Rabies Vaccination and Public Health Insights in the Extended Arabian Gulf and Saudi Arabia: A Systematic Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Research Questions
2.4. Trial Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Analysis of Outcome Measures
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Baseline Characteristics
3.4. Vaccination Schedules
3.5. Safety of the Included Vaccines
3.6. Quality Assessment
4. Discussion
4.1. Demographics of the Included Patients
4.2. Protocols of Vaccination
4.3. Different Practices of Post-Exposure Prophylaxis (PEP)
4.4. WHO’s Global Rabies Elimination Strategy (2018)
4.5. Safety of Vaccines
5. Limitations
6. Implications and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Neglected Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Gan, H.; Hou, X.; Wang, Y.; Xu, G.; Huang, Z.; Zhang, T.; Lin, R.; Xue, M.; Hu, H.; Liu, M.; et al. Global burden of rabies in 204 countries and territories, from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Int. J. Infect. Dis. 2023, 126, 136–144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; World Health Organization: Geneva, Switzerland, 2018; Volume 1012. [Google Scholar]
- Hankins, D.G.; Rosekrans, J.A. Overview, prevention, and treatment of rabies. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2004; pp. 671–676. [Google Scholar]
- Wertheim, H.F.L.; Nguyen, T.Q.; Nguyen, K.A.T.; de Jong, M.D.; Taylor, W.R.J.; Le, T.V.; Nguyen, H.H.; Nguyen, H.T.H.; Farrar, J.; Horby, P.; et al. Furious rabies after an atypical exposure. PLoS Med. 2009, 6, e1000044. [Google Scholar] [CrossRef]
- Wilde, H.; Hemachudha, T.; Wacharapluesadee, S.; Lumlertdacha, B.; Tepsumethanon, V. Rabies in Asia: The classical zoonosis. In One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases: The Concept and Examples of a One Health Approach; Springer: Berlin/Heidelberg, Germany, 2013; pp. 185–203. [Google Scholar]
- World Health Organization. Rabies vaccines: WHO position paper, April 2018–Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef]
- Hampson, K.; Cleaveland, S.; Briggs, D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLOS Neglected Trop. Dis. 2011, 5, e982. [Google Scholar] [CrossRef]
- Kasem, S.; Hussein, R.; Al-Doweriej, A.; Qasim, I.; Abu-Obeida, A.; Almulhim, I.; Alfarhan, H.; Hodhod, A.A.; Abel-Latif, M.; Hashim, O.; et al. Rabies among animals in Saudi Arabia. J. Infect. Public Health 2019, 12, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Warrell, M.J. Current rabies vaccines and prophylaxis schedules: Preventing rabies before and after exposure. Travel Med. Infect. Dis. 2012, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U. Zoonoses in the Arabian peninsula. Saudi Med. J. 2014, 35, 1455. [Google Scholar]
- Memish, Z.A.; Assiri, A.M.; Gautret, P. Rabies in Saudi Arabia: A need for epidemiological data. Int. J. Infect. Dis. 2015, 34, 99–101. [Google Scholar] [CrossRef]
- Al-Dubaib, M. Rabies in camels at Qassim region of central Saudi Arabia. J. Camel Pract. Res. 2007, 14, 101–103. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bay, V.; Rezapour, A.; Jafari, M.; Maleki, M.R.; Asl, I.M. Healthcare utilization patterns and economic burden of animal bites: A cross-sectional study. J. Acute Dis. 2021, 10, 142–146. [Google Scholar] [CrossRef]
- Kilic, B.; Unal, B.; Semin, S.; Konakci, S.K. An important public health problem: Rabies suspected bites and post-exposure prophylaxis in a health district in Turkey. Int. J. Infect. Dis. 2006, 10, 248–254. [Google Scholar] [CrossRef]
- Taghvaii, M.R.E.; Seyednozadi, S.M. An epidemiologic survey on animal bite cases referred to health centers in mashhad during 2006 to 2009. Biomed. Pharmacol. J. 2015, 6, 301–306. [Google Scholar] [CrossRef]
- Khazaei, S.; Shiraz Medical University; Karami, M.; Veisani, Y.; Solgi, M.; Goodarzi, S. Epidemiology of animal bites and associated factors with delay in post-exposure prophylaxis; a cross-sectional study. Bull. Emerg. Trauma 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Roncero, M.I.G.; Cerdá-Olmedo, E. Genetics of carotene biosynthesis in phycomyces. Curr. Genet. 1982, 5, 5–8. [Google Scholar] [CrossRef]
- Sari, T.; Tulek, N.; Bulut, C.; Oral, B.; Ertem, G.T. Adverse events following rabies post-exposure prophylaxis: A comparative study of two different schedules and two vaccines. Travel Med. Infect. Dis. 2014, 12, 659–666. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Shirzadi, M.R.; Ramezankhani, A.; Poor, M.J. A comparative study on the adverse reactions of purified chick embryo cell vaccine (PCECV) and purified vero cell rabies vaccine (PVRV). Arch. Iran Med. 2016, 19, 502–507. [Google Scholar]
- Khoubfekr, H.; Jokar, M.; Rahmanian, V.; Blouch, H.; Shirzadi, M.R.; Bashar, R. Fatal cases in pediatric patients after post-exposure prophylaxis for rabies: A report of two cases. Asian Pac. J. Trop. Med. 2024, 17, 39–42. [Google Scholar] [CrossRef]
- Ansari, M.; Shafiei, M.; Kordi, R. Dog bites among off-road cyclists: A report of two cases. Asian J. Sports Med. 2012, 3, 60. [Google Scholar] [CrossRef]
- Tabbara, K.F.; Al-Omar, O. Eyelid laceration sustained in an attack by a rabid desert fox. Arch. Ophthalmol. 1995, 119, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, A.; Pourhossein, B.; Bashar, R.; Shirzadi, M.R.; Amiri, B.; Kheiri, E.V.; Mostafazadeh, B.; Fazeli, M. Case Report: Investigating Possible Etiologies of Post-Exposure Prophylaxis Failure and Deaths From Rabies Infection. Int. J. Med. Toxicol. Forensic Med. 2020, 10, 27378. [Google Scholar] [CrossRef]
- Farahtaj, F.; Fayaz, A.; Howaizi, N.; Biglari, P.; Gholami, A. Human rabies in Iran. Trop. Dr. 2014, 44, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Bizri, A.R.; Azar, A.; Salam, N.; Mokhbat, J. Human rabies in Lebanon: Lessons for control. Epidemiol. Infect. 2000, 125, 175–179. [Google Scholar] [CrossRef]
- Davarani, E.R.; Domari, A.A.; Mahani, A.H.; Samakkhah, S.A.; Raesi, R.; Daneshi, S. Epidemiological characteristics, injuries, and rabies post-exposure prophylaxis among children in Kerman county, Iran during 2019–2021. Open Public Health J. 2023, 16, e187494452303272. [Google Scholar]
- Khazaei, S.; Shirzadi, M.R.; Amiri, B.; Pourmozafari, J.; Ayubi, E. Epidemiologic aspects of animal bite, rabies, and predictors of delay in post-exposure prophylaxis: A national registry-based study in Iran. J. Res. Health Sci. 2023, 23, e187494452303272. [Google Scholar] [CrossRef]
- Yıldırım, A.A.; Doğan, A.; Kurt, C.; Çetinkol, Y. Evaluation of Our Rabies Prevention Practices: Is Our Approach Correct? Iran. J. Public Health 2022, 51, 2128–2134. [Google Scholar] [CrossRef]
- Porsuk, A.O.; Cerit, C. An increasing public health problem: Suspected rabies exposures. J. Infect. Dev. Ctries. 2021, 15, 1694–1700. [Google Scholar] [CrossRef]
- Amiri, S.; Maleki, Z.; Nikbakht, H.-A.; Hassanipour, S.; Salehiniya, H.; Ghayour, A.-R.; Kazemi, H.; Ghaem, H. Epidemiological Patterns of Animal Bites in the Najafabad, Center of Iran (2012–2017). Ann. Glob. Health 2020, 86, 38. [Google Scholar] [CrossRef]
- Janatolmakan, M.; Delpak, M.; Abdi, A.; Mohamadi, S.; Andayeshgar, B.; Khatony, A. Epidemiological study on animal bite cases referred to Haji Daii health Center in Kermanshah province, Iran during 2013–2017. BMC Public Health 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Sarbazi, E.; Sarbazi, M.; Ghaffari-Fam, S.; Babazadeh, T.; Heidari, S.; Aghakarimi, K.; Jamali, I.; Sherini, A.; Babaie, J.; Darghahi, G. Factors related to delay in initiating post-exposure prophylaxis for rabies prevention among animal bite victims: A cross-sectional study in Northwest of Iran. Bull. Emerg. Trauma 2020, 8, 236. [Google Scholar] [PubMed]
- Kassiri, H.; Ebrahimi, A.; Lotfi, M. Animal Bites: Epidemiological Considerations in the East of Ahvaz County, Southwestern Iran (2011–2013). Arch. Clin. Infect. Dis. 2018, 13, 1–8. [Google Scholar] [CrossRef]
- Babazadeh, T.; Nikbakhat, H.A.; Daemi, A.; Yegane-Kasgari, M.; Ghaffari-Fam, S.; Banaye-Jeddi, M. Epidemiology of acute animal bite and the direct cost of rabies vaccination. J. Acute Dis. 2016, 5, 488–492. [Google Scholar] [CrossRef]
- Mohtasham-Amiri, Z.; Pourmarzi, D.; Razi, M. Epidemiology of dog bite, a potential source of rabies in Guilan, north of Iran. Asian Pac. J. Trop. Dis. 2015, 5, S104–S108. [Google Scholar] [CrossRef]
- Celiloglu, C.; OzdemIr, U.; Tolunay, O.; Sucu, A.; Celik, U. Post-exposure Rabies Prophylaxis for Children in Southern Turkey. J. Coll. Physicians Surg. Pak. 2021, 31, 1219–1223. [Google Scholar]
- Riabi, H.R.A.; Ghorbannia, R.; Mazlum, S.B.; Atarodi, A. A Three-year (2011–2013) Surveillance on Animal Bites and Victims Vaccination in the South of Khorasan-e-Razavi Province, Iran. J. Clin. Diagn. Res. 2015, 9, LC01–LC05. [Google Scholar] [CrossRef]
- Poorolajal, J.; Babaee, I.; Yoosefi, R.; Farnoosh, F. Animal bite and deficiencies in rabies post-exposure prophylaxis in Tehran, Iran. Arch. Iran. Med. 2015, 18, 822–826. [Google Scholar]
- Charkazi, A.; Behnampour, N.; Fathi, M.; Esmaeili, A.; Shahnazi, H.; Heshmati, H. Epidemiology of animal bite in Aq Qala city, northen of Iran. J. Educ. Health Promot. 2013, 2, 13. [Google Scholar] [CrossRef]
- Ghannad, M.S.; Roshanaei, G.; Rostampour, F.; Fallahi, A. An epidemiologic study of animal bites in Ilam Province, Iran. Arch. Iran. Med. 2012, 15, 356–360. [Google Scholar]
- Oztoprak, N.; Berk, H.; Kizilates, F. Preventable public health challenge: Rabies suspected exposure and prophylaxis practices in southwestern of Turkey. J. Infect. Public Health 2021, 14, 221–226. [Google Scholar] [CrossRef]
- Can, F.K.; Tekin, E.; Sezen, S.; Clutter, P. Assessment of rabies prophylaxis cases in an emergency service. J. Emerg. Nurs. 2020, 46, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, V.; Shakeri, H.; Jahromi, A.S.; Shakeri, M.; Khoubfekr, H.; Hatami, I. Epidemiological Characteristic of Animal Bite and Direct Economic Burden of Rabies Vaccination in the Southern of Iran. Am. J. Anim. Veter-Sci. 2020, 15, 245–251. [Google Scholar] [CrossRef]
- Bijari, B.; Sharifzade, G.R.; Abbasi, A.; Salehi, S. Epidemiological survey of animal bites in east of Iran. Arch. Clin. Infect. Dis. 2011, 6, 90–92. [Google Scholar]
- Sengoz, G.; Yasar, K.K.; Karabela, S.N.; Yildirim, F.; Vardarman, F.T.; Nazlican, O. Evaluation of cases admitted to a center in Istanbul, Turkey in 2003 for rabies vaccination and three rabies cases followed up in the last 15 years. Jpn. J. Infect. Dis. 2006, 59, 254–257. [Google Scholar] [CrossRef]
- Hamta, A.; Saghafipour, A.; Hosseinalipour, S.A.; Rezaei, F. Forecasting delay times in post-exposure prophylaxis to human animal bite injuries in Central Iran: A decision tree analysis. Veter-World 2019, 12, 965–971. [Google Scholar] [CrossRef]
- Najafi, N.; Ghasemian, R. Animal bites and rabies in northern Iran, 2001–2005. Iran. J. Clin. Infect. Dis. 2009, 4, 224–227. [Google Scholar]
- Karbeyaz, K.; Ayranci, U. A Forensic and Medical Evaluation of Dog Bites in a Province of W estern T urkey. J. Forensic Sci. 2014, 59, 505–509. [Google Scholar] [CrossRef]
- Sheikholeslami, N.Z.; Rezaeian, M.; Salem, Z. Epidemiology of animal bites in Rafsanjan, southeast of Islamic Republic of Iran, 2003–05. East. Mediterr. Health J. 2009, 15, 455–457. [Google Scholar] [CrossRef]

| Study ID | Study Design | Setting (Country) | Date (Period of the Study) | Sample Size | PEP: Vaccine (Dose/Route of Administration/Number of Cycles)/Immunoglobulin. N (%) | |
|---|---|---|---|---|---|---|
| Khoubfekr et al., 2024 [22] | Two case reports | Iran | NA | 2 | 1st case: The boy received RIG (CSL Behring 300 IU/2 mL, Marburg, Germany, Serums and Vaccines Ltd., Ambernath) at a dose of 20 IU/kg, totaling approximately 100 IU. Additionally, Verorab 0.5 mL was administered IM in the deltoid region. On day 3, he received the second dose of the vaccine, followed by the third dose on day 7. Additionally, he received a 4th dose of the vaccine on the 14th day post-exposure. 2nd case: Swift action was taken, and within 30 min of washing the affected areas, rabies immunoglobulin (CSL Behring 300 IU/2 mL, Marburg, Germany Serums and Vaccines Ltd., Ambernath) at a dose of 20 IU/kg, totaling approximately 100 IU, was administered. A rabies vaccine, Verorab 0.5 mL, was also administered IM in the deltoid region. | |
| Davarani et al., 2023 [28] | A cross-sectional descriptive-analytical study | Rabies prevention and treatment centers in Kerman, Iran | From April 2019 to March 2021 | 933 | In patients who received the immunoglobulin injection before the end of the first 12 h: 381 (68.9) cases. In patients who received the immunoglobulin injection within 12–72 h: 151 (27.3) cases. Patients who received the immunoglobulin injection within 4–7 days 21 (3.8) cases. Patients who received rabies vaccine before the end of the first 48 h 868 (93) cases. Patients who received rabies vaccine within 48–72 h: 52 (5.6) cases. Patients who received rabies vaccine within 4–10 days: 12 (1.3) cases. Patients who received rabies vaccine within 11–20 days: 1 (0.1) case. | |
| Khazaei et al., 2023 [18] | Registry-based cross-sectional study | The rabies treatment centers located in the health centers of Iran | From March 2021 to March 2022 | 260,470 | Patients who received vaccinations before the end of the first 48 h: 253,185 (97.20) cases. Patients who received vaccination after the first 48 h: 7285 (2.80) cases. | |
| Yıldırım et al., 2022 [30] | A retrospective cross-sectional study | Medical Faculty Rabies Vaccine Center, Ordu University, Ordu, Turkey | From 2014 to 2018 | 748 | Patients who received the rabies vaccine: 477 (63.8) cases. Patients who did not complete or did not receive tetanus immunization: 2 (0.3) cases. Patients whose tetanus immunization was not known: 742 (99.2) cases. Patients who had complete tetanus immunization: 4 (0.5) cases. | |
| Bay et al., 2021 [15] | A cross-sectional study | Golestan Province, Iran | From March 2019 and March 2020 | 12,181 | Patients who needed vaccines: 10,675 (99.7) cases. Patients who received a complete vaccine series: 1591 (13.1) cases. Patients who received the full vaccine four times: 10,608 (87.2) cases. Patients who received HRIG: 9840 (81.1) cases. Patients who received the DT vaccine: 6831 (56.1) cases. | |
| Celiloglu et al., 2021 [38] | Cross-sectional study | Adana City Training and Research Hospital, Turkey | From September 2017 to September 2018 | 2068 | In terms of serial prophylaxis vaccinations, 761 (83.99) families were in full compliance, and 145 (16) families had discontinued vaccinations. RIG was administered to 447 (49.3) and not administered to 459 (50.7). | |
| Oztoprak et al., 2021 [43] | A retrospective descriptive study | Health Science University, Antalya Training and Research Hospital, Department of Infectious Diseases and Clinical Microbiology, Antalya, Turkey | From 2010 to 2013 | 2513 | Vaccination was needed in 2015 cases (80.2). Rabies vaccine was administered to 1017 (40.5) cases as 5-dose. Rabies vaccine was administered to 626 (24.9) cases as 4-dose. Rabies vaccine was administered to 372 (14.8) cases as 2-1-1 schema. | |
| Porsuk et al., 2021 [31] | A descriptive cross-sectional study | Hospital’s emergency service in Turkey | From 2015 to 2019 | 3378 | Post-exposure prophylaxis was applied to nearly all cases, 3352 (99.2), but 26 (0.8) of them were found to be inappropriate according to guidelines. HRIG treatment was applied together with the vaccine. One dose of vaccine to 256 (7.6) cases. Two doses were applied to 215 (6.4) cases. Three doses were applied to 1129 (33.4) cases. Four doses were applied to 653 (19.3) cases. Five doses were applied to 1125 (33.3) cases. | |
| Amiri et al., 2020 [32] | A cross-sectional study | Health centers of Najafabad in Isfahan province, Iran | From 2012 to 2017 | 4104 | Patients who received the anti-rabies vaccine three times: 2197 (53) cases. Patients who received the anti-rabies vaccine five times: 1597 (38) cases. In terms of serum therapy, 3951 (96) of the injured did not need to receive rabies serum. | |
| Can et al., 2020 [44] | A retrospective cohort Study | Erzurum Palandöken State Hospital Emergency Service located in northeast Turkey | From August 2013 to June 2017 | 691 | Human diploid cell vaccine. Single dose occurred for 114 (16.5) cases. There were two doses for 71 (10.3) cases. Three doses were administered to 150 (21.7) cases. 128 (18.5) cases received 4 doses. In 227 (32.9) cases, 5 doses were administered. In addition, human rabies immunoglobulin was applied to 540 (78.1) cases in the prophylaxis program. | |
| Janatolma Kan et al., 2020 [33] | A cross-sectional study | Kermanshah Province, Iran | From 2013 to 2017 | 5618 | Most victims (n = 4594 (82)) had been vaccinated with the rabies vaccine three times. The remaining victims (n = 1024 (18)) had been vaccinated with the rabies vaccine five times. | |
| Rahmanian et al., 2020 [22] | A Retrospective Observational Study | Larestan County in the south Fars province, Iran | From 21 March 2018 to 20 March 2019 | 375 | Patients who received three doses of vaccine: 268 (71.4) cases. Patients who received 5 doses of vaccine: 107 (28.5) cases. Patients who received 1 dose of RIG: 109 (29.06) cases. | |
| Rasooli et al., 2020 [25] | Case Series | Different regions of Iran | From 2014 to 2018 | 7 | PVRV is an inactivated vaccine derived from the rabies virus cultured in PVRV cells. Combining this rabies vaccine and RIG as the recommended PEP protocol based on WHO guidelines to prevent rabies after exposure. | |
| Sarbazi et al., 2020 [34] | A cross-sectional study | Rabies center of Tabriz, Tabriz, Iran | From 1 March 2013 to 29 February 2019 | 3032 | Patients who received vaccinations before the end of the first 48 h: 2773 (92.5). Patients who received vaccinations after the first 48 h: 259 (8.5). | |
| Hamta et al., 2019 [48] | A descriptive study | Centers for Disease Control and Prevention unit of Qom Provincial Health Center, Iran | From January 2017 to December 2018 | 2414 | A delay of more than 48 h in the initiation of PEP was estimated among 305 (12.73) animal bite victims. Patients who received vaccinations before the end of the first 48 h: 2109 (87.36) victims. | |
| Kassiri et al., 2018 [35] | Cross-sectional study | Disease Prevention and Control Department of East Ahvaz Health Center, Southwestern Iran | From 2011 to 2013 | 2493 | Treatment in 61.4% of victims suspected of having rabies who visited rabies treatment and prevention centers was done with three doses of anti-rabies vaccine and stopped after ten days. Treatment with five doses of anti-rabies vaccine was conducted in 38.6% of cases per year. | |
| Khazaei et al., 2018 [29] | A cross-sectional study | Nahavand district, Iran | From March 2015 to March 2017 | 1448 | Patients who received vaccinations before the end of the first 48 h: 1175 (81.14) cases. Patients who received vaccination after the end of the first 48 h: 273 (18.85) cases. | |
| BabazadeH. et al., 2016 [36] | A cross-sectional study | Chalderan City, West Azerbaijan province, Iran | From 21 March 2008 to 20 March 2014 | 1449 (747 vs. 702). | Patients who received 3 doses of vaccine: 1607 (93.2). Patients who received 5 doses of vaccine: 117 (6.8). | |
| Ramezankhani et al., 2016 [21] | A double-blind randomized clinical trial | 9 cities of Iran | 2010 | 1449 (747 vs. 702) | PCECV (Rabipur®, Novartis, Germany) | PVRV (Verorab®, Mérieux Institute, France) |
| Amiri et al., 2015 [37] | A cross-sectional study | Guilan Province, north of Iran | From June 2011 to May 2012 | 1771 | Only 810 cases (49.3%) were aware that they should receive the rabies vaccine after a dog bite. About 477 cases (29) were referred to RVCs after attending rural or urban health centers for receiving the tetanus vaccine or wound treatments. Then, 171 cases (10.4%) and 71 cases (4.7%) were referred to RVCs after attending private clinics and hospitals for receiving wound treatment, respectively. About 102 cases (6.2%) attended RVCs because their family or friends recommended them, and 6 cases (0.4%) attended RVCs after receiving advice from pharmacies, schools, or veterinarian clinic staff. | |
| Riabi et al., 2015 [39] | Cross-sectional study | Gonabad, Iran | From 2011 to 2013 | 616 | 517 (83.9) victims had been incompletely vaccinated, and 99 (16.1) received a complete vaccination. | |
| Poorolajal et al., 2015 [40] | Six-year population-based cross-sectional study | The Research Committee of Hamadan University of Medical Sciences, Tehran Province, Iran | From April 2006 to March 2012 | 22,766 | Patients affected by animal bites and referred to health centers within 48 h received intramuscular doses of 0.5 mL. Those who were referred to health centers after 48 h received an IM dose of 1 mL, given in 5 doses over 4 weeks. Doses should be received on days 0, 3, 7, 14, and 30. Single or multiple dermal bites or wounds in the head and neck require immediate vaccination and RIG administration. Human RIG is given in a single dose of 20 IU per kg of body weight. | |
| Farahtaj et al., 2014 [26] | Case series | Multiple villages in Iran | From 2002 to 2011 | 16 | Patients who received vaccination as a single shot: 1 (6.25) case. Patients who received vaccination as three shots: 2 (12.50) cases. Patients who received four vaccination shots: 13 (81.25) cases. | |
| Karbeyaz et al., 2014 [50] | A descriptive study | Director of Forensic Medicine, Eskisehir, Western Turkey | From 1 January 2006 to 31 December 2010 | 328 | Patients who received vaccination against rabies: 42 (12.8) cases. 38 (11.6) cases of tetanus vaccination have been reported. Patients who did not receive vaccination: 16 (4.9) cases. There were 270 (82.3) cases of unknown vaccination status. | |
| Seri et al., 2014 [20] | A prospective trial. | The Infectious Diseases and Clinical Microbiology Outpatient Clinic of the Ministry of Health Ankara Training and Research Hospital, Turkey | From February 2010 to December 2010 | 1685 (761 vs. 924). | Abhayrab vaccine (Human Biologicals Institute, Ooty, India), each dose of vaccine as 0.5 mL into the deltoid muscle (924 patients). Vaccination at days 0, 3, 7, 14, and 28, plus immunoglobulin at initiation by scheduling 5 doses of vaccine and ERIG (Equirab; Bharat Serums and Vaccines Ltd., Navi Mumbai, India). OR Vaccination at days 0, 7, and 21 by a schedule of 2-1-1. | Verorab vaccine (Pasteur Merieux, Lyon, France), each dose of vaccine as 0.5 mL into the deltoid muscle (761 patients). Vaccination at days 0, 3, 7, 14, and 28, plus immunoglobulin at initiation by scheduling 5 doses of vaccine and ERIG (Equirab; Bharat Serums and Vaccines Ltd., India). OR Vaccination at days 0, 7, and 21 by a schedule of 2-1-1. |
| Charkazi et al., 2013 [41] | A descriptive cross-sectional study | Rabies Center of AqQala city, Northen of Iran | From 1998 to 2009 | 13,142 | Patients who received a complete vaccination: 6463 (72) cases. Patients who receive incomplete vaccinations: 6679 (28) cases. | |
| Taghvaii et al., 2013 [17] | A cross-sectional study | Three health centers in Mashhad, Iran | From 2006 to 2009 | 14,037 | Patients who received incomplete vaccinations: 11,672 (83.1) cases. Patients who received a complete vaccination: 2365 (16.9) cases. | |
| Ghannad et al., 2012 [42] | A cross-sectional descriptive study | Health centers located in Ilam Province, Iran | From April 1999 to March 2008 | 4420 | There were 3596 (81.3) cases of incomplete vaccinations. Patients who received complete vaccination comprised 824 (18.7) cases. | |
| Ansari et al., 2011 [23] | Case reports | Sports Medicine Research Center, Tehran University of Medical Sciences, Tehran, Iran | NA | 2 | PVRV and HRIG, 20 IU/kg. Prophylaxis with doxycycline (100 mg, twice daily) and clindamycin (450 mg, three times daily) were administered for 7 days. The 5-dose regimen of the PEP rabies vaccine was completed (on days 0, 3, 7, 14, and 28). | PVRV and HRIG (20 IU/kg) were administered. The patient received antibiotic prophylaxis with oral amoxicillin-clavulanate (675/125 mg, twice daily). The 5-dose regimen of the PEP rabies vaccine was completed. |
| Bijari et al., 2011 [46] | A retrospective study | Health Center of Birjand University of Medical Sciences, Ghafari St., Birjand, Iran | From April 2002 to April 2009 | 1662 | 1597 (96.1) of cases were vaccinated within the first 24 h. 1361 (81.9) had incomplete vaccination. 301 (18.1) had complete vaccination. | |
| Najafi et al., 2009 [49] | Descriptive study | Mazandaran Province, northern Iran | From 2001 to 2005 | 32,079 | Either immune globulin prophylaxis or a vaccine was used for PEP. Both HRIG and vaccines were given in 5263 cases (16.4%). Only vaccine was given in 26,816 cases (83.6). | |
| Sheikholeslami et al., 2009 [51] | Cross-sectional study | Rafsanjan, southeast of the Islamic Republic of Iran | From 2003 to 2005 | 1542 | Rabies vaccine was given to 1311 cases (85). Rabies vaccine plus rabies immunoglobulin was given to 23 cases (15). The tetanus toxoid vaccine was given to 1018 cases (66). | |
| Kilic et al., 2006 [16] | A descriptive study | Narlidere District, Turkey | From 1999 to 2001 | 1569 | 1067 cases (68) were included in a post-exposure rabies vaccination program. | |
| Sengoz et al., 2006 [47] | A retrospective study | Hospital’s Center for Rabies Vaccination, Haseki Hospital for Training and Research, Istanbul, Turkey | From January 2003 to December 2003 | 7266 | Patients who received vaccines with the first day: 5320 (72) cases. Patients who received vaccines with the 1st–5th day: 1890 (26) cases. Patients who received vaccines after the 5th day: 146 (2) cases. Patients who received 3 doses in 10 days: 2690 (37) cases. Patients who received 5 doses in 30 days: 1050 (14) cases. Patients who received the 2-1-1 schedule: 1770 (24). Number of patients not completing the standard vaccination schedule: 1750 (24) cases. Number of patients who did not require vaccine: 6 (<1) cases. | |
| Bizri et al., 2000 [27] | Case series | The American University of Beirut Medical Center (AUBMC), in Lebanon | From 1991 to 1996 | 8 | Patients who received vaccination: 1 (12.5) case. There were 7 (87.5) cases of patients who did not receive vaccination. | |
| Tabbara et al., 1995 [24] | Two case reports | Department of Ophthalmology, College of Medicine, King Saud University | NA | 2 | The patient was given tetanus prophylaxis and 20 IU/kg of body weight of HRIG, one-half around the bite wound and the other half IM. She was also given HDCV and prophylactic antibiotics in the form of oral ampicillin. | |
| Study ID | Age (Year), N (%) | Male Population, N (%) | Type of Area, N (%) | Type of Animal, N (%) | Bitten Organ, N (%) | Regimen of Vaccine, N (%) | Type of Injury, N (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Khoubfekr et al., 2024 [22] | The age ranged from 7 to 12 years old | 2 (100) | NA | Dog 2 (100) | Upper limb 2 (100) Head injury 1 (50) | >24 h: 1 (50) <24 h: 1 (50) | NA | ||||
| Davarani et al., 2023 [28] | Group 1 (<3) 99 (10.6) Group 2 (4–6) 225 (24.1) Group 3 (7–12) 609 (65.3) | 620 (66.5) | Urban 835 (89.5) Rural 98 (10.5) | Dog 632 (67.7) Cat 237 (25.4) Hamster 44 (4.7) Other 20 (2.1) | Hand (including fingertips to wrist) 394 (42.2) Lower limbs (including legs/buttocks) 295 (31.6) Hands (including forearm, arm, shoulder) 84 (9) Head, face, and neck 86 (9.2) Chest, abdomen, and back 25 (2.7) Two organs of the body 39 (4.2) Three organs and more 10 (1.1) | <48 h: 868 (93) 48–72 h: 52 (5.6) 4–10 days: 12 (1.3) 11–20 days: 1 (0.1) | Puncture and scratching 573 (61.4) Scratch 345 (36.9) Perforation 8 (0.8) Crushing a part of the body 6 (0.6) Bone fracture 1 (0.10) | ||||
| Khazaei et al., 2023 [18] | Group 1 (<5) 11,231 (4.31) Group 2 (5–15) 45,377 (17.42) Group 3 (16–30) 67,193 (25.80) Group 4 (31–45) 72,849 (27.97) Group 5 (46–60) 39,655 (15.22) Group 6 (+60) 24,165 (9.28) | 201,164 (77.23) | Urban 146,868 (56.39) Rural 90,140 (34.61) Mobile 23,462 (9.01) | Domestic 256,138 (98.34) Wild 1614 (0.62) Redundant 2718 (1.04) | NA | <48 h: 253,185 (97.20) >48 h: 7285 (2.80) | Bone fracture 66 (0.03) Perforation 49,133 (18.89) Rupture 6634 (2.55) Segregation of the tissue 6259 (2.40) | ||||
| Yıldırım et al., 2022 [30] | The mean and standard deviation 28.12 ± 21.60 | 466 (62.3) | NA | Dog 463 (61.9) Cat 259 (31.6) Wild animal 15 (2) Bat 2 (0.3) Other 9 (4.2) | NA | Administered vaccination: 477 (63.8) Not administered vaccination: 271 (36.2) | NA | ||||
| Bay et al., 2021 [15] | The highest number was related to the age groups of 1 to 19 years, occupying 4629 (38) | 9562 (78.5) | NA | NA | NA | No need for vaccines: 32 (0.3) Incomplete vaccine: 10,591 (86.9) Full vaccine (4 times): 1558 (12.8) | NA | ||||
| Celiloglu et al., 2021 [38] | 565 (62.4%) | NA | Cat 478 (52.8) Dog 413 (45.6) Bat 4 (0.4) Other species 11 (1.2) | NA | <24 h: 720 (79.4) | Scratch 428 (47.2) Bite 388 (42.8) Open wound contact 11 (1.2) Other types of trauma (e.g., abrasions) 79 (8.7) | |||||
| Oztoprak et al., 2021 [43] | Group 1 (0–15) 730 (29.04) Group 2 (≥15) 1783 (70.95) | 1599 (63.6) | Urban 2304 (91.68) Rural 209 (8.3) | Dogs 1539 (61.2) Cats 877 (34.9) Cattle 59 (2.3) Sheep/goat 13 (0.5) Bat 15 (0.6) Foxes 2 (0.1) | Upper extremity 1447 (57.58) Lower extremity 997 (39.67) Head and neck 112 (4.5) Abdomen 33 (1.3) Thorax 16 (0.6) Genital area 2 (0.08) | Five doses: 1017 (40.5) Four doses: 626 (24.9) 2-1-1 schema of vaccination: 372 (14.8) | Bite 1867 (74.3) Scratch 549 (21.8) Contact to open wound 21 (0.8) Other exposures 76 (3) | ||||
| Porsuk et al., 2021 [31] | Group 1 (≤6) 458 (13.55) Group 2 (7–14) 563 (16.66) Group 3 (15–64) 2096 (62.04) Group 4 (≥65) 261 (7.72) | 2097 (62.1) | Urban 2713 (80.3) Suburban 236 (7.0) Rural 429 (12.7) | Dogs 1865 (55.2) Cats 1483 (43.9) Mice 10 (0.3) Horses 3 (0.1) Bats 3 (0.1) Monkey and seagull 2 (0.05) Human 12 (0.4) | NA | One dose: 256 (7.6) Two doses: 215 (6.4) Three doses: 1129 (33.4) Four doses: 653 (19.3) Five doses: 1125 (33.3) | NA | ||||
| Amiri et al., 2020 [32] | Group 1 (<7) 178 (4.33) Group 2 (7–18) 570 (13.8) Group 3 (19–30) 1564 (38.11) Group 4 (31–45) 1077 (26.24) Group 5 (46–60) 518 (12.6) Group 6 (>61) 197 (4.8) | 3648 (88.89) | NA | Domestic 4081 (99.4) Wild and feral 23 (0.6) Dogs 2909 (70.88) Cats 997 (24.29) Others 198 (4.82) | Head and Neck 6 (0.1) Chest and abdomen 11 (0.3) Shoulder and hand 166 (4) Hips and buttocks 19 (0.5) Thighs and legs 211 (5.1) No 2997 (73) | Vaccinated: 1841 (44.9) Not vaccinated: 2263 (55.1) | NA | ||||
| Can et al., 2020 [44] | Group 1 (0–18) 232 (33.5) Group 2 (19–36) 261 (37.6) Group 3 (37–54) 113 (16.3) Group 4 (55–72) 85 (12.3) | 547 (79.2) | Urban 506 (73.2) Rural 185 (26.8) | Dogs 483 (69.9) Cats 171 (24.7) Horses 14 (2) Foxes 11 (1.6) Cattle 10 (1.4) Sheep 2 (0.3) Cow 10 (1.4) | Head 23 (3.3) Arm 385 (55.7) Body 90 (13.0) Leg 193 (27.9) | Complete vaccination: 227 (32.9) Incomplete vaccination: 464 (67.14) | Bite 510 (73.8) Scratch 159 (23.0) Contact 22 (3.2) | ||||
| Janatolma Kan et al., 2020 [33] | Group 1 (1–9) 599 (11.0) Group 2 (10–19) 772 (14.0) Group 3 (20–29) 1347 (24.0) Group 4 (30–39) 1050 (19.0) Group 5 (40–49) 706 (13.0) Group 6 (50–59) 576 (10.2) Group 7 (>60) 568 (10.1) | 4277 (76.3) | Urban 4239 (76.0) Rural 1365 (24.0) | Dog 4032 (72.0) Cats 1194 (21.3) Livestock 41 (0.7) Others 335 (6.0) | Upper limbs 2776 (49.5) Lower limbs 2666 (47.6) Both limbs 162 (3.0) | Three doses: 4594 (82.0) Five doses: 1009 (18.0) | NA | ||||
| Rahmanian et al., 2020 [22] | Group 1 (<4) 8 (2.10) Group 2 (5–9) 27 (7.20) Group 3 (10–19) 74 (19.70) Group 4 (20–29) 84 (22.40) Group 5 (30–39) 86 (22.90) Group 6 (40–49) 96 (25.60) | 314 (83.70) | Urban 203 (54.10) Rural 172 (45.90) | Dog 255 (68.00) Cat 101 (26.90) Hours 5 (1.30) Monkey (pet) 2 (0.50) Sheep 1 (0.30) Donkey 1 (0.30) Hamster 3 (0.80) Wild pigs 3 (0.80) Fox 2 (0.50) Wolf 1 (0.30) Hedgehog 1 (0.30) | Hand 103 (27.4) Leg 97 (25.8) Arm and forearm 85 (22.6) Ankle 56 (14.9) Shoulder 17 (4.53) Trunk 10 (2.66) Head/face 7 (1.86) | Three doses of vaccine: 268 (71.4) Five doses of vaccine: 107 (28.5) One dose of RIG: 109 (29.06) | NA | ||||
| Rasooli et al., 2020 [25] | Ages ranged from 10 to 67 years old | 7 (100) | NA | NA | The most common sites of injury were the hands and face, affecting 5 (71.43) of the individuals | NA | NA | ||||
| Sarbazi et al., 2020 [34] | Group 1 (<10) 382 (12.6) Group 2 (10–20) 339 (11.2) Group 3 (20–30) 706 (23.3) Group 4 (30–40) 599 (19.8) Group 5 (40–50) 409 (13.5) Group 6 (>50) 597 (19.7) | 2438 (80.4) | Urban 2094 (69.1) Rural 938 (30.9) | Carnivorous (Dog, Jackal, Fox) 1793 (59.2) Cat 1092 (36.0) Other 146 (4.8) | Upper limb 2006 (66.2) Lower limb 1026 (33.8) | <48 h: 2773 (92.5) >48 h: 259 (8.5) | Puncture of the wound 266 (8.8) No Puncture of the wound 2766 (91.2) | ||||
| Hamta et al., 2019 [48] | Group 1 (<10) 231 (9.56) Group 2 (10–20) 377 (15.6) Group 3 (20–30) 612 (25.35) Group 4 (30–40) 532 (22.04) Group 5 (40–50) 286 (11.84) Group 6 (>50) 376 (15.57) | 2009 (83.22) | Rural 474 (19.6) Urban 1897 (78.58) | Cattle (Horse, Donkey, Cow, Sheep, Camel, Goat) 75 (3.10) Carnivorous (Dog, Jackal, Pig, Fox) 1187 (49.17) Cat 1100 (45.56) Other 52 (2.15) | NA | >48 h: 305 (12.6) <48 h: 2109 (87.36) | Puncture wounds 283 (11.7) Scratches 2212 (91.6) Crush injuries 39 (1.6) | ||||
| Kassiri et al., 2018 [35] | Group 1 (0–4) 180 (7.2) Group 2 (5–10) 333 (13.4) Group 3 (11–20) 436 (17.5) Group 4 (21–30) 543 (21.8) Group 5 (31–40) 410 (16.4) Group 6 (41–50) 266 (10.7) Group 7 (51–60) 235 (9.4) Group 8 (>61) 90 (3.6) | 1910 (76.6) | Urban 1620 (65.0) Rural 873 (35.0) | Dog 1954 (78.4) Cat 432 (17.3) Others 107 (4.3) | Hands 1036 (41.6) Feet 1168 (46.9) Head and neck 143 (5.7) Trunk 146 (5.8) | Incomplete vaccination: 1531 (61.4) Complete vaccination: 962 (38.6) | NA | ||||
| Khazaei et al., 2018 [29] | Group 1 (<5) 76 (5.25) Group 2 (5–15) 314 (21.69) Group 3 (16–30) 402 (27.76) Group 4 (31–45) 302 (20.86) Group 5 (46–60) 231 (15.95) Group 6 (>60) 123 (8.49) | 1067 (80.60) | Urban 472 (32.60) Rural 976 (67.40) | Dogs 1088 (75.13) Cats 316 (21.8) Donkeys 16 (1.10) Rats 8 (0.55) Wolves 7 (0.48) Sheep 5 (0.35) Others 8 (0.55) | NA | <48 h: 1175 (81.14) >48 h: 273 (18.85) | NA | ||||
| Babazade H. et al., 2016 [36] | The mean age of men (20.52 ± 14.72) and women (22.20 ± 14.11) | 1240 (71.9) | Urban 409 (23.7) Rural 1 234 (71.5) Unknown 81 (4.8) | Pet dogs 1642 (95.2) Stray dogs 44 (2.6) Cats 21 (1.2) wolves 5 (0.3) Foxes 2 (0.1) other animals 10 (0.6) | Lower extremity 1 337 (77.6) Shoulders and hands 280 (16.2) Chest 66 (3.8) Head and neck 41 (2.4) | Three doses: 1607 (93.2) Five doses: 117 (6.8) | NA | ||||
| Ramezankhani et al., 2016 [21] | Mean age 26.8 years (SD, ±13.1 years) | Mean age: 27.4 years (SD, ±13.9 years) | 554 (78.9) | 616 (82.4) | NA | NA | Head and neck 4 (30.8) Chest and abdomen 9 (52.9) Back 12 (50.0) Upper extremity 246 (49.3) Lower extremity 396 (47.5) Multiple locations 35 (56.5) | Head and neck 9 (69.2) Chest and abdomen 8 (47.1) Back 12 (50.0) Upper extremity 253 (50.7) Lower extremity 438 (52.5) Multiple locations 27 (43.5) | (Five doses of vaccine + Ig) 702 (48.45) | (2 + 1 + 1) 747 (51.55) | NA |
| Amiri et al., 2015 [37] | Group 1 (0–9) 127 (7.7) Group 2 (10–19) 228 (13.9) Group 3 (20–29) 330 (20.1) Group 4 (30–39) 295 (18.0) Group 5 (40–49) 266 (16.2) Group 6 (50–59) 228 (13.9) Group 7 (60) 169 (10.3) | 1328 (75) | Urban 645 (39.3) Rural 998 (60.7) | History of dog bite was mentioned by 151 victims (9.2%) and 107 of them (70.9%) received rabies vaccine in previous bite | Lower extremity 899 (50.76) Upper extremity 723 (40.8) Abdominal and trunk 116 (6.5) Head, face, and neck 23 (1.30) Genital 2 (0.1) | NA | NA | ||||
| Riabi et al., 2015 [39] | Group 1 (1–10) 49 (8) Group 2 (11–20) 82 (13.3) Group 3 (21–30) 168 (27.3) Group 4 (31–40) 78 (14.1) Group 5 (41–50) 70 (11.4) Group 6 (>50) 160 (26) | 485 (78.7) | Urban 367 (59.57) Rural 230 (37.33) | Domestic dog 411 (66.7) Domestic cat 66 (10.7) Stray cat 60 (9.7) Stray dog 33 (5.4) Other animals 145 (23.5) | Hand 302 (49) Foot 228 (37) Hands and feet 22 (3.6) Legs and buttocks 3 (0.5) Hips 23 (3.7) Arm 3 (0.5) Side 6 (1.0) Neck 2 (0.3) Back 5 (0.8) Face 10 (1.6) Hands and face 1 (0.2) Abdomen 3 (0.5) Testis 1 (0.2) Head 2 (0.3) Thigh 1 (0.2) Leg and waist 1 (0.2) Unknown 3 (0.5) | Uncompleted vaccination: 517 (83.9) Completed vaccination: 99 (16.1) | NA | ||||
| Poorolajal et al., 2015 [40] | Group 1 (<10) 2050 (9.08) Group 2 (11–20) 4145 (18.37) Group 3 (21–30) 7206 (31.93) Group 4 (31–40) 3741 (16.58) Group 5 (41–50) 2624 (11.63) (Group 6 (51–60) 1689 (7.48 Group 7 (61–70) 783 (3.47) | 19,216 (84.51) | Urban 14,921 (66.27) Rural 7596 (33.73) | Dog 18,157 (81.71) Cat 2964 (13.34) Wolf 39 (0.18) Fox 69 (0.31) Jackal 8 (0.04) Donkey 67 (0.30) Cow 30 (0.14) Others 886 (3.99) | Head and neck 448 (2.06) Legs 9916 (45.62) Hands 9424 (43.36) Trunk 511 (2.35) Hand and foot 854 (3.93) Multiple organs 581 (2.67) | Three doses: 17,971 (78.94) Five doses: 4795 (21.06) | NA | ||||
| Farahtaj et al., 2014 [26] | The age ranged from 11 months to 80 years (median, 21.5 years) | 12 (75) | Urban 5 (31.25) Rural 11 (68.75) | Fox 2 (12.5) Dog 11 (68.75) Wolf 3 (18.75) | Head, face, trunk 10 (62.5) Upper extremities 8 (50) Abdomen, back, buttock 3 (18.75) | One dose: 1 (6.25) Three doses: 2 (12.50) Four doses: 13 (81.25) | NA | ||||
| Karbeyaz et al., 2014 [50] | Group 1 (0–18) 159 (48.5) Group 2 (19–34) 96 (29.3) Group 3 (≥35) 73 (22.2) | 235 (71.6) | NA | Dog 328 (100) | Lower extremity 161 (49.1) Upper extremity 102 (31.1) Head/neck/face 44 (13.4) Chest/abdomen/back 53 (16.1) | Vaccinated against rabies: 42 (12.8) Vaccinated against tetanus: 38 (11.6) No vaccination: 16 (4.9) Unknown: 270 (82.3) | Ecchymosis/abrasions/bruising/break, such as scratch or puncture in the skin, 240 (73.2) Laceration 59 (18.0) Flap-style injury 23 (7.0) Vascular Injury 6 (1.8) | ||||
| Seri et al., 2014 [20] | Group 1 (0–15) 532 (31.6) Group 2 (15–60) 1030 (61.1) Group 3 (>60) 123 (7.3) | 1089 (64.6) | NA | Dog 1133 (67.2) Cat 531 (31.5) Wild animal 15 (0.9) Rodent/Other 3 (0.2) | Head/neck 103 (6.1) Upper extremity 896 (53.2) Lower extremity 531 (31.5) Body 119 (7.1) Multiple sites 36 (2.1) | (2 + 1 + 1): 1420 (84.3) (Five doses of vaccine + Ig): 265 (15.7) | Bite 1380 (81.9) Scratch 298 (17.7) Lick 1 (0.1) Other 6 (0.4) | ||||
| Charkazi et al., 2013 [41] | The mean and standard deviation of age 25.0 ± 17.8 years. The highest cases of bites were related to the age range of 11–15 years (2320 cases) (17.7) | 9479 (72.1) | Rural 11,038 (84) Urban 2104 (16) | Dogs 12,895 (98.8) Cows 210 (1.6) Cats 39 (0.3) Camels 13 (0.1) Horses 13 (0.1) Donkeys 13 (0.1) | Leg 9136 (69.6) Body 2416 (12) Hand 1938 (9.2) Head, face, and neck 279 (2.1) Several organs 75 (0.6) Unclear 4 (0.03) | Complete vaccination: 6463 (72) Incomplete vaccination: 6679 (28) | NA | ||||
| Taghvaii et al., 2013 [17] | Group 1 (<5) 392 (8.2) Group 2 (5–10) 1029 (7. 3) Group 3 (10–20) 3331 (23.7) Group 4 (20–30) 3488 (24.8) Group 5 (30–40) 2147 (15.3) Group 6 (40–50) 1496 (10.7) Group 7 (50 and >50) 2154 (15.4) | 11,482 (81.8) | Urban 8633 (61.5) Rural 5404 (38.5) | Dog 10,848 (77.3) Cat 2504 (17.8) Wolf 11 (1) Jackal 6 (0.04) Fox 22 (2) | Hands 6197 (44.1) Legs 6166 (43.9) Body 1197 (8.5) Head and face 427 (3) Neck 50 (0.3%) | Incomplete vaccination: 11,672 (83.1) Complete vaccination: 2365 (16.9) | NA | ||||
| Ghannad et al., 2012 [42] | The majority of animal bites were reported among the 10–19-year-old (143.9 per 10,000 population) age group | 3032 (68.3) | Urban 1017 (23) Rural 3227 (73) | Dog 3942 (89.2) Cats 221 (5) Wolves 31 (0.7) Jackals 27 (0.6) Foxes 13 (0.3) Other animals 186 (4.2) | Lower extremities 3165 (71.6) Hands 884 (20) Trunk 265 (6) Head and face 88 (2) Neck 18 (0.4) | Incomplete vaccination: 3596 (81.3) Complete vaccination: 824 (18.7) | NA | ||||
| Ansari et al., 2011 [23] | Ages ranged from 22 to 25 years old | 2 (100%) | NA | Dogs 2 (100) | Lower extremity 2 (100) | Five doses: 2 (100) | Bite 2 (100) | ||||
| Bijari et al., 2011 [46] | Mean age of 33.4± 19.5 years (range from 1 to 90 years) | 1300 (78.2) | Rural 595 (35.8) Urban 1067 (64.2) | Domestic 1435 (86.3%) Wild 73 (4.4) Stray 154 (9.3) | NA | Complete: 301 (18.1) Incomplete: 1361 (81.9) | NA | ||||
| Najafi et al., 2009 [49] | Group 1 (0–4) 466 (2.01) Group 2 (5–9) 1822 (7.86) Group 3 (10–19) 6605 (28.50) Group 4 (20–29) 5064 (21.85) Group 5 (30–39) 3387 (14.61) Group 6 (40–49) 2816 (12.15) Group 7 (>50) 3008 (12.98) | 18,445 (79.6) | Urban 11,797 (36.77) Rural 20,282 (63.22) | NA | Lower extremities 16,979 (52.93%) Upper extremities 12,671 (39.5) Trunk 1841 (5.74) Face 577(1.8) Neck 119 (0.37) | NA | Bite 32,077 (99.99) Scratch 2 (0.006) | ||||
| Sheikholeslami et al., 2009 [51] | Children: 277 (18) Adults: 1265 (82) | 1310 (85) | Urban 694 (45) Rural 848 (55) | Dogs 1141 (74) Cats 355 (23) Other animals 46 (3) | Hand 62 (4) Foot 524 (34) Head 77 (5) Trunk 62 (4) Mixed sites of injury 817 (53) | The mean time delay from injury to initial management for both sexes was 15.1 (SD 29.8) hours | NA | ||||
| Kilic et al., 2006 [16] | The ages ranged from 0 to 85 years (median, 25 years), and 43.5% of them were younger than 20 years | 1046 (66.7) | Urban 690 (44) Rural 879 (56) | Dogs 1087 (96.2) Cats 298 (19) Other (cattle, rat, etc.) 112 (79.5) | Upper extremities 493 (68.3) Lower extremities 457 (68.7) Other 232 (71.1) | Vaccinated: 1067 (68) Not vaccinated: 502 (32) | NA | ||||
| Sengoz et al., 2006 [47] | One-third of the cases (2422) were under 15 years of age | 5450 (75) | NA | Dog bite 5390 (74) Cat bite 1850 (25) Other animals 25 (0.3) | Head/neck wounds 298 (4) Trunk/extremity wounds 4610 (63) Hand wounds 2360 (32) | Complete vaccination: 1750 (24) Incomplete vaccination: 5516 (76) | NA | ||||
| Bizri et al., 2000 [27] | The ages ranged from 11 to 77 years. | 5 (62.5) | NA | Dog 5 (62.5) Other 3 (37.5) | NA | Vaccinated: 1 (12.5) Not vaccinated: 7 (87.5) | NA | ||||
| Tabbara et al., 1995 [24] | The age ranged from 1.5 to 7 years old | 0 (0) | Desert 2 (100) | Fox 2 (100) | Face and eyelid 2 (100) | >48 h: 2 (100) | NA | ||||
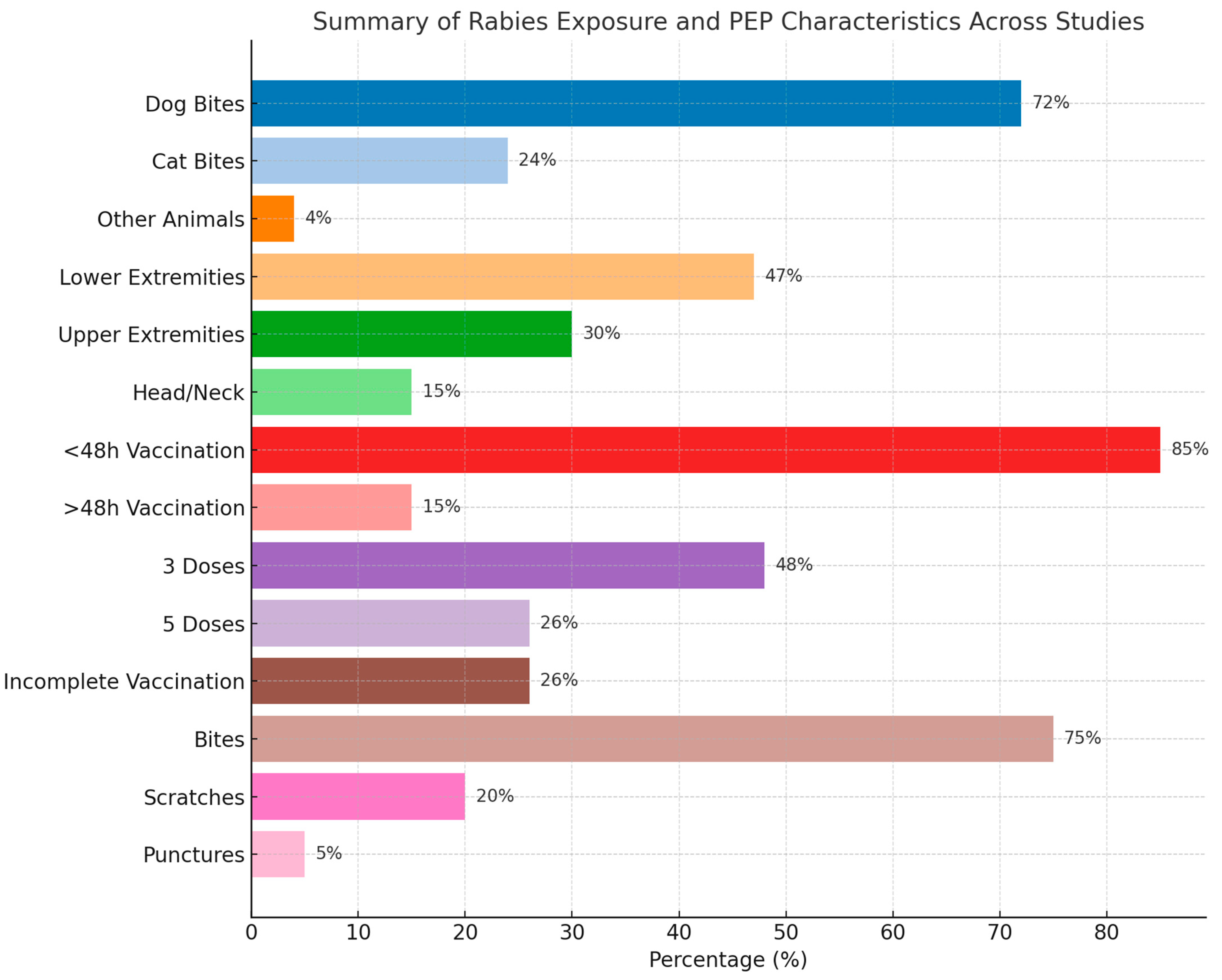
| Category | Most Common Findings | Range/Notes |
|---|---|---|
| Age Groups Affected | Highest exposure: young adults (20–39 years) consistently showed the highest rabies exposure rates, likely due to occupational or outdoor activities Children (0–12 years): significant exposure, especially in regions with stray dog populations Elderly (60+): lower but non-negligible exposure, possibly due to reduced mobility or rural residency | Many studies used age groupings; children <15 highly represented in several cases |
| Sex (Male %) | Mean ~70–80% male | Male predominance observed in nearly all studies |
| Type of Area | Urban (dominant in most studies) | Mixed urban/rural settings also reported |
| Main Animal Involved | Dogs (most common), followed by cats | Other animals: foxes, bats, monkeys, livestock |
| Most Bitten Body Parts | Lower extremities, upper limbs, hands | Some studies also reported multiple sites or head/neck injuries |
| Time to Vaccination | Majority < 48 h | Some delays >48 h in rural or underserved areas |
| Vaccination Regimen | 3- or 5-dose regimens most frequent | Some also reported incomplete vaccination and RIG administration |
| Injury Type | Bites (most common), scratches, punctures | Severe injuries (fractures, crush injuries) reported in a few studies |
| Study ID | Seri et al., 2014 [20] | Ramezankhani et al., 2016 [21] | ||
|---|---|---|---|---|
| Vaccine | Verorab | Abhayrab | PVRV | PCECV |
| Local pain, N (%) | 23 (3.02) | 87 (9.4) | 27 (3.9) | 28 (3.8) |
| Local redness and swelling, N (%) | 3 (0.39) | 19 (2.06) | 9 (1.3) | 8 (1.1) |
| Local numbness, N (%) | 0 (0) | 8 (0.86) | NA | |
| Itching at site of admin., N (%) | 3 (0.39) | 9 (0.97) | 7 (1) | 1 (0.1) |
| Swelling, N (%) | NA | 4 (0.6) | 2 (0.3) | |
| Bruising, N (%) | NA | 0 (0) | 4 (0.5) | |
| Headache, N (%) | 27 (3.55) | 95 (10.28) | 8 (1.4) | 16 (2.5) |
| Dizziness, N (%) | 6 (0.78) | 42 (4.54) | 5 (0.8) | 6 (0.9) |
| Fever, N (%) | 38 (4.99) | 229 (24.78) | 11 (1.9) | 10 (1.6) |
| Itching, N (%) | 5 (0.65) | 13 (1.4) | 0 (0) | 1 (0.2) |
| Weakness, N (%) | 38 (4.99) | 140 (15.15) | 5 (0.8) | 11 (1.7) |
| Abdominal pain, N (%) | 13 (1.71) | 30 (3.24) | 4 (0.7) | 3 (0.5) |
| Lymphadenopathy, N (%) | 0 (0) | 3 (0.324) | NA | |
| Nausea, N (%) | 19 (2.49) | 52 (5.62) | 4 (0.7) | 7 (1.1) |
| Vomiting, N (%) | 2 (0.26) | 16 (1.73) | 4 (0.7) | 7 (1.1) |
| Myalgia, N (%) | 7 (0.91) | 57 (6.17) | 6 (1.0) | 5 (0.8) |
| Cough, N (%) | 9 (1.18) | 11 (1.19) | NA | |
| Insomnia, N (%) | 0 (0) | 19 (2.05) | NA | |
| Numbness, N (%) | 0 (0) | 12 (1.29) | NA | |
| Irregular menstruation, N (%) | 0 (0) | 3 (0.324) | NA | |
| Decreased libido, N (%) | 0 (0) | 1 (0.11) | NA | |
| Rash, N (%) | 2 (0.26) | 9 (0.97) | NA | |
| Sore throat, N (%) | 8 (1.05) | 19 (2.05) | NA | |
| Nasal discharge, N (%) | 8 (1.05) | 2 (2.27) | NA | |
| Arthralgia, N (%) | 14 (1.83) | 69 (7.47) | 6 (1) | 3 (0.5) |
| Sweating, N (%) | NA | 3 (0.5) | 4 (0.6) | |
| Hypotension, N (%) | NA | 0 (0) | 1 (0.2) | |
| Study ID | Clearly Stated Aim | Consecutive Patients | Prospective Collection of Data | Endpoints | Assessment of Endpoint | Follow-Up Period | Loss < 5% | Study Size | Adequate Control Group | Contemporary Group | Baseline Control | Statistical Analyses | MINORS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Khoubfekr et al., 2024 [22] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 13 |
| Davarani et al., 2023 [28] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Khazaei et al., 2023 [18] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Yıldırım et al., 2022 [30] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Bay et al., 2021 [15] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Celiloglu et al., 2021 [38] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Oztoprak et al., 2021 [43] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Porsuk et al., 2021 [31] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Amiri et al., 2020 [32] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Can et al., 2020 [44] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Janatolma Kan et al., (2020) [33] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Rahmanian et al., 2020 [22] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Rasooli et al., 2020 [25] | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 12 |
| Sarbazi et al., 2020 [34] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Hamta et al., 2019 [48] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Kassiri et al., 2018 [35] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Khazaei et al., 2018 [29] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| BabazadeH. et al., 2016 [36] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 23 |
| Ramezankhani et al., 2016 [21] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 24 |
| Amiri et al., 2015 [37] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Riabi et al., 2015 [39] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Poorolajal et al., 2015 [40] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Farahtaj et al., 2014 [26] | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 12 |
| Karbeyaz et al., 2014 [50] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Seri et al., 2014 [20] | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 23 |
| Charkazi et al., 2013 [41] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Taghvaii et al., 2013 [17] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 |
| Ghannad et al., 2012 [42] | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 14 |
| Ansari et al., 2011 [23] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 13 |
| Bijari et al., 2011 [46] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 | |
| Najafi et al., 2009 [49] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 | |
| Sheikholeslami et al., 2009 [51] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 | |
| Kilic et al., 2006 [16] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 | |
| Sengoz et al., 2006 [47] | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 15 | |
| Bizri et al., 2000 [27] | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 12 | |
| Tabbara et al., 1995 [24] | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetta, H.F.; Albalawi, K.S.; Almalki, A.M.; Albalawi, N.D.; Albalawi, A.S.; Al-Atwi, S.M.; Alatawi, S.E.; Alharbi, M.J.; Albalawi, M.F.; Alharbi, A.A.; et al. Rabies Vaccination and Public Health Insights in the Extended Arabian Gulf and Saudi Arabia: A Systematic Scoping Review. Diseases 2025, 13, 124. https://doi.org/10.3390/diseases13040124
Hetta HF, Albalawi KS, Almalki AM, Albalawi ND, Albalawi AS, Al-Atwi SM, Alatawi SE, Alharbi MJ, Albalawi MF, Alharbi AA, et al. Rabies Vaccination and Public Health Insights in the Extended Arabian Gulf and Saudi Arabia: A Systematic Scoping Review. Diseases. 2025; 13(4):124. https://doi.org/10.3390/diseases13040124
Chicago/Turabian StyleHetta, Helal F., Khalid S. Albalawi, Amal M. Almalki, Nasser D. Albalawi, Abdulmajeed S. Albalawi, Suleiman M. Al-Atwi, Saleh E. Alatawi, Mousa J. Alharbi, MeshaL F. Albalawi, Ahmad A. Alharbi, and et al. 2025. "Rabies Vaccination and Public Health Insights in the Extended Arabian Gulf and Saudi Arabia: A Systematic Scoping Review" Diseases 13, no. 4: 124. https://doi.org/10.3390/diseases13040124
APA StyleHetta, H. F., Albalawi, K. S., Almalki, A. M., Albalawi, N. D., Albalawi, A. S., Al-Atwi, S. M., Alatawi, S. E., Alharbi, M. J., Albalawi, M. F., Alharbi, A. A., Elfadil, H., Albalawi, A. S., & Sayad, R. (2025). Rabies Vaccination and Public Health Insights in the Extended Arabian Gulf and Saudi Arabia: A Systematic Scoping Review. Diseases, 13(4), 124. https://doi.org/10.3390/diseases13040124









