Benchmark for Setting ACTH Cell Dosage in Clinical Regenerative Medicine for Post-Operative Hypopituitarism
Abstract
1. Introduction
2. Materials and Methods
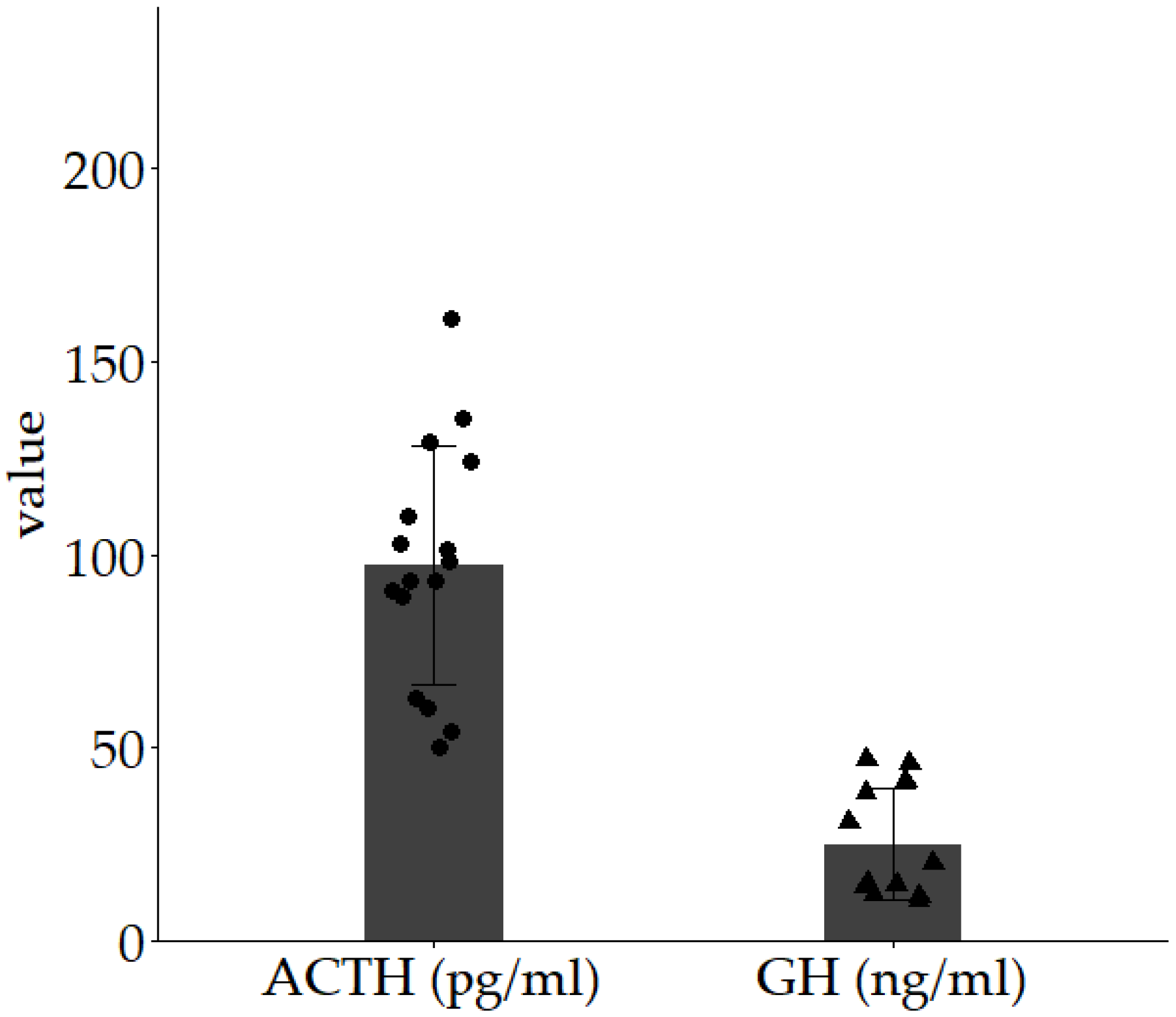
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ooi, G.T.; Tawadros, N.; Escalona, R.M. Pituitary cell lines and their endocrine applications. Mol. Cell Endocrinol. 2004, 228, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Regal, M.; Palamo, C.; Garcia-Mayor, R.V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. 2001, 55, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Puar, T.H.K.; Stikkelbroeck, N.M.M.L.; Smans, L.C.C.J.; Zelissen, P.M.J.; Hermus, A.R.M.M. Adrenal Crisis: Still a Deadly Event in the 21st Century. Am. J. Med. 2016, 129, 339.e1–339.e9. [Google Scholar] [CrossRef] [PubMed]
- Hahner, S.; Spinnler, C.; Fassnacht, M.; Burger-Stritt, S.; Lang, K.; Milovanovic, D.; Beuschlein, F.; Willenberg, H.S.; Quinkler, M.; Allolio, B. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: A prospective study. J. Clin. Endocrinol. Metab. 2015, 100, 407–416. [Google Scholar] [CrossRef]
- Carroll, P.V.; Christ, E.R.; Bengtsson, B.A.; Carlsson, L.; Christiansen, J.S.; Clemmons, D.; Hintz, R.; Ho, K.; Laron, Z.; Sizonenko, P.; et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. J. Clin. Endocrinol. Metab. 1998, 83, 382–395. [Google Scholar] [CrossRef]
- Amato, G.; Mazziotti, G.; Somma, C.D.; Lalli, E.; Felice, G.D.; Conte, M.; Rotondi, M.; Pietrosante, M.; Lombardi, G.; Bellastella, A.; et al. Recombinant growth hormone (GH) therapy in GH-deficient adults: A long-term controlled study on daily versus thrice weekly injections. J. Clin. Endocrinol. Metab. 2000, 85, 3720–3725. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human es and ips cells by dual inhibition of smad signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Gotoh, S.; Ito, I.; Nagasaki, T.; Yamamoto, Y.; Konishi, S.; Korogi, Y.; Matsumoto, H.; Muro, S.; Hirai, T.; Funato, M.; et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 2014, 3, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Vilar, E.; Tsai, S.-Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; McGivern, J.V.; Van Dyke, J.M.; Ebert, A.D.; Suzuki, M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl. Med. 2014, 3, 564–574. [Google Scholar] [CrossRef]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Matsumoto, R.; Takahashi, Y. Human pituitary development and application of iPSCs for pituitary disease. Cell Mol. Life Sci. 2021, 78, 2069–2079. [Google Scholar] [CrossRef]
- Zhu, X.; Gleiberman, A.S.; Rosenfeld, M.G. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol. Rev. 2007, 87, 933–963. [Google Scholar] [CrossRef]
- Watanabe, K.; Kamiya, D.; Nishiyama, A.; Katayama, T.; Nozaki, S.; Kawasaki, H.; Watanabe, Y.; Mizuseki, K.; Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005, 8, 288–296. [Google Scholar] [CrossRef]
- Eiraku, M.; Watanabe, K.; Takasaki, M.M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef]
- Wataya, T.; Ando, S.; Muguruma, K.; Ikeda, H.; Watanabe, K.; Eiraku, M.; Kawada, M.; Takahashi, J.; Hashimoto, N.; Sasai, Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 11796–11801. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kadoshima, T.; Minaguchi, M.; Ohgushi, M.; Soen, M.; Nakano, T.; Takata, N.; Wataya, T.; Muguruma, K.; Miyoshi, H.; et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 2011, 480, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ozone, C.; Suga, H.; Eiraku, M.; Kadoshima, T.; Yonemura, S.; Takata, N.; Oiso, Y.; Tsuji, T.; Sasai, Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun. 2016, 7, 10351. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Suga, H.; Sakakibara, M.; Ozone, C.; Matsumoto, R.; Kano, M.; Mitsumoto, K.; Ogawa, K.; Kodani, Y.; Nagasaki, H.; et al. Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3d-cultured human iPS cells. Cell Rep. 2020, 30, 18–24. [Google Scholar] [CrossRef]
- Dincer, Z.; Piao, J.; Niu, L.; Ganat, Y.; Kriks, S.; Zimmer, B.; Shi, S.H.; Tabar, V.; Studer, L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep. 2013, 5, 1387–1402. [Google Scholar] [CrossRef]
- Zimmer, B.; Piao, J.; Ramnarine, K.; Tomishima, M.J.; Tabar, V.; Studer, L. Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Rep. 2016, 6, 858–872. [Google Scholar] [CrossRef]
- Taga, S.; Suga, H.; Nakano, T.; Kuwahara, A.; Inoshita, N.; Kodani, Y.; Nagasaki, H.; Sato, Y.; Tsumura, Y.; Sakakibara, M.; et al. Generation and purification of ACTH-secreting hPSC-derived pituitary cells for effective transplantation. Stem Cell Rep. 2023, 18, 1657–1671. [Google Scholar] [CrossRef]
- Sasaki, H.; Suga, H.; Takeuchi, K.; Nagata, Y.; Harada, H.; Kondo, T.; Ito, E.; Maeda, S.; Sakakibara, M.; Soen, M.; et al. Subcutaneous transplantation of human embryonic stem cells-derived pituitary organoids. Front. Endocrinol. 2023, 14, 1130465. [Google Scholar] [CrossRef]
- Matsuyama, H.; Ruhmann-Wennhold, A.; Nelson, D.H. Radioimmunoassay of Plasma ACTH in Intact Rats. Endocrinology 1971, 88, 692–695. [Google Scholar] [CrossRef]
- Schalch, D.S.; Reichlin, S. Plasma growth hormone concentration in the rat determined by radioimmunoassay: Influence of sex, pregnancy, lactation, anesthesia, hypophysectomy and extrasellar pituitary transplants. Endocrinology 1966, 79, 275–280. [Google Scholar] [CrossRef]
- Eden, S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology 1979, 105, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, W.; Boelke, T.; Bahr, V. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1-39) or human corticotropin-releasing hormone in man. J. Clin. Endocrinol. Metab. 1988, 66, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bowers, C.Y.; Reynolds, G.A.; Durham, D.; Barrera, C.M.; Pezzoli, S.S.; Thorner, M.O. Growth hormone (GH)-releasing peptide stimulates GH release in normal men and acts synergistically with GH-releasing hormone. J. Clin. Endocrinol. Metab. 1990, 70, 975–982. [Google Scholar] [CrossRef] [PubMed]
| Median (IQR) | |
|---|---|
| Age | 63 (55.8–69) |
| Sex (M:F) | 11:5 |
| ACTH (pg/mL) | 24.2 (18.0–30.7) |
| Cortisol (µg/mL) | 10.6 (8.0–11.7) |
| Tumor type | NFPA 14, TSHoma 2 |
| Median (IQR) | |
|---|---|
| Age | 58.5 (51.3–65.8) |
| Sex (M:F) | 5:7 |
| GH (ng/mL) | 0.58 (0.18–1.25) |
| IGF-1 (ng/mL) | 140.5 (118.8–149.8) |
| Tumor type | NFPA 1 9, TSHoma 2 2, PRLoma 3 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, T.; Suga, H.; Takeuchi, K.; Fuse, Y.; Sato, Y.; Hirose, T.; Hideyuki, H.; Nagata, Y.; Saito, R. Benchmark for Setting ACTH Cell Dosage in Clinical Regenerative Medicine for Post-Operative Hypopituitarism. Diseases 2025, 13, 112. https://doi.org/10.3390/diseases13040112
Kondo T, Suga H, Takeuchi K, Fuse Y, Sato Y, Hirose T, Hideyuki H, Nagata Y, Saito R. Benchmark for Setting ACTH Cell Dosage in Clinical Regenerative Medicine for Post-Operative Hypopituitarism. Diseases. 2025; 13(4):112. https://doi.org/10.3390/diseases13040112
Chicago/Turabian StyleKondo, Tatsuma, Hidetaka Suga, Kazuhito Takeuchi, Yutaro Fuse, Yoshiki Sato, Toshiaki Hirose, Harada Hideyuki, Yuichi Nagata, and Ryuta Saito. 2025. "Benchmark for Setting ACTH Cell Dosage in Clinical Regenerative Medicine for Post-Operative Hypopituitarism" Diseases 13, no. 4: 112. https://doi.org/10.3390/diseases13040112
APA StyleKondo, T., Suga, H., Takeuchi, K., Fuse, Y., Sato, Y., Hirose, T., Hideyuki, H., Nagata, Y., & Saito, R. (2025). Benchmark for Setting ACTH Cell Dosage in Clinical Regenerative Medicine for Post-Operative Hypopituitarism. Diseases, 13(4), 112. https://doi.org/10.3390/diseases13040112






