapy for hypertension (%) | 70.2 | 75.8 | 79.3 | 83.2 ^^ | <0.001 |
| Clinic systolic BP (mmHg) | 141 ± 17.5 | 151 ± 18.1 ^^ | 144 ± 16.6 | 156 ± 20.5 ^^ | <0.001 |
| Clinic diastolic BP (mmHg) | 84 ± 12.3 | 91 ± 11.9 ^^ | 88 ± 13.5 * | 95 ± 15.8 ^^ | <0.001 |
| 24 h systolic BP (mmHg) | 118 ± 6.4 | 127 ± 6.8 ^^ | 125 ± 5.7 ^^ | 138 ± 11.6 ^^ | <0.001 |
| 24 h diastolic BP (mmHg) | 84 ± 12.3 | 91 ± 11.9 ^^ | 88 ± 13.5 ^^ | 95 ± 15.8 ^^ | <0.001 |
| 24 h heart rates (b/min) | 73 ± 9.1 | 76 ± 7.6 * | 73 ± 9.4 | 75 ± 10.3 ^^ | 0.001 |
| Daytime systolic BP (mmHg) | 122 ± 6.9 | 135 ± 7.9 ^^ | 126 ± 5.6 ^^ | 144 ± 10.7 ^^ | <0.001 |
| Daytime diastolic BP (mmHg) | 75 ± 6.4 | 86 ± 6.7 ^^ | 79 ± 5.0 ^^ | 95 ± 15.8 ^^ | <0.001 |
| Nighttime systolic BP (mmHg) | 109 ± 7.9 | 110 ± 6.4 | 121 ± 8.5 ^^ | 131 ± 12.8 ^^ | <0.001 |
| Nighttime diastolic BP (mmHg) | 62 ± 5.6 | 65 ± 5.3 ** | 74 ± 5.2 ^^ | 81 ± 9.5 ^^ | <0.001 |
| Ambulatory Blood Pressure Phenotypes | |||||
|---|---|---|---|---|---|
| (A) Normal Daytime and Nocturnal BP (n = 273) | (B) Isolated Daytime HTN (n = 94) | (C) Isolated Nocturnal HTN (n = 152) | (D) Daytime and Nocturnal HTN (n = 820) | ANOVA or X2 Test (p) | |
| No CKD (%) (n = 919) | 82.8 | 88.4 | 68.4 ^ | 61.6 ^^ | <0.0001 |
| Stage 1 CKD (%) (n = 187) | 5.5 | 3.2 | 9.2 | 18.9 ^^ | <0.0001 |
| Stage 2 CKD (%) (n = 89) | 3.7 | 2.1 | 4.6 | 8.5 ** | 0.005 |
| Stage 3a CKD (%) (n = 66) | 3.7 | 4.2 | 6.6 | 5.1 | 0.5749 |
| Stage 3b CKD (%) (n = 45) | 2.2 | 1.1 | 5.9 | 3.5 | 0.1194 |
| Stage 4-5 CKD (%) (n = 34) | 2.2 | 1.1 | 5.3 | 2.3 | 0.127 |
| Ambulatory Blood Pressure Phenotypes | |||||
|---|---|---|---|---|---|
| (A) Normal Daytime and Nocturnal BP (n = 273) | (B) Isolated Daytime HTN (n = 94) | (C) Isolated Nocturnal HTN (n = 152) | (D) Daytime and Nocturnal HTN (n = 820) | ANOVA or X2 test (p) | |
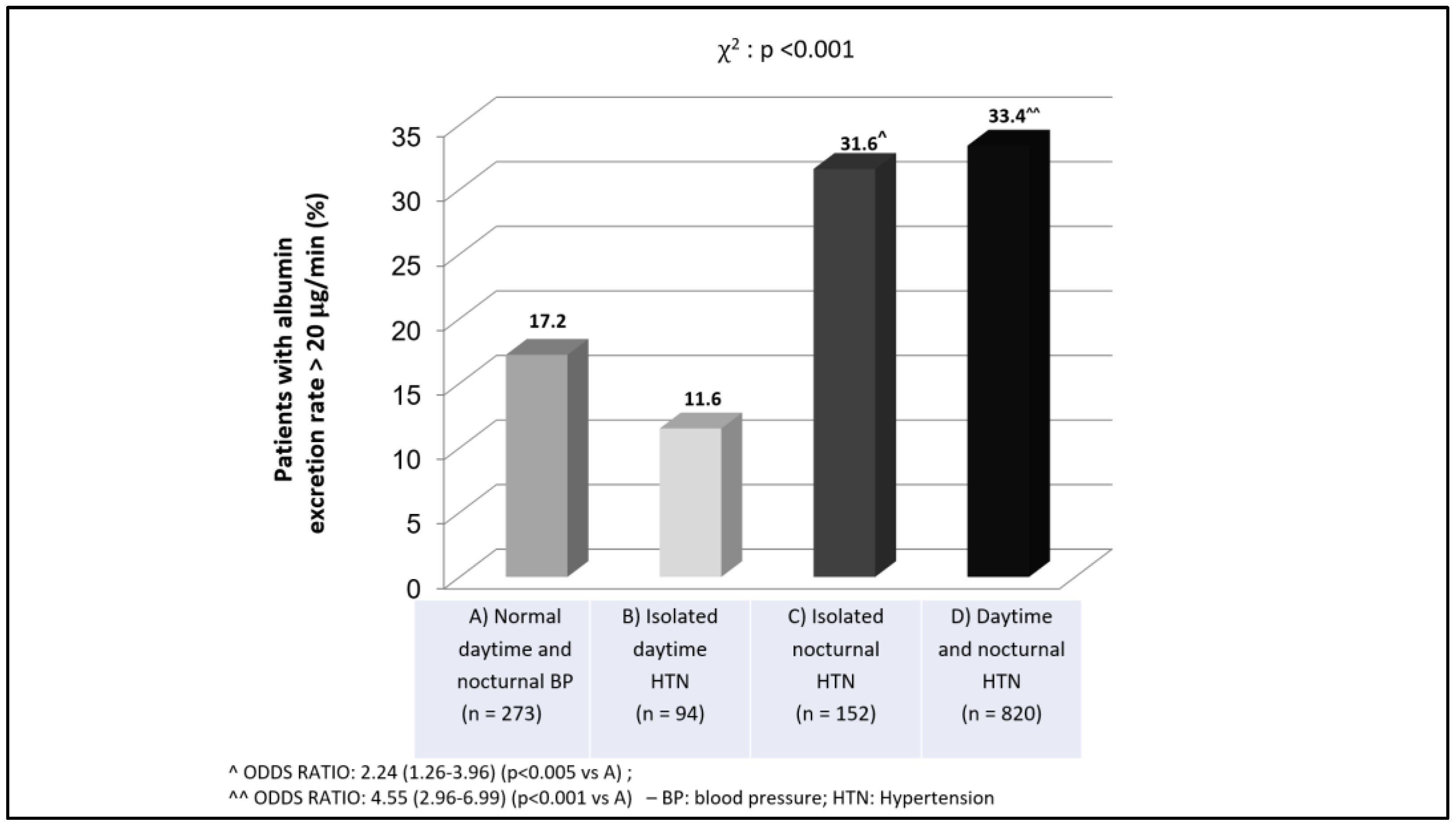
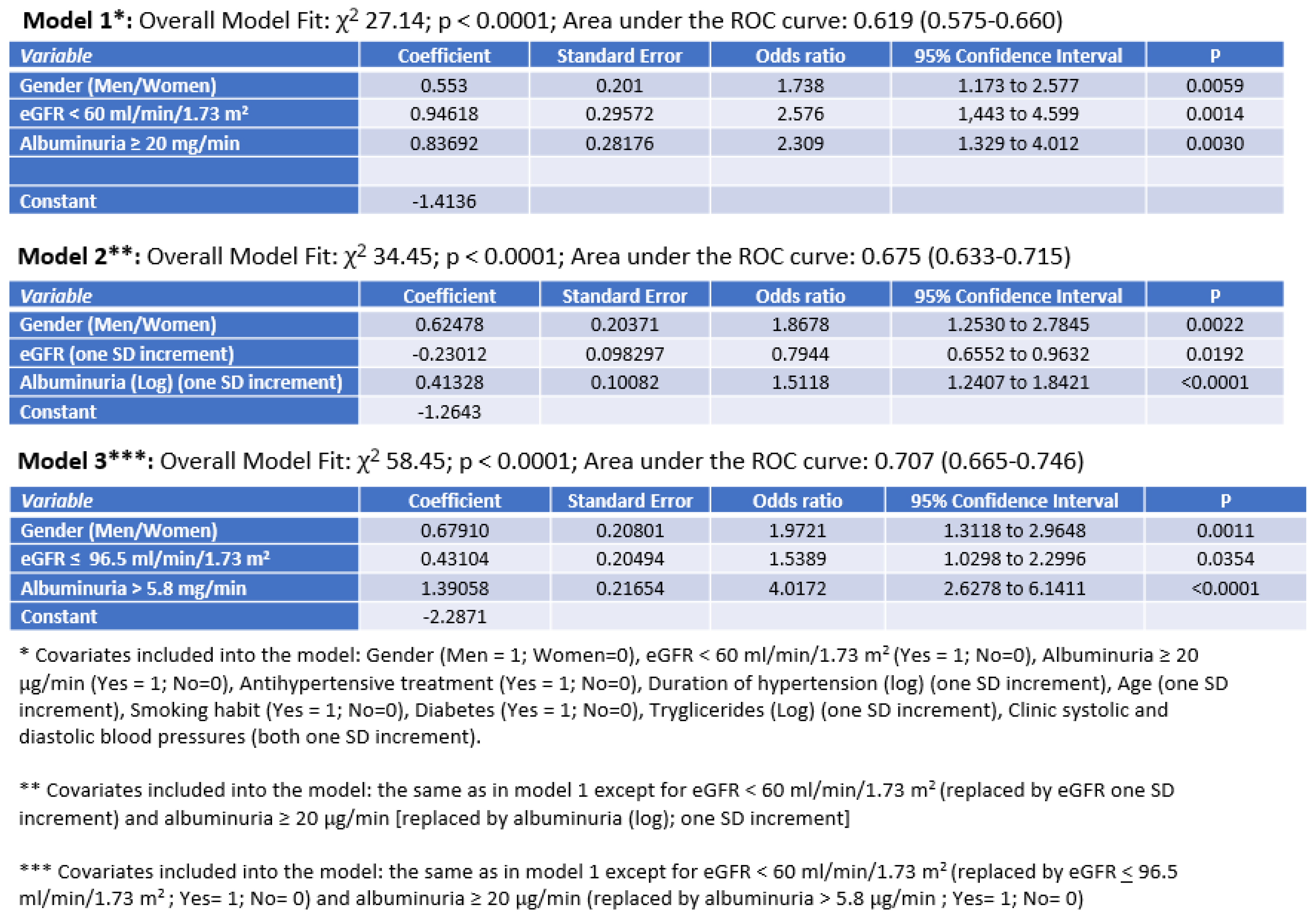
| Albuminuria (μg/min) | 5.0 (3.0–9.7) | 5.6 (3.3–10.0) | 9.1(5.4–17.9) ^^ | 14.7(8–30.3) ^^ | <0.0001 |
| Patients with high albuminuria (%) | 9.5 | 7.4 | 19.1 ^ | 32.3 ^^ | <0.0001 |
| Serum creatinine (mg/dL) | 0.89 ± 0.42 | 0.87 ± 0.29 * | 1.06 ± 0.67 ^ | 0.99 ± 0.52 | 0.002 |
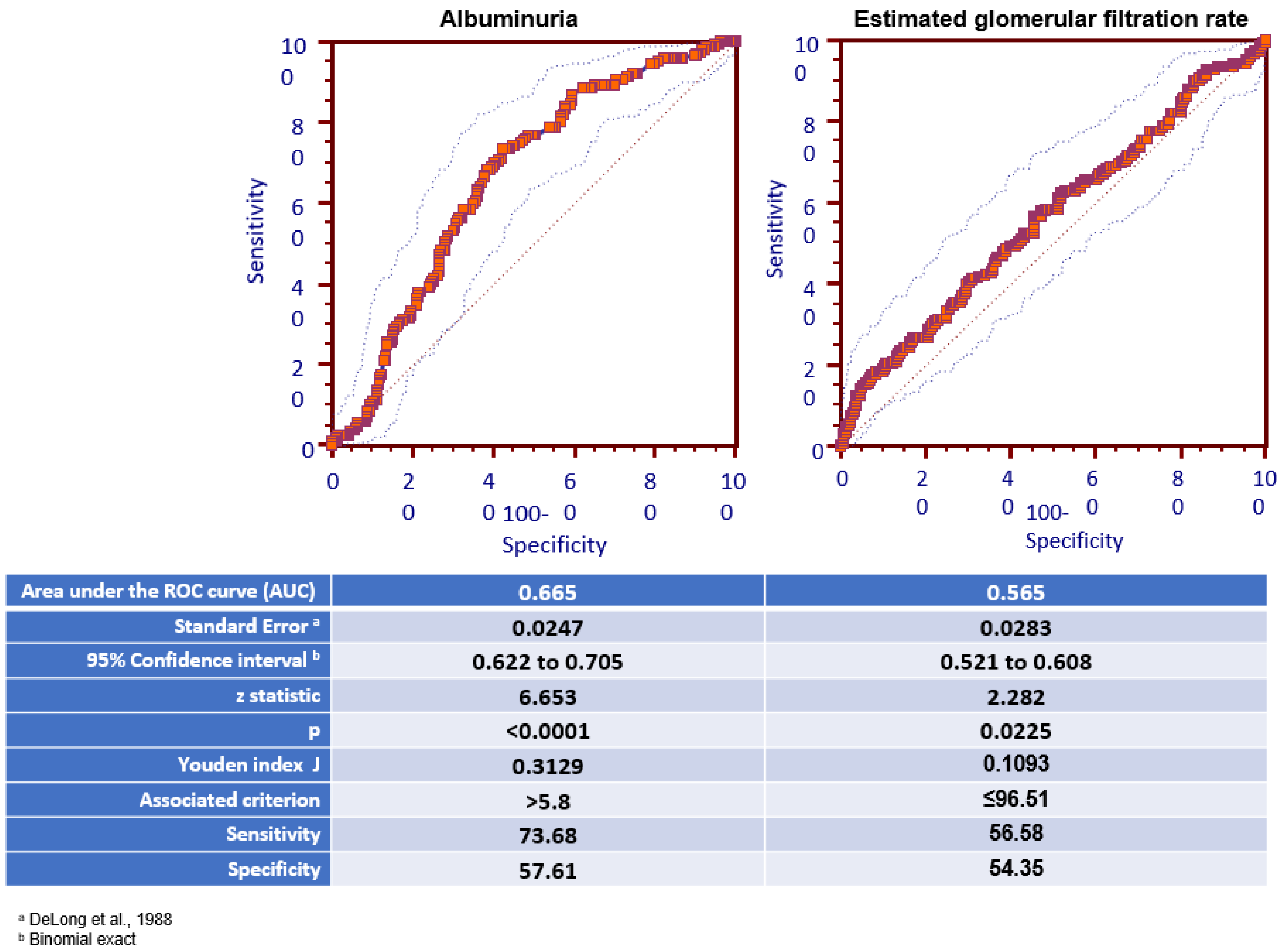
| Estimated GFR (ml/min/1.73 m2) | 93.6 ± 24.2 | 95.8 ± 20.8 ^ | 87.4 ± 28.8 * | 89.7 ± 23.4 * | 0.005 |
| Patients with eGFR 60 ml/min (%) | 8.1 | 6.3 | 17.8 ^ | 11 | 0.01 |
| Ambulatory Blood Pressure Phenotypes | |||||
|---|---|---|---|---|---|
| (A) Normal Daytime and Nocturnal BP (n = 102) | (B) Isolated Daytime HTN (n = 8) | (C) Isolated Nocturnal HTN (n = 47) | (D) Daytime and Nocturnal HTN (n = 106) | ANOVA or X2 Test (p) | |
| Albuminuria (μg/min) | 5.0 (3.0–11) | 3.4 (2.0-3.9) | 9.7 (6.0–19.0) * | 10.5 (7.4–20.7) ^^ | <0.0001 |
| Patients with high albuminuria (%) | 8.8 | 0 | 21.3 * | 24.5 ^ | 0.002 |
| Serum creatinine (mg/dL) | 0.91 ± 0.55 | 0.88 ± 0.37 * | 1.23 ± 0.97 ^ | 1.05 ± 0.43 | 0.02 |
| Estimated GFR (mL/min/1.73 m2) | 97.4 ± 26.4 | 95.0 ± 23.5 ^ | 82.8 ± 33.4 ^ | 86.1 ± 24.9 ^ | 0.004 |
| Patients with eGFR 60 mL/min (%) | 9.8 | 12.5 | 21.3 | 15.1 | 0.194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, C.; Geraci, G.; Sorce, A.; Morreale Bubella, R.; Cirafici, E.; Ciuppa, M.E.; Evola, S.; Mulè, G. Relationships of Isolated Nocturnal Hypertension with Glomerular Filtration Rate and Albuminuria. Diseases 2025, 13, 107. https://doi.org/10.3390/diseases13040107
Carollo C, Geraci G, Sorce A, Morreale Bubella R, Cirafici E, Ciuppa ME, Evola S, Mulè G. Relationships of Isolated Nocturnal Hypertension with Glomerular Filtration Rate and Albuminuria. Diseases. 2025; 13(4):107. https://doi.org/10.3390/diseases13040107
Chicago/Turabian StyleCarollo, Caterina, Giulio Geraci, Alessandra Sorce, Raffaella Morreale Bubella, Emanuele Cirafici, Maria Elena Ciuppa, Salvatore Evola, and Giuseppe Mulè. 2025. "Relationships of Isolated Nocturnal Hypertension with Glomerular Filtration Rate and Albuminuria" Diseases 13, no. 4: 107. https://doi.org/10.3390/diseases13040107
APA StyleCarollo, C., Geraci, G., Sorce, A., Morreale Bubella, R., Cirafici, E., Ciuppa, M. E., Evola, S., & Mulè, G. (2025). Relationships of Isolated Nocturnal Hypertension with Glomerular Filtration Rate and Albuminuria. Diseases, 13(4), 107. https://doi.org/10.3390/diseases13040107






