Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern
Abstract
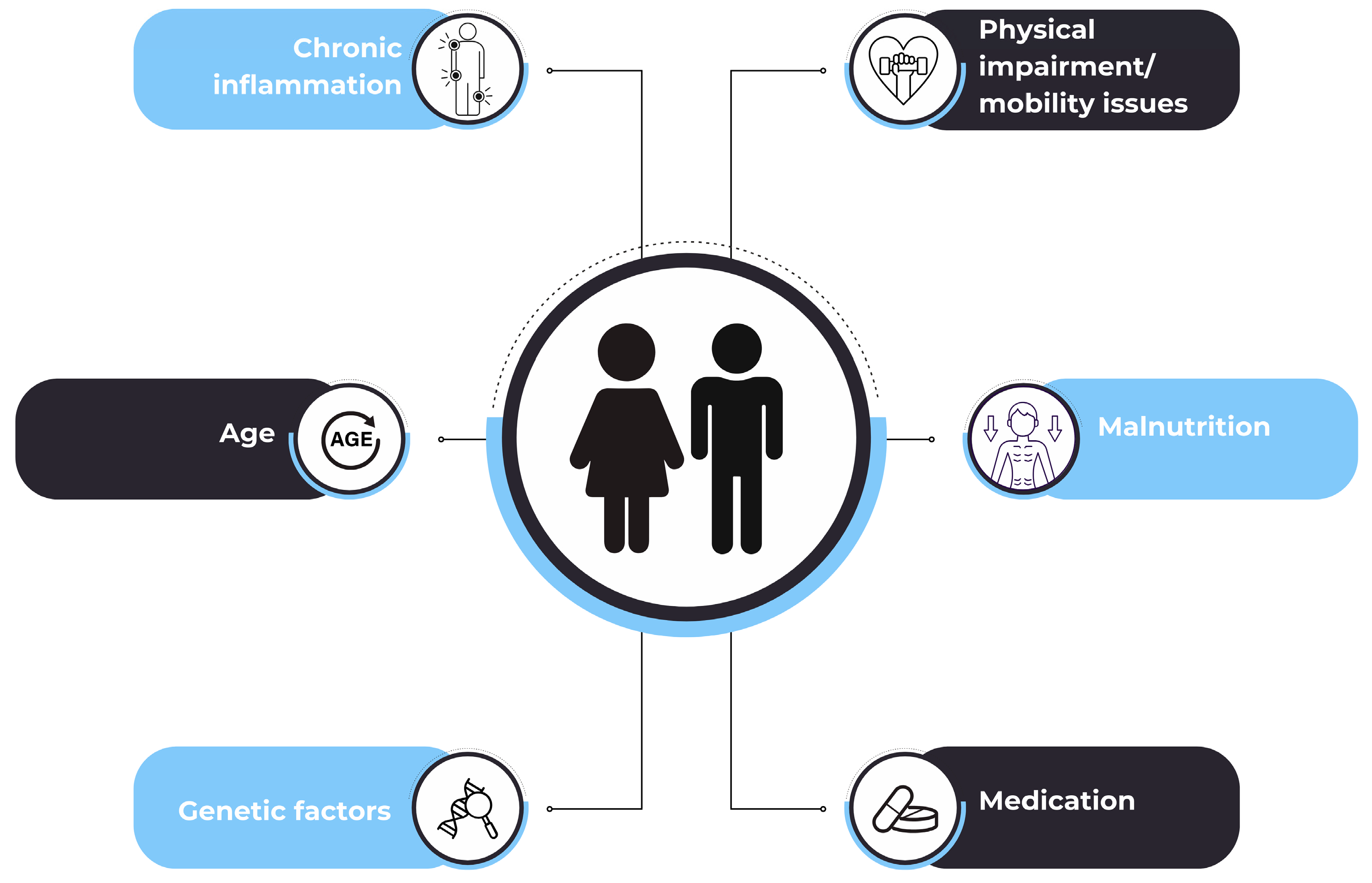
:1. Introduction
2. Literature Search
3. Sarcopenia Definitions
4. Inflammation Biomarkers in Sarcopenia
5. Sarcopenia and RDs
5.1. Sarcopenia in RA
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Barone [51] | 76 | RA | 21.0 | EWGSOP 2010 | MMI Ñ, HGS | 10.75 kg/m2 for men and 6.75 kg/m2 for women | BIA |
| Brance [47] | 105 | RA | 19 | EWGSOP 2019 | SMI §, HGS, 4MWS, sit-to-stand, TUG | 7.0 kg/m2 for men and 5.5 kg/m2 for women | DXA |
| Cano-García [59] | 76 | RA | 15.8 | EWGSOP 2019 | SMI §, HGS, SPPB | 7.0 kg/m2 for men and 5.5 kg/m2 for women | DXA |
| Chen [57] | 238 | RA | 58.4 | AWGS 2014, EWGSOP 2010 | SMI § | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Chu [63] | 188 | RA | 63.8 | AWGS 2014 | SMI Ð | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Dietzel [53] | 289 | RA | 4.5/2.8 | EWGSOP 2019/FNIH | SMI §, SMI ₩, HGS, 6.45MWS, chair rise test | EWGSOP: 7.0 kg/m2 for men and 5.7 kg/m2 for women FNIH: 0.789 for men and 0.512 for women | DXA |
| Dobrovolskaya [65] | 91 | RA | 27.5 | EWGSOP 2019 | SMI § | 6 kg/m2 for women | DXA |
| Ekici [66] | 54 | RA | 31.5 | EWGSOP 2019 | SMMI œ, HGS | 6.76 kg/m2 for women and 10.76 kg/m2 for men | BIA |
| Fang [67] | 64 | RA | 20.3 | AWGS 2019 | SMI §, HGS, 6MWS | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Guzmán-Guzmán [54] | 223 | RA | 86 | EWGSOP 2010 | SMI §, HGS | 7.26 kg/m2 for men and 5.45 kg/m2 for women | ΒΙA |
| Lian [48] | 549 | RA | 61.7 | AWGS 2014 | SMI Ð | based on the AWGS criteria | BIA |
| Lozada-Mellado [44] | 165 | RA | 15.8 | EWGSOP 2019 | SMMI œ, HGS | 6.38 kg/m2 for women | ΒΙA |
| Mena-Vázquez [52] | 94 | RA | 24.5 | SMI | SMI § | 7.26 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Mochizuki [62] | 240 | RA | 29.6 | AWGS 2014 | SMI §, HGS, walking speed | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Mochizuki [68] | 87 | RA | 10.3 | AWGS 2014 | SMI §, HGS, walking speed | NR | BIA |
| Moschou [69] | 80 | RA | 39 | EWGSOP 2019 | SMI §, HGS, SPPB | 5.45 kg/m2 for women | DXA |
| Nakayama [70] | 2416 | RA | 14.1 | SARC-F | N/A | N/A | N/A |
| Pardali [71] | 79 | RA | 7.6 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Qu [56] | 480 | RA | 19.4 | AWGS 2019 | NR | NR | NR |
| Santos [72] | 89 | RA | 4.5 | FFMI | FFMI ¤ | ≤2 SD below the mean of a reference Caucasian sample | BIA |
| Tada [73] | 100 | RA | 28 | AWGS 2014 | SMI §, HGS, 3MWS | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Tekgoz [50] | 100 | RA | 35 * | EWGSOP 2019 | SMMI Ø, HGS, 6MWS | 9.2 kg/m2 for men and 7.4 kg/m2 for women | BIA |
| Tong [74] | 474 | RA | 62.4 * | AWGS 2014 | SMI § | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Torii [58] | 388 | RA | 37.1 | EWGSOP 2010 | SMI §, HGS, 6MWS | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Tournadre [46] | 74 | RA | 7.8 | EWGSOP 2010 | SMI §, HGS, walking speed | 7.26 kg/m2 for men and 5.45 kg/m2 for women | DXA |
| Tournadre [75] | 21 | RA | 28.6 | SMI | SMI § | 7.26 kg/m2 for men and 5.5 kg/m2 for women | DXA |
| Valencia-Muntalà [45] | 67 | RA | 43.3/16.4/62.7 | EWGSOP 2010/EWGSOP 2019/SARC-F | SMI §, HGS, 6MWS | 5.67 kg/m2 for women | DXA |
| Vlietstra [76] | 82 | RA | 17.1 | FNIH | SMI ₩, HGS, 40MWS, sit-to-stand, TUG | 0.789 for men and 0.512 for women | DXA |
| Wiegmann [60] | 238 | RA | 4.6/2.9 | EWGSOP 2019/FNIH | SMI §, SMI ₩, HGS, SPPB, 6.45MWS, TUG | SMI §: 7.0 kg/m2 for men and 5.5 kg/m2 for women SMI ₩: 0.789 for men and 0.512 for women | DXA |
| Yamada [18] | 100 | RA | 13.4 | AWGS 2014 | SMI §, HGS, 3MWS | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
| Yun [49] | 320 | RA | 2.2/6.6/11.9 | AWGS 2019/EWGSOP 2010/SARC-F | SMI §, HGS, 4MWS | AWGS: 7.0 kg/m2 for men and ≤5.7 kg/m2 for women EWGSOP: 8.87 kg/m2 for men and 6.42 kg/m2 for women | BIA |
| Zhang [61] | 130 | RA | 43.1 | AWGS 2014 | SMI § | 7.0 kg/m2 for men and 5.7 kg/m2 for women | BIA |
5.2. Sarcopenia in Systemic Sclerosis (SSc)
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Ajdynan [89] | 43 | SSc | 33.3 | EWGSOP 2019 | SMI §, HGS, sit-to-stand | NR | DXA |
| Caimmi [85] | 140 | SSc | 20.7 | SMI | SMI § | 7.26 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Corallo [82] | 62 | SSc | 42 | EWGSOP 2010 | SMI §, HGS | 7.26 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Hax [87] | 94 | SSc | 15.9/ 22.3/ 21.3/ 21.3/36.2 | EWGSOP 2019/ SARC-F/ SARC-CalF/ SARC-F + EBM/Ishii | SMI §, HGS, 4MWS, SPPB | 7.0 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Paolino [84] | 43 | SSc | 23.3 | EWGSOP 2010 | SMI § | 7.26 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Pardali [71] | 17 | SSc | 52.9 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Rincón [90] | 27 | SSc | 33.3 | EWGSOP 2010 | SMI §, HGS, 4MWS | 7.26 kg/m2 for men and 5.50 kg/m2 for women | DXA |
| Sangaroon [86] | 180 | SSc | 22.8 | AWGS 2019 | SMI §, FFMI ¤, HGS, 6MWS | 7.0 kg/m2 for men and 5.4 kg/m2 for women | DXA |
| Sari [81] | 93 | SSc | 10.7 | EWGSOP 2010 | ASMI  , HGS , HGS | 7.26 kg/m2 for men and 5.50 kg/m2 for women | BIA |
| Siegert [83] | 129 | SSc | 22.5 | EWGSOP 2010 | SMI §, HGS | 7.26 kg/m2 for men and 5.50 kg/m2 for women | BIA |
 ASMΙ: ALM/height2 where ALM = −4.211 + (0.267 × height2/resistance) + (0.095 × BW) + (1.909 × sex [men = 1, women = 0]) + (−0.012 × age) + (0.058 × reactance); § SMI: the sum of upper and lower limb muscle mass (ALM) divided by squared height (kg/m2); ¤ FFMI: FFM divided by the square of the height (kg/m2).
ASMΙ: ALM/height2 where ALM = −4.211 + (0.267 × height2/resistance) + (0.095 × BW) + (1.909 × sex [men = 1, women = 0]) + (−0.012 × age) + (0.058 × reactance); § SMI: the sum of upper and lower limb muscle mass (ALM) divided by squared height (kg/m2); ¤ FFMI: FFM divided by the square of the height (kg/m2).5.3. Sarcopenia in Spondyloarthritides (SpAs)
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnoses | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Aguiar [101] | 36/ 24 | AS/ PsA | 62 | MMI | MMI ª | Grade I: 8.51 < MMI < 10.75 for men and 5.76 < MMI < 6.75 for women; Grade II: MMI < 8.51 for men and <5.76 for women | Skinfold thickness |
| El Maghraoui [98] | 67 | AS | 34.3/50.4 * | EWGSOP 2010 | SMI §, HGS, TUG | 7.25 kg/m2 for men | DXA |
| Kanjanavaikoon [93] | 104 | SpA | 22.1 | AWGS 2019, SARC-F, SARC-CalF | SMI §, calf circumference, 6MWS, sit-to-stand | 7.0 kg/m2 for men 5.4 kg/m2 for women | DXA |
| Krajewska-Wlodarczyk [102] | 51 | PsA | 13.7 (SMI §) 43.1 (SMI š) | EWGSOP 2010 | SMI §, SMI š, TUG | SMI §: 5.4 kg/m2 for women SMI š: 27.6% for women | BIA |
| Merle [92] | 103 | SpA | 5 | EWGSOP 2019 | SMI §, HGS, 4MWS | 7.0 kg/m2 for men and 5.5 kg/m2 for women | DXA |
| Pardali [71] | 21 | PsA | 14.3 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Pardali [71] | 17 | AS | 11.8 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Takami [100] | 156 | PsA | 5.1/16.7 * | AWGS 2019 | SMI §, HGS | 7.0 kg/m2 for men and 5.4 kg/m2 for women | DXA |
| Tournadre [46] | 11 | PsA | 9.1 | EWGSOP 2010 | SMI §, HGS, walking speed | 7.26 kg/m2 for men and 5.45 kg/m2 for women | DXA |
| Tournadre [46] | 63 | SpA | 1.7 | EWGSOP 2010 | SMI §, HGS, walking speed | 7.26 kg/m2 for men and 5.45 kg/m2 for women | DXA |
5.4. Sarcopenia in Systemic Lupus Erythematosus (SLE)
5.5. Sarcopenia in Juvenile Idiopathic Arthritis (JIA)
5.6. Sarcopenia in Primary Sjögren’s Syndrome (pSS)
5.7. Sarcopenia in Myositis
5.8. Sarcopenia in Fibromyalgia Syndrome (FMS)
5.9. Sarcopenia in Vasculitides
5.10. Sarcopenia in Other RDs and Mixed Patient Samples
5.11. Sarcopenic Obesity
6. Sarcopenia and Pharmacological Treatments in RDs
6.1. The Class of DMARDs
6.2. Steroids and Muscle Wasting
7. Lifestyle Approaches to Managing Sarcopenia
7.1. Nutritional Interventions
7.2. Exercise as an Intervention
8. Conclusions
9. Future Directions and Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALM | Appendicular lean mass |
| AS | Ankylosing spondylitis |
| AWSG | Asian Working Group for Sarcopenia |
| BIA | Bioelectrical impedance analysis |
| bDMARDs | Biologic disease-modifying anti-rheumatic drugs |
| BMI | Body mass index |
| CHIKARA | Correlation research of sarcopenia, skeletal muscle, and disease activity in rheumatoid arthritis |
| CRP | C-reactive protein |
| csDMARDs | Conventional synthetic disease-modifying anti-rheumatic drugs |
| CCP | Cyclic Citrullinated Peptide |
| CT | Computed tomography |
| DXA | Dual-energy X-ray absorptiometry |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| ESR | Erythrocyte sedimentation rate |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| FFMI | Fat-free mass index |
| FMS | Fibromyalgia syndrome |
| FNIH | Foundation For the National Institutes of Health Sarcopenia Project |
| GC | Glucocorticoids |
| HGS | Handgrip strength |
| HMB | Β-hydroxy-β-methylbutyrate |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| JAK | Janus kinase |
| JIA | Juvenile idiopathic arthritis |
| MMI | Muscle mass index |
| MRI | Magnetic resonance imaging |
| MSRA | Mini sarcopenia risk assessment |
| NF-kB | Nuclear factor-kappa b |
| PsA | Psoriatic arthritis |
| pSS | Primary Sjögren’s syndrome |
| RA | Rheumatoid arthritis |
| RDs | Rheumatic diseases |
| SARC-F | Strength, Assistance with Walking, Rise from a Chair, Climb Stairs, and Falls |
| SARC-F + EBM | SARC-F plus elderly and BMI |
| sIL6R | Soluble form of IL6 receptor |
| SLE | Systemic lupus erythematosus |
| SMI | Skeletal mass index |
| SpA | Spondyloarthritides |
| SPPB | Short physical performance battery |
| SSc | Systemic sclerosis |
| TNF-α | Tumor-necrosis factor-α |
| tsDMARDs | Targeted synthetic disease-modifying anti-rheumatic drugs |
| TUG | Timed up-and-go |
References
- Rosenberg, I.H. Summary Comments: Epidemiological and Methodological Problems in Determining Nutritional Status of Older Persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.G.; Bauer, J.J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [CrossRef]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip Strength, Body Composition, and Mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Ferreira, S.; Amaral, T.F. Financial Impact of Sarcopenia on Hospitalization Costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef]
- Geenen, R.; Overman, C.L.; Christensen, R.; Åsenlöf, P.; Capela, S.; Huisinga, K.L.; Husebø, M.E.P.; Köke, A.J.A.; Paskins, Z.; Pitsillidou, I.A.; et al. EULAR Recommendations for the Health Professional’s Approach to Pain Management in Inflammatory Arthritis and Osteoarthritis. Ann. Rheum. Dis. 2018, 77, 797–807. [Google Scholar] [CrossRef]
- da Rocha, D.S.; Tessari, J.A.; Mainardi, N.B.; Hax, V.; Gasparin, A.A.; de Oliveira, C.A.V.; Garcia, T.S.; Xavier, R.M.; Chakr, R.M.d.S. Assessment of Muscle Mass Using Chest Computed Tomography-Based Quantitative and Qualitative Measurements in Patients with Systemic Sclerosis: A Retrospective Study with Cross-Sectional and Longitudinal Analyses. Semin. Arthritis Rheum. 2023, 59, 152168. [Google Scholar] [CrossRef]
- Lin, J.-Z.; Chen, C.-T.; Ma, J.-D.; Mo, Y.-Q.; Li, Q.-H.; Chen, L.-F.; Yang, Z.-H.; Cheng, W.-M.; He, X.-L.; Zheng, D.-H.; et al. Neglected Extra-Articular Manifestations in Rheumatoid Arthritis Patients with Normal Body Mass Index: Reduced Skeletal Muscle Overlapping Overfat. Ther. Adv. Chronic Dis. 2020, 11, 2040622320975241. [Google Scholar] [CrossRef]
- Efthymiou, E.; Grammatikopoulou, M.G.; Gkiouras, K.; Efthymiou, G.; Zafiriou, E.; Goulis, D.G.; Sakkas, L.I.; Bogdanos, D.P. Time to Deal with Rheumatoid Cachexia: Prevalence, Diagnostic Criteria, Treatment Effects and Evidence for Management. Mediterr. J. Rheumatol. 2022, 33, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ding, K.; Jiang, W.; Zhu, W.; Gao, Y. Molecular Crosstalk and Potential Causal Mechanisms of Rheumatoid Arthritis and Sarcopenia Co-Morbidity: A Gene Integration Analysis. Exp. Gerontol. 2025, 203, 112729. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Sica, A.; Selmi, C. Frailty in Rheumatic Diseases. Front. Immunol. 2020, 11, 576134. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-Alpha with Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Kuzuya, M. Drug-related Sarcopenia as a Secondary Sarcopenia. Geriatr. Gerontol. Int. 2024, 24, 195–203. [Google Scholar] [CrossRef]
- Hoes, J.N.; Jacobs, J.W.G.; Boers, M.; Boumpas, D.; Buttgereit, F.; Caeyers, N.; Choy, E.H.; Cutolo, M.; Da Silva, J.A.P.; Esselens, G.; et al. EULAR Evidence-Based Recommendations on the Management of Systemic Glucocorticoid Therapy in Rheumatic Diseases. Ann. Rheum. Dis. 2007, 66, 1560–1567. [Google Scholar] [CrossRef]
- Güler-Yüksel, M.; Hoes, J.N.; Bultink, I.E.M.; Lems, W.F. Glucocorticoids, Inflammation and Bone. Calcif. Tissue Int. 2018, 102, 592–606. [Google Scholar] [CrossRef]
- Yamada, Y.; Tada, M.; Mandai, K.; Hidaka, N.; Inui, K.; Nakamura, H. Glucocorticoid Use Is an Independent Risk Factor for Developing Sarcopenia in Patients with Rheumatoid Arthritis: From the CHIKARA Study. Clin. Rheumatol. 2020, 39, 1757–1764. [Google Scholar] [CrossRef]
- Villalobos-Sánchez, L.; Blanco-Cáceres, B.; Bachiller-Corral, J.; Rodríguez-Serrano, M.T.; Vázquez-Díaz, M.; de Mercado, P.L.Y. Quality of Life of Patients with Rheumatic Diseases. Reumatol. Clínica 2024, 20, 59–66. [Google Scholar] [CrossRef]
- Coletta, G.; Phillips, S.M. An Elusive Consensus Definition of Sarcopenia Impedes Research and Clinical Treatment: A Narrative Review. Ageing Res. Rev. 2023, 86, 101883. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus Definition of Sarcopenia, Cachexia and Pre-Cachexia: Joint Document Elaborated by Special Interest Groups (SIG) “Cachexia-Anorexia in Chronic Wasting Diseases” and “Nutrition in Geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Stewart Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia With Limited Mobility: An International Consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Jo, E.; Lee, S.-R.; Park, B.-S.; Kim, J.-S. Potential Mechanisms Underlying the Role of Chronic Inflammation in Age-Related Muscle Wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; He, M.; Wang, J.; Wu, Y.; Li, Y. Immune System and Sarcopenia: Presented Relationship and Future Perspective. Exp. Gerontol. 2022, 164, 111823. [Google Scholar] [CrossRef]
- Alway, S.E.; Siu, P.M. Nuclear Apoptosis Contributes to Sarcopenia. Exerc. Sport Sci. Rev. 2008, 36, 51–57. [Google Scholar] [CrossRef]
- McKinnell, I.W.; Rudnicki, M.A. Molecular Mechanisms of Muscle Atrophy. Cell 2004, 119, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.-Y.; Baldwin, A.S., Jr. NF-ΚB-Induced Loss of MyoD Messenger RNA: Possible Role in Muscle Decay and Cachexia. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Al-Shanti, N.; Stewart, C.E. Inhibitory Effects of IL-6 on IGF-1 Activity in Skeletal Myoblasts Could Be Mediated by the Activation of SOCS-3. J. Cell. Biochem. 2012, 113, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory Markers and Loss of Muscle Mass (Sarcopenia) and Strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and Sarcopenia: A Systematic Review and Meta-Analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The Forgotten Key Players in Rheumatoid Arthritis: IL-8 and IL-17—Unmet Needs and Therapeutic Perspectives. Front. Med. 2023, 10, 956127. [Google Scholar] [CrossRef] [PubMed]
- Westbury, L.D.; Fuggle, N.R.; Syddall, H.E.; Duggal, N.A.; Shaw, S.C.; Maslin, K.; Dennison, E.M.; Lord, J.M.; Cooper, C. Relationships Between Markers of Inflammation and Muscle Mass, Strength and Function: Findings from the Hertfordshire Cohort Study. Calcif. Tissue Int. 2018, 102, 287–295. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; La Rocca, C.; Formisano, L.; Matarese, G. Role of Adipokines Signaling in the Modulation of T Cells Function. Front. Immunol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Öztürk, Z.A.; Kul, S.; Türkbeyler, İ.H.; Sayıner, Z.A.; Abiyev, A. Is Increased Neutrophil Lymphocyte Ratio Remarking the Inflammation in Sarcopenia? Exp. Gerontol. 2018, 110, 223–229. [Google Scholar] [CrossRef]
- Van Atteveld, V.A.; Van Ancum, J.M.; Reijnierse, E.M.; Trappenburg, M.C.; Meskers, C.G.M.; Maier, A.B. Erythrocyte Sedimentation Rate and Albumin as Markers of Inflammation Are Associated with Measures of Sarcopenia: A Cross-Sectional Study. BMC Geriatr. 2019, 19, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving Concepts of Rheumatoid Arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Markaki, A.G.; Gkiouras, K.; Papakitsos, C.; Grammatikopoulou, M.G.; Papatsaraki, A.; Ioannou, R.; Tsagkari, A.; Papamitsou, T.; Bogdanos, D.P. Disease Activity, Functional Ability and Nutritional Status in Patients with Rheumatoid Arthritis: An Observational Study in Greece. Mediterr. J. Rheumatol. 2020, 31, 406–411. [Google Scholar] [CrossRef]
- Lozada-Mellado, M.; Llorente, L.; Hinojosa-Azaola, A.; Ogata-Medel, M.; Valdez-Echeverría, R.D.; Lira-Reyes, A.R.; Castillo-Martínez, L. Inflammatory Profile in Patients with Rheumatoid Arthritis and Sarcopenia. Clin. Rheumatol. 2024, 43, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Muntalà, L.; Gómez-Vaquero, C.; Mora, M.; Berbel-Arcobé, L.; Benavent, D.; Narváez, J.; Juanola, X.; Nolla, J.M. Evaluating Sarcopenia Prevalence and SARC-F Effectiveness in Elderly Spanish Women with RA: A Comparative Study of EWGSOP Criteria. Front. Med. 2024, 11, 1392604. [Google Scholar] [CrossRef]
- Tournadre, A.; Jaffeux, P.; Frayssac, T.; Fan, A.; Couderc, M.; Dubost, J.; Malochet-Guinamand, S.; Mathieu, S.; Tatar, Z.; Jourdy, J.; et al. SAT0682 Prevalence of Sarcopenia in Patients with Chronic Inflammatory Rheumatic Diseases. Ann. Rheum. Dis. 2017, 76, 1033. [Google Scholar] [CrossRef]
- Brance, M.L.; Di Gregorio, S.; Pons-Estel, B.A.; Quagliato, N.J.; Jorfen, M.; Berbotto, G.; Cortese, N.; Raggio, J.C.; Palatnik, M.; Chavero, I.; et al. Prevalence of Sarcopenia and Whole-Body Composition in Rheumatoid Arthritis. J. Clin. Rheumatol. 2021, 27, S153–S160. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Wang, J.-X.; Xu, Y.-C.; Zong, H.-X.; Teng, Y.-Z.; Xu, S.-Q. Sarcopenia May Be a Risk Factor for Osteoporosis in Chinese Patients with Rheumatoid Arthritis. Int. J. Gen. Med. 2022, 15, 2075–2085. [Google Scholar] [CrossRef]
- Yun, H.-W.; Kim, C.-J.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H.; Jung, J.-Y. The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 3458. [Google Scholar] [CrossRef]
- Tekgoz, E.; Colak, S.; Ozalp Ates, F.S.; Sonaeren, I.; Yilmaz, S.; Cinar, M. Sarcopenia in Rheumatoid Arthritis: Is It a Common Manifestation? Int. J. Rheum. Dis. 2020, 23, 1685–1691. [Google Scholar] [CrossRef]
- Barone, M.; Viggiani, M.T.; Anelli, M.G.; Fanizzi, R.; Lorusso, O.; Lopalco, G.; Cantarini, L.; Di Leo, A.; Lapadula, G.; Iannone, F. Sarcopenia in Patients with Rheumatic Diseases: Prevalence and Associated Risk Factors. J. Clin. Med. 2018, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Mena-Vázquez, N.; Manrique-Arija, S.; Ordoñez-Cañizares, M.C.; Redondo-Rodriguez, R.; Rioja Villodres, J.; Cano-Garcia, L.; Godoy-Navarrete, F.J.; Jiménez Nuñez, F.G.; Diaz-Cordovés Rego, G.; Ureña Garnica, I.; et al. Relationship between Polyautoimmunity and Sarcopenic Obesity in Rheumatoid Arthritis Patients. Reumatol. Clínica 2022, 18, 531–537. [Google Scholar] [CrossRef]
- Dietzel, R.; Wiegmann, S.; Borucki, D.; Detzer, C.; Zeiner, K.N.; Schaumburg, D.; Buehring, B.; Buttgereit, F.; Armbrecht, G. Prevalence of Sarcopenia in Patients with Rheumatoid Arthritis Using the Revised EWGSOP2 and the FNIH Definition. RMD Open 2022, 8, e002600. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, I.P.; Zaragoza-García, O.; Navarro-Zarza, J.E.; Ramírez, M.; Parra-Rojas, I. FRI0048 Sarcopenia and Sarcopenic Obesity Are Associated to Glucocorticoid Use and Clinical and Serological Markers in Rheumatoid Arthritis. Ann. Rheum. Dis. 2019, 78, 685–686. [Google Scholar] [CrossRef]
- Lin, J.; Liang, J.; Ma, J.; Li, Q.; Mo, Y.; Cheng, W.; He, X.; Li, N.; Cao, M.; Xu, D.; et al. Myopenia Is Associated with Joint Damage in Rheumatoid Arthritis: A Cross-sectional Study. J. Cachexia Sarcopenia Muscle 2019, 10, 355–367. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, L.; Liu, Y.; Fu, Y.; Wang, M.; Liu, C.; Wang, X.; Wan, Y.; Xu, B.; Zhang, Q.; et al. Development and Validation of a Predictive Model Assessing the Risk of Sarcopenia in Rheumatoid Arthritis Patients. Front. Immunol. 2024, 15, 1437980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Zong, H.-X.; Xu, S.-Q.; Chu, Y.-R.; Wang, J.-X.; Li, W.-J.; Chen, K.-M. Synergistic Effect of Sarcopenia and Poor Balance on Osteoporotic Vertebral Fracture in Chinese Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2021, 40, 3627–3637. [Google Scholar] [CrossRef]
- Torii, M.; Hashimoto, M.; Hanai, A.; Fujii, T.; Furu, M.; Ito, H.; Uozumi, R.; Hamaguchi, M.; Terao, C.; Yamamoto, W.; et al. Prevalence and Factors Associated with Sarcopenia in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2019, 29, 589–595. [Google Scholar] [CrossRef]
- Cano-García, L.; Manrique-Arija, S.; Domínguez-Quesada, C.; Vacas-Pérez, J.C.; Armenteros-Ortiz, P.J.; Ruiz-Vilchez, D.; Martín-Martín, J.M.; Redondo-Rodríguez, R.; García-Studer, A.; Ortiz-Márquez, F.; et al. Sarcopenia and Nutrition in Elderly Rheumatoid Arthritis Patients: A Cross-Sectional Study to Determine Prevalence and Risk Factors. Nutrients 2023, 15, 2440. [Google Scholar] [CrossRef]
- Wiegmann, S.; Armbrecht, G.; Borucki, D.; Buehring, B.; Buttgereit, F.; Detzer, C.; Schaumburg, D.; Zeiner, K.N.; Dietzel, R. Association between Sarcopenia, Physical Performance and Falls in Patients with Rheumatoid Arthritis: A 1-Year Prospective Study. BMC Musculoskelet. Disord. 2021, 22, 885. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, D.; Zhang, Q. Correlation between Sarcopenia and Nailfold Microcirculation, Serum 25-Hydroxycholecalciferol (Vitamin D3) and IL-17 Levels in Female Patients with Rheumatoid Arthritis. Biomed. Pap. 2021, 165, 264–269. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Okazaki, K. Sarcopenia-Associated Factors in Japanese Patients with Rheumatoid Arthritis: A Cross-Sectional Study. Geriatr. Gerontol. Int. 2019, 19, 907–912. [Google Scholar] [CrossRef]
- Chu, Y.-R.; Xu, S.-Q.; Wang, J.-X.; Zong, H.-X.; Chen, K.-M.; Wang, C.; Tong, W.-Q.; Wang, X.-L. Synergy of Sarcopenia and Vitamin D Deficiency in Vertebral Osteoporotic Fractures in Rheumatoid Arthritis. Clin. Rheumatol. 2022, 41, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.; Zhao, L.-J.; Wu, K.; Zhou, Y.; Chen, T.; Deng, H.-W. Race and Socioeconomic Effect on Sarcopenia and Sarcopenic Obesity in the Louisiana Osteoporosis Study (LOS). JCSM Clin. Rep. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.V.; Toroptsova, N.V.; Demin, N.V.; Feklistov, A.Y.; Nikitinskaya, O.A. Obesity and Sarcopenia in Patients with Rheumatoid Arthritis: A Cross-Sectional Study. Pharmateca 2020, 27, 57–63. [Google Scholar] [CrossRef]
- Ekici, R.; Erden, A.; Güven, S.C.; Armağan, B.; Özdemir, B.; Karakaş, Ö.; Gök, K.; Omma, A.; Küçükşahin, O.; Erten, Ş. Prevalence of Sarcopenia and Clinical Implications in Patients with Newly Diagnosed Rheumatoid Arthritis. Nutrition 2021, 90, 111353. [Google Scholar] [CrossRef]
- Fang, T.J.; Chiu, M.H.; Huang, M.S.; Dai, C.Y.; Yeh, Y.T.; Yen, J.H. Increased Serum Adipokines Are Associated with Sarcopenia in Non-Obese Women with Rheumatoid Arthritis. Kaohsiung J. Med. Sci. 2024, 40, 489–498. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Okazaki, K. Sarcopenia in Japanese Younger Patients with Rheumatoid Arthritis: A Cross-Sectional Study. Mod. Rheumatol. 2021, 31, 504–505. [Google Scholar] [CrossRef]
- Moschou, D.; Krikelis, M.; Georgakopoulos, C.; Mole, E.; Chronopoulos, E.; Tournis, S.; Mavragani, C.; Makris, K.; Dontas, I.; Gazi, S. Prevalence and Factors Associated with Sarcopenia in Post-Menopausal Women with Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2024, 35, 438–447. [Google Scholar] [CrossRef]
- Nakayama, M.; Furuya, T.; Inoue, E.; Tanaka, E.; Ikari, K.; Yamanaka, H.; Harigai, M. Factors Associated with Sarcopenia in Japanese Patients with Rheumatoid Arthritis: Results from the IORRA Cohort Study. Clin. Rheumatol. 2024, 43, 521–526. [Google Scholar] [CrossRef]
- Pardali, E.C.; Kontouli, K.-M.; Gkouvi, A.; Tsakmaki, I.A.; Patrikiou, E.; Karapli, M.; Liaskos, C.; Liapis, N.M.; Syrmou, V.; Alexiou, I.; et al. Screening and Diagnosis of Sarcopenia in Rheumatic and Musculoskeletal Diseases: Findings from a Cross-Sectional Study. Rheumatol. Int. 2025, 45, 67. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Vinagre, F.; Canas da Silva, J.; Gil, V.; Fonseca, J.E. Body Composition Phenotypes in Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Comparative Study of Caucasian Female Patients. Clin. Exp. Rheumatol. 2011, 29, 470–476. [Google Scholar] [PubMed]
- Tada, M.; Yamada, Y.; Mandai, K.; Hidaka, N. Matrix Metalloprotease 3 Is Associated with Sarcopenia in Rheumatoid Arthritis—Results from the CHIKARA Study. Int. J. Rheum. Dis. 2018, 21, 1962–1969. [Google Scholar] [CrossRef]
- Tong, J.-J.; Xu, S.-Q.; Wang, J.-X.; Zong, H.-X.; Chu, Y.-R.; Chen, K.-M.; Teng, Y.-Z. Interactive Effect of Sarcopenia and Falls on Vertebral Osteoporotic Fracture in Patients with Rheumatoid Arthritis. Arch. Osteoporos. 2021, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet-Guinamand, S.; Soubrier, M. Changes in Body Composition and Metabolic Profile during Interleukin 6 Inhibition in Rheumatoid Arthritis. J. Cachexia Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef]
- Vlietstra, L.; Stebbings, S.; Meredith-Jones, K.; Haxby Abbott, J.; Treharne, G.J.; Waters, D.L. Sarcopenia in Osteoarthritis and Rheumatoid Arthritis: The Association with Self-Reported Fatigue, Physical Function and Obesity. PLoS ONE 2019, 14, e0217462. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a Simple Screening Test for Sarcopenia in Older Adults. Geriatr. Gerontol. Int. 2014, 14, 93–101. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Boström, C.; Mattsson, M.; Kouidi, E.; Dimitroulas, T.; Liem, S.I.E.; Vlieland, T.P.M.V.; de Vries-Bouwstra, J.K.; Jacobsen, S.; Cuomo, G.; et al. Exploring the Effects of a Combined Exercise Programme on Pain and Fatigue Outcomes in People with Systemic Sclerosis: Study Protocol for a Large European Multi-Centre Randomised Controlled Trial. Trials 2022, 23, 962. [Google Scholar] [CrossRef]
- Cuomo, G.; Santoriello, C.; Polverino, F.; Ruocco, L.; Valentini, G.; Polverino, M. Impaired Exercise Performance in Systemic Sclerosis and Its Clinical Correlations. Scand. J. Rheumatol. 2010, 39, 330–335. [Google Scholar] [CrossRef]
- Tu, X.; Lin, T.; Ju, Y.; Shu, X.; Jiang, T.; Ge, N.; Yue, J. Sarcopenia in Systemic Sclerosis: Prevalence and Impact—A Systematic Review and Meta-Analysis. BMJ Open 2024, 14, e078034. [Google Scholar] [CrossRef]
- Sari, A.; Esme, M.; Aycicek, G.S.; Armagan, B.; Kilic, L.; Ertenli, A.I.; Halil, M.G.; Akdogan, A. Evaluating Skeletal Muscle Mass with Ultrasound in Patients with Systemic Sclerosis. Nutrition 2021, 84, 110999. [Google Scholar] [CrossRef]
- Corallo, C.; Fioravanti, A.; Tenti, S.; Pecetti, G.; Nuti, R.; Giordano, N. Sarcopenia in Systemic Sclerosis: The Impact of Nutritional, Clinical, and Laboratory Features. Rheumatol. Int. 2019, 39, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Siegert, E.; March, C.; Otten, L.; Makowka, A.; Preis, E.; Buttgereit, F.; Riemekasten, G.; Müller-Werdan, U.; Norman, K. Prevalence of Sarcopenia in Systemic Sclerosis: Assessing Body Composition and Functional Disability in Patients with Systemic Sclerosis. Nutrition 2018, 55–56, 51–55. [Google Scholar] [CrossRef]
- Paolino, S.; Goegan, F.; Cimmino, M.A.; Casabella, A.; Pizzorni, C.; Patanè, M.; Schenone, C.; Tomatis, V.; Sulli, A.; Gotelli, E.; et al. Advanced Microvascular Damage Associated with Occurence of Sarcopenia in Systemic Sclerosis Patients: Results from a Retrospective Cohort Study. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 1), 65–72. [Google Scholar] [PubMed]
- Caimmi, C.; Caramaschi, P.; Venturini, A.; Bertoldo, E.; Vantaggiato, E.; Viapiana, O.; Ferrari, M.; Lippi, G.; Frulloni, L.; Rossini, M. Malnutrition and Sarcopenia in a Large Cohort of Patients with Systemic Sclerosis. Clin. Rheumatol. 2018, 37, 987–997. [Google Scholar] [CrossRef]
- Sangaroon, A.; Foocharoen, C.; Theerakulpisut, D.; Srichompoo, K.; Mahakkanukrauh, A.; Suwannaroj, S.; Seerasaporn, P.; Pongchaiyakul, C. Prevalence and Clinical Association of Sarcopenia among Thai Patients with Systemic Sclerosis. Sci. Rep. 2022, 12, 18198. [Google Scholar] [CrossRef]
- Hax, V.; do Espírito Santo, R.C.; dos Santos, L.P.; Farinon, M.; de Oliveira, M.S.; Três, G.L.; Gasparin, A.A.; de Andrade, N.P.B.; Bredemeier, M.; Xavier, R.M.; et al. Practical Screening Tools for Sarcopenia in Patients with Systemic Sclerosis. PLoS ONE 2021, 16, e0245683. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.C.K.; Vasi, İ.; Kılıç, H.K.; Erden, A.; Gündoğdu, O.; Kardaş, R.C.; Küçük, H.; Alp, G.T.; Bölek, E.Ç.; Kesen, S.; et al. Is Myopenia or Myosteatosis Clinically Relevant in Systemic Sclerosis? Skeletal Muscle Assessment Using Computed Tomography. Acad. Radiol. 2025. [Google Scholar] [CrossRef]
- Ajdynan, M.; Melkonyan, N.; Zhuravleva, N.; Guryanova, E.; Diomidova, V. AB0865 Body Composition Assessment in Patients with Systemic Scleroderma. Ann. Rheum. Dis. 2023, 82, 1645–1646. [Google Scholar]
- Rincón, I.D.R.; Alak, M.; Alsina, G.; Quevedo, P.; Rivero, M.; Duartes, D. Sarcopenia in Systemic Sclerosis. In Proceedings of the 2019 ACR/ARP Annual Meeting, Atlanta, GA, USA, 8–13 November 2019; Volume 71, p. 1657. [Google Scholar]
- Dougados, M.; Baeten, D. Spondyloarthritis. Lancet 2011, 377, 2127–2137. [Google Scholar] [CrossRef]
- Merle, B.; Cottard, M.; Sornay-Rendu, E.; Szulc, P.; Chapurlat, R. Spondyloarthritis and Sarcopenia: Prevalence of Probable Sarcopenia and Its Impact on Disease Burden: The Saspar Study. Calcif. Tissue Int. 2023, 112, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Kanjanavaikoon, N.; Saisirivechakun, P.; Chaiamnuay, S. Age, Body Mass Index, and Function as the Independent Predictors of Sarcopenia in Axial Spondyloarthritis: A Cross-Sectional Analysis. Clin. Rheumatol. 2023, 42, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.; Torres, R.P.; Ramiro, S.; Sardoo, A.; Rodrigues-Manica, S.; Lagoas-Gomes, J.; Domingues, L.; Crespo, C.L.; Teixeira, D.; Sepriano, A.; et al. Muscle Dysfunction in Axial Spondylarthritis: The MyoSpA Study. Clin. Exp. Rheumatol. 2022, 40, 267–273. [Google Scholar] [CrossRef]
- Yurdakul, O.V.; Ince, O.E.; Bagcier, F.; Kara, M.; Kultur, E.; Aydin, T. Evaluating the Strength of Spinal and Proximal Girdle Muscles in Patients with Axial Spondyloarthritis: Correlation with Activity, Disability, and Functionality. Int. J. Rheum. Dis. 2021, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Breban, M.; Lories, R.; Schett, G.; Sieper, J. Are Spondylarthritides Related but Distinct Conditions or a Single Disease with a Heterogeneous Phenotype? Arthritis Rheum. 2013, 65, 12–20. [Google Scholar] [CrossRef]
- Kao, C.-I.; Liau, B.-Y.; Lai, K.-L.; Kuo, F.-C. Correlation Among Disease Activity, Musculoskeletal Function, and Quality of Life in Patients with Ankylosing Spondylitis with Mild to Moderate Radiographic Signs. J. Med. Biol. Eng. 2023, 43, 147–155. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Ebo’O, F.B.; Sadni, S.; Majjad, A.; Hamza, T.; Mounach, A. Is There a Relation between Pre-Sarcopenia, Sarcopenia, Cachexia and Osteoporosis in Patients with Ankylosing Spondylitis? BMC Musculoskelet. Disord. 2016, 17, 268. [Google Scholar] [CrossRef]
- Scotti, L.; Franchi, M.; Marchesoni, A.; Corrao, G. Prevalence and Incidence of Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2018, 48, 28–34. [Google Scholar] [CrossRef]
- Takami, K.; Higashiyama, M.; Tsuji, S. Sarcopenia and Osteoporosis in Patients with Psoriatic Arthritis: A Single-Center Retrospective Study. Nutrition 2025, 129, 112595. [Google Scholar] [CrossRef]
- Aguiar, R.; Sequeira, J.; Meirinhos, T.; Ambrósio, C.; Barcelos, A. SARCOSPA—Sarcopenia in Spondyloarthritis Patients. Acta Reumatol. Port. 2014, 39, 322–326. [Google Scholar]
- Krajewska-Wlodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Changes in Body Composition and Bone Mineral Density in Postmenopausal Women with Psoriatic Arthritis. Reumatologia 2017, 55, 215–221. [Google Scholar] [CrossRef]
- Takami, K.; Higashiyama, M.; Tsuji, S. Osteoporosis and Osteopenia in Patients with Psoriatic Arthritis: A Single-Centre Retrospective Study. Mod. Rheumatol. 2024, 34, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Pena, É.; dos Santos, L.P.; do Espírito Santo, R.C.; Guaresi, S.; Hirakata, V.N.; Karnopp, T.E.; Xavier, R.M.; Monticielo, O.A. Systemic Lupus Erythematosus: A Systematic Review with Meta-Analysis on Muscle Strength, Muscle Mass, and Physical Function. Clin. Rheumatol. 2023, 42, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Bilici, R.; Candemir, B.; Satış, H.; Alp, G.T.; Borazan, F.Y.; Deniz, O.; Guler, A.A.; Karadeniz, H.; Varan, H.D.; Tufan, A.; et al. Frequency of Sarcopenia in Turkish Women with Systemic Lupus Erythematosus. Lupus Sci. Med. 2024, 11, e001204. [Google Scholar] [CrossRef]
- Pena, E.; Santo, R.C.D.E.; Dos Santos, L.; Denardi Dória, L.; Pilotti, S.; Nóbrega de Moraes, D.; Mallmann, A.L.; Gasparin, A.A.; Hax, V.; Muniz Fighra, T.; et al. POS1497 Prevalence of Sarcopenia in Women with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2023, 82, 1105–1106. [Google Scholar] [CrossRef]
- Lieber, S.; Paget, S.; Berman, J.; Barbhaiya, M.; Sammaritano, L.; Kirou, K.; Carrino, J.; Sheira, D.; Finik, J.; Mandl, L. Frailty and Sarcopenia in Women with Systemic Lupus Erythematosus. In Proceedings of the 2019 ACR/ARP Annual Meeting, Atlanta, GA, USA, 8–13 November 2019; Volume 71. [Google Scholar]
- Ravelli, A.; Martini, A. Juvenile Idiopathic Arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Thatayatikom, A.; Modica, R.; De Leucio, A. Juvenile Idiopathic Arthritis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Grammatikopoulou, M.G.; Gkiouras, K.; Syrmou, V.; Vassilakou, T.; Simopoulou, T.; Katsiari, C.G.; Goulis, D.G.; Bogdanos, D.P. Nutritional Aspects of Juvenile Idiopathic Arthritis: An A to Z for Dietitians. Children 2023, 10, 203. [Google Scholar] [CrossRef]
- Kulyk, M.; Dzhus, M. Factors Associated with Sarcopenia among Young Adults with Juvenile Idiopathic Arthritis: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2024, 25, 923. [Google Scholar] [CrossRef]
- Slitine, I.; Hittinger, A.; Charlot-Lambrecht, I.; Loïs, B.; Geoffroy, M.; Salmon, J.H. AB1440 Assessment of the Prevalence of Sarcopenia in Adult with Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2023, 82, 1948. [Google Scholar] [CrossRef]
- Fox, R.I. Sjögren’s Syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Llobell-Uriel, A.; Gonzalez-Mazon, I.; Retamozo, S.; Secada-Gómez, C.; Martín-Gutiérrez, A.; García Castaño, A.; Gallardo-Diaz, E.; Gómez-Centeno, A.; Fernandez-Morales, L.; Gratacós, J.; et al. AB1537 Multicentric Analysis of Rheumatologic Adverse Events Related to Cancer Immunotherapy in Two Spanish Hospitals. Ann. Rheum. Dis. 2024, 83, 2139–2140. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Pellicano, C.; Colalillo, A.; Alunni-Fegatelli, D.; Muscaritoli, M. Phase Angle, Nutritional Status, and Mortality in Systemic Sclerosis: An Exploratory Pilot Study. Nutrition 2023, 107, 111946. [Google Scholar] [CrossRef]
- Öztürk, Ö.; Feyzioğlu, Ö.; Sarıtaş, F. Pre-Sarcopenia Is Associated with Health-Related Quality of Life in Patients with Primary Sjögren’s Syndrome. Clin. Rheumatol. 2023, 42, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Tekgoz, E.; Hayme, S.; Sonaeren, I.M.; Cinar, M.; Yilmaz, S. The Risk of Presarcopenia Is Increased Among Female Patients With Primary Sjögren’s Syndrome. JCR J. Clin. Rheumatol. 2022, 28, e161–e165. [Google Scholar] [CrossRef]
- Alunno, A.; Ruscio, E.D.; Cappannari, S.; Carugno, M.E.; Matone, E.; Olivieri, I.; Serio, L.; Mariani, F.M.; Altieri, P.; Ferri, C.; et al. AB0814 Sarcopenia and Sarcopenic Obesity in Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2024, 83, 1702. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Immune Disorders and Disability. Selected Immune Disorders and Disability; National Academies Press: Washington, DC, USA, 2022; ISBN 978-0-309-68949-6. [Google Scholar]
- Giannini, M.; Charles, A.L.; Debrut, L.; Goupilleau, F.; Blaess, J.; Javier, R.M.; Geny, B.; Meyer, A. POS1206 Sarcopenia Is a Marker of Muscle Damage Associated with Disease Severity and Disability in Patients with Inflammatory Myopathies. Ann. Rheum. Dis. 2023, 82, 936. [Google Scholar] [CrossRef]
- Giannini, M.; Debrut, L.; Charles, A.-L.; Pizzimenti, M.; Javier, R.-M.; Geny, B.; Meyer, A. Sarcopenia in Myositis Patients: A Marker of Muscle Damage Associated with Myositis Severity and Disability. Arthritis Rheumatol. 2022, 74, 0160. [Google Scholar]
- Giannini, M.; Charles, A.L.; Pizzimenti, M.; Debrut, L.; Levy, D.A.; Javier, R.M.; Geny, B.; Meyer, A. POS0876 Sarcopenia in Myositis Patients: A Marker of Muscle Damage Associated with Handicap. Ann. Rheum. Dis. 2021, 80, 694. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Tang, X.; Huang, S.; Qiu, L. Quantitative Assessment of Muscle Properties in Polymyositis and Dermatomyositis Using High-Frequency Ultrasound and Shear Wave Elastography. Quant. Imaging Med. Surg. 2023, 13, 428–440. [Google Scholar] [CrossRef]
- McLeish, E.; Slater, N.; Sooda, A.; Wilson, A.; Coudert, J.D.; Lloyd, T.E.; Needham, M. Inclusion Body Myositis: The Interplay between Ageing, Muscle Degeneration and Autoimmunity. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101761. [Google Scholar] [CrossRef]
- Yamashita, S. Late-Onset Primary Muscle Diseases Mimicking Sarcopenia. Geriatr. Gerontol. Int. 2024, 24, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Fang, Y.-H.D.; Jones, C.; McConathy, J.E.; Raman, F.; Lapi, S.E.; Younger, J.W. Evidence of Neuroinflammation in Fibromyalgia Syndrome: A [18F]DPA-714 Positron Emission Tomography Study. Pain 2023, 164, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Beiner, E.; Lucas, V.; Reichert, J.; Buhai, D.-V.; Jesinghaus, M.; Vock, S.; Drusko, A.; Baumeister, D.; Eich, W.; Friederich, H.-C.; et al. Stress Biomarkers in Individuals with Fibromyalgia Syndrome: A Systematic Review with Meta-Analysis. Pain 2023, 164, 1416–1427. [Google Scholar] [CrossRef]
- Gkouvi, A.; Tsiogkas, S.G.; Bogdanos, D.P.; Gika, H.; Goulis, D.G.; Grammatikopoulou, M.G. Proteomics in Patients with Fibromyalgia Syndrome: A Systematic Review of Observational Studies. Curr. Pain Headache Rep. 2024, 28, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- da Cruz, D.S.; Almanza, A.P.M.C.; de Oliveira-Júnior, S.A.; Coelho-Ravagnani, C.d.F.; Martinez, P.F. Evaluation of Functional Capacity and Sarcopenia in Adult Women with and without Fibromyalgia. Braz. J. Phys. Ther. 2024, 28, 100697. [Google Scholar] [CrossRef]
- Koca, I.; Savas, E.; Ozturk, Z.A.; Boyaci, A.; Tutoglu, A.; Alkan, S.; Yildiz, H.; Kimyon, G. The Evaluation in Terms of Sarcopenia of Patients with Fibromyalgia Syndrome. Wien. Klin. Wochenschr. 2016, 128, 816–821. [Google Scholar] [CrossRef]
- Góes, S.M.; Leite, N.; Shay, B.L.; Homann, D.; Stefanello, J.M.F.; Rodacki, A.L.F. Functional Capacity, Muscle Strength and Falls in Women with Fibromyalgia. Clin. Biomech. 2012, 27, 578–583. [Google Scholar] [CrossRef]
- Kapuczinski, A.; Soyfoo, M.S.; De Breucker, S.; Margaux, J. Assessment of Sarcopenia in Patients with Fibromyalgia. Rheumatol. Int. 2022, 42, 279–284. [Google Scholar] [CrossRef]
- Jatwani, S.; Goyal, A. Vasculitis; StatPearls, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Henriquez, S.; Dunogué, B.; Porcher, R.; Régent, A.; Cohen, P.; Berezne, A.; Kolta, S.; Le Jeunne, C.; Mouthon, L.; Roux, C.; et al. Handgrip Strength Is a Comorbidity Marker in Systemic Necrotizing Vasculitides and Predicts the Risk of Fracture and Serious Adverse Events. Rheumatology 2020, 59, 2581–2590. [Google Scholar] [CrossRef]
- Conticini, E.; D’Alessandro, M.; Al Khayyat, S.G.; D’Alessandro, R.; D’Ignazio, E.; Pata, A.P.; Vallifuoco, G.; Falsetti, P.; Baldi, C.; Bardelli, M.; et al. Inflammatory Muscle Involvement in Systemic Vasculitis: A Systematic Review. Autoimmun. Rev. 2022, 21, 103029. [Google Scholar] [CrossRef]
- Pinto, M.V.; Warrington, K.J.; Soontrapa, P.; Koster, M.J.; Naddaf, E. Vasculitic Myopathy. Neurology 2024, 103, e210141. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Kikuchi, J.; Hiramoto, K.; Akiyama, M.; Saito, S.; Kondo, Y.; Kaneko, Y. Sarcopenia in Patients with Rheumatic Musculoskeletal Diseases. Int. J. Rheum. Dis. 2023, 26, 2007–2013. [Google Scholar] [CrossRef]
- Castañeda Ureña, M.; Cerpa Cruz, S.; Martinez Bonilla, G.E.; Gutierrez Ureña, S.; Bernard Medina, A.G.; Rodriguez Orozco, V.; Lopez Rodriguez, A.; Ayala Arzola, M.; Veloz Hernandez, C.; Perez Romero, M.; et al. AB0718 Autoimmune Sarcopenia in Patients with Rheumatoid Arthritis and Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2012, 71, 679. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic Obesity: Epidemiology, Pathophysiology, Cardiovascular Disease, Mortality, and Management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Baker, J.F.; Giles, J.T.; Weber, D.; George, M.D.; Leonard, M.B.; Zemel, B.S.; Long, J.; Katz, P. Sarcopenic Obesity in Rheumatoid Arthritis: Prevalence and Impact on Physical Functioning. Rheumatology 2022, 61, 2285–2294. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, S685–S699. [Google Scholar] [CrossRef]
- Hasegawa, E.; Ito, S.; Kurosawa, Y.; Kobayashi, D.; Otani, H.; Abe, A.; Nakazono, K.; Murasawa, A.; Narita, I.; Ishikawa, H. The Efficacy of Biological Disease-Modifying Antirheumatic Drugs on Sarcopenia in Patients with Rheumatoid Arthritis. Intern. Med. 2023, 62, 373–379. [Google Scholar] [CrossRef]
- Hein, T.R.; Peterson, L.; Bartikoski, B.J.; Portes, J.; Espírito Santo, R.C.; Xavier, R.M. The Effect of Disease-Modifying Anti-Rheumatic Drugs on Skeletal Muscle Mass in Rheumatoid Arthritis Patients: A Systematic Review with Meta-Analysis. Arthritis Res. Ther. 2022, 24, 171. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Ben Tekaya, A.; Mehmli, T.; Ben Sassi, M.; Teyeb, Z.; Bouden, S.; Rouached, L.; Mahmoud, I.; Dziri, C.; Abdelmoula, L. Effects of Biologic and Target Synthetic Disease-Modifying Anti-Rheumatic Drugs on Sarcopenia in Spondyloarthritis and Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2023, 42, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Kirk, B.; Phu, S.; Vogrin, S.; Duque, G. Prevalence of Sarcopenia and Its Association with Antirheumatic Drugs in Middle-Aged and Older Adults with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Calcif. Tissue Int. 2021, 109, 475–489. [Google Scholar] [CrossRef]
- Bermejo-Álvarez, I.; Pérez-Baos, S.; Gratal, P.; Medina, J.P.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Effects of Tofacitinib on Muscle Remodeling in Experimental Rheumatoid Sarcopenia. Int. J. Mol. Sci. 2023, 24, 13181. [Google Scholar] [CrossRef]
- Chen, S.-E.; Jin, B.; Li, Y.-P. TNF-α Regulates Myogenesis and Muscle Regeneration by Activating P38 MAPK. Am. J. Physiol. Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Vial, G.; Lambert, C.; Pereira, B.; Couderc, M.; Malochet-Guinamand, S.; Mathieu, S.; Pickering, M.E.; Soubrier, M.; Tournadre, A. The Effect of TNF and Non-TNF-Targeted Biologics on Body Composition in Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Wong-Pack, M.; Liu, T.; Adachi, J.; Lau, A.; Ma, J.; Papaioannou, A.M.; Rodrigues, I.B.P. Risk Factors and Clinical Outcomes Associated With Sarcopenia in Rheumatoid Arthritis. JCR J. Clin. Rheumatol. 2024, 30, 18–25. [Google Scholar] [CrossRef]
- Hanaoka, B.Y.; Zhao, J.; Heitman, K.; Khan, F.; Jarjour, W.; Volek, J.; Brock, G.; Gower, B.A. Interaction Effect of Systemic Inflammation and Modifiable Rheumatoid Cachexia Risk Factors on Resting Energy Expenditure in Patients with Rheumatoid Arthritis. JCSM Clin. Rep. 2022, 7, 12–23. [Google Scholar] [CrossRef]
- Mok, C.C.; To, C.H.; Ma, K.M. Changes in Body Composition after Glucocorticoid Therapy in Patients with Systemic Lupus Erythematosus. Lupus 2008, 17, 1018–1022. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between Dietary Nutrient Intake and Sarcopenia in the SarcoPhAge Study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does Nutrition Play a Role in the Prevention and Management of Sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Nutrition and Sarcopenia: A Review of the Evidence of Nutritional Influences. Crit. Rev. Food Sci. Nutr. 2019, 59, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Henríquez Sánchez, P.; Ruano, C.; De Irala, J.; Ruiz-Canela, M.; Martínez-González, M.A.; Sánchez-Villegas, A. Adherence to the Mediterranean Diet and Quality of Life in the SUN Project. Eur. J. Clin. Nutr. 2012, 66, 360–368. [Google Scholar] [CrossRef]
- Shahar, D.R.; Houston, D.K.; Hue, T.F.; Lee, J.S.; Sahyoun, N.R.; Tylavsky, F.A.; Geva, D.; Vardi, H.; Harris, T.B. Adherence to Mediterranean Diet and Decline in Walking Speed over 8 Years in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2012, 60, 1881–1888. [Google Scholar] [CrossRef]
- Hanach, N.I.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A High Whey Protein, Vitamin D and E Supplement Preserves Muscle Mass, Strength, and Quality of Life in Sarcopenic Older Adults: A Double-Blind Randomized Controlled Trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Chang, M.C.; Choo, Y.J. Effects of Whey Protein, Leucine, and Vitamin D Supplementation in Patients with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Lemmey, A.B.; Jones, J.G.; Sheikh, F.; Ahmad, Y.A.; Chitale, S.; Maddison, P.J.; O’Brien, T.D. Can Creatine Supplementation Improve Body Composition and Objective Physical Function in Rheumatoid Arthritis Patients? A Randomized Controlled Trial. Arthritis Care Res. 2016, 68, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with Eicosapentaenoic Acid and Docosahexaenoic Acid Reduces High Levels of Circulating Proinflammatory Cytokines in Aging Adults: A Randomized, Controlled Study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Liang, J.; Xiao, W.; Li, Y. Relationship Between Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids Level and Sarcopenia. A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 8, 738083. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Chiu, W.-C.; Hsu, Y.-P.; Lo, Y.-L.; Wang, Y.-H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef]
- Therdyothin, A.; Prokopidis, K.; Galli, F.; Witard, O.C.; Isanejad, M. The Effects of Omega-3 Polyunsaturated Fatty Acids on Muscle and Whole-Body Protein Synthesis: A Systematic Review and Meta-Analysis. Nutr. Rev. 2025, 83, e131–e143. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Cordingley, D.M.; Shaw, K.A.; Forbes, S.C.; Leonhardt, T.; Bristol, A.; Candow, D.G.; Chilibeck, P.D. Effects of Omega-3 Supplementation Alone and Combined with Resistance Exercise on Skeletal Muscle in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2221. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus Paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2022, 14, 4566. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef]
- Yang, C.; Song, Y.; Li, T.; Chen, X.; Zhou, J.; Pan, Q.; Jiang, W.; Wang, M.; Jia, H. Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Older Adults with Sarcopenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Nutr. Health Aging 2023, 27, 329–339. [Google Scholar] [CrossRef]
- Su, H.; Zhou, H.; Gong, Y.; Xiang, S.; Shao, W.; Zhao, X.; Ling, H.; Chen, G.; Tong, P.; Li, J. The Effects of β-Hydroxy-β-Methylbutyrate or HMB-Rich Nutritional Supplements on Sarcopenia Patients: A Systematic Review and Meta-Analysis. Front. Med. 2024, 11, 1348212. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between Sarcopenia and Physical Activity in Older People: A Systematic Review and Meta-Analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- The IOF-ESCEO Sarcopenia Working Group; Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; et al. Nutrition and Physical Activity in the Prevention and Treatment of Sarcopenia: Systematic Review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, X.; Feng, Y.; Ainsworth, B.E.; Liu, Y. Effects of Resistance Training in Healthy Older People with Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Rev. Aging Phys. Act. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Lourenzi, F.M.; Jones, A.; Pereira, D.F.; dos Santos, J.H.C.A.; Furtado, R.N.V.; Natour, J. Effectiveness of an Overall Progressive Resistance Strength Program for Improving the Functional Capacity of Patients with Rheumatoid Arthritis: A Randomized Controlled Trial. Clin. Rehabil. 2017, 31, 1482–1491. [Google Scholar] [CrossRef]
- Baillet, A.; Vaillant, M.; Guinot, M.; Juvin, R.; Gaudin, P. Efficacy of Resistance Exercises in Rheumatoid Arthritis: Meta-Analysis of Randomized Controlled Trials. Rheumatology 2012, 51, 519–527. [Google Scholar] [CrossRef]
- Hagfors, L.; Leanderson, P.; Sköldstam, L.; Andersson, J.; Johansson, G. Antioxidant Intake, Plasma Antioxidants and Oxidative Stress in a Randomized, Controlled, Parallel, Mediterranean Dietary Intervention Study on Patients with Rheumatoid Arthritis. Nutr. J. 2003, 2, 5. [Google Scholar] [CrossRef]
- Andersson, S.E.M.; Lange, E.; Kucharski, D.; Svedlund, S.; Önnheim, K.; Bergquist, M.; Josefsson, E.; Lord, J.M.; Mårtensson, I.-L.; Mannerkorpi, K.; et al. Moderate-to High Intensity Aerobic and Resistance Exercise Reduces Peripheral Blood Regulatory Cell Populations in Older Adults with Rheumatoid Arthritis. Immun. Ageing 2020, 17, 12. [Google Scholar] [CrossRef]
- Liao, C.-D.; Chen, H.-C.; Huang, S.-W.; Liou, T.-H. Exercise Therapy for Sarcopenia in Rheumatoid Arthritis: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. Clin. Rehabil. 2022, 36, 145–157. [Google Scholar] [CrossRef]
- Yun, H.W.; Kim, C.J.; Ahn, J.A.; Schlenk, E.A. Effects of a Self-Determination Theory-Based Physical Activity Programme for Postmenopausal Women with Rheumatoid Arthritis: A Randomized Controlled Trial. Int. J. Nurs. Pract. 2023, 29, e13199. [Google Scholar] [CrossRef]
- Siqueira, U.S.; Orsini Valente, L.G.; de Mello, M.T.; Szejnfeld, V.L.; Pinheiro, M.M. Effectiveness of Aquatic Exercises in Women With Rheumatoid Arthritis. Am. J. Phys. Med. Rehabil. 2017, 96, 167–175. [Google Scholar] [CrossRef] [PubMed]
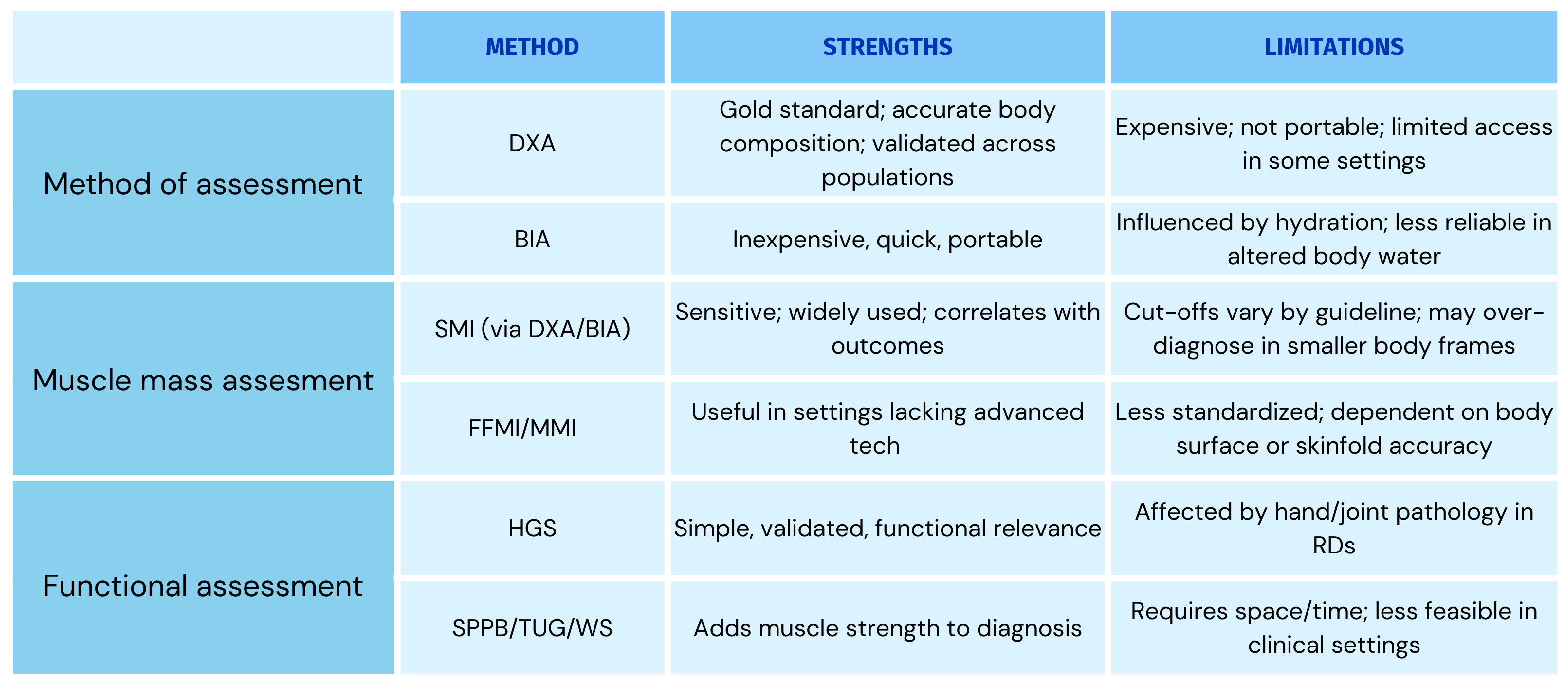

| Diagnostic Criteria | Muscle Mass Determination | Threshold Values | Method(s) of Assessment | |
|---|---|---|---|---|
| Men | Women | |||
| EWGSOP 2010 | SMMI œ | - | 6.42 kg/m2 | BIA |
| SMI § | 7.23–8.87 kg/m2 | 5.45–6.75 kg/m2 | DXA, BIA | |
| FFMI ¤ | 18 kg/m2 | 15 kg/m2 | Skinfold thickness, DXA | |
| MMI Ñ | 10.75 kg/m2 | 6.75 kg/m2 | BIA | |
| SMI š | - | 27.6% | BIA | |
| ASMI | 7.26 kg/m2 | 5.5 kg/m2 | BIA | |
| EWGSOP 2019 | SMI § | 7.0 kg/m2 | 5.5–5.7 kg/m2 | DXA, BIA |
| SMMI œ | 10.76 kg/m2 | 6.76 kg/m2 | BIA | |
| SMMI Ø | 9.2 kg/m2 | 7.4 kg/m2 | BIA | |
| SMI § | 6.0 kg/m2 | 5.5 kg/m2 | DXA | |
| AWGS 2014 | SMI § | 7.0 kg/m2 | 5.7 kg/m2 | BIA or DXA |
| AWGS 2019 | SMI § | 7.0 kg/m2 | 5.4–5.7 kg/m2 | BIA or DXA |
| FNIH | SMI ₩ | 0.789 kg/m2 | 0.512 kg/m2 | DXA |
| SARC-F | N/A | N/A | N/A | N/A |
| SMI | SMI § | 7.26 kg/m2 | 5.5 kg/m2 | DXA |
| MMI | MMI ª | Grade I: 8.51–10.75 kg/m2 Grade II: <8.51 kg/m2 | Grade I: 5.76–6.75 kg/m2 Grade II: <5.76 kg/m2 | Skinfold thickness |
| FFMI | FFMI ¤ | ≤2 SD below the mean of a reference Caucasian sample | BIA | |
| Diagnostic Criteria | Biological Function | Association with Sarcopenia |
|---|---|---|
| IL-6 | cytokine involved in inflammation and myokine signaling | suppresses IGF-1 and activates JAK/STAT pathway, increasing myolytic enzyme expression and muscle catabolism |
| TNF-α | pro-inflammatory cytokine | activation of NF-κB, muscle apoptosis, and breakdown via downregulation of myogenic and upregulation of myolytic enzymes |
| IL-1β | pro-inflammatory cytokine involved in immune signaling | promotes catabolic activity in muscle, contributing to muscle wasting and sarcopenia |
| IL-8 | chemokine involved in inflammation and neutrophil recruitment | elevated levels linked to neutrophil dysregulation, muscle damage, and sarcopenia |
| CRP | acute-phase protein; marker of systemic inflammation | elevated levels associated with muscle degradation, sarcopenia, and frailty |
| ESR | non-specific marker of systemic inflammation | elevated levels linked to sarcopenia, reduced strength, and poor physical performance |
| Adiponectin-to-leptin ratio | anti- and pro-inflammatory adipokines | adiponectin inhibits NF-kB, while leptin stimulates the secretion of IL-6 and TNF-α |
| Neutrophil-to-lymphocyte ratio | indicator of immune system status and systemic inflammation | a greater ratio is negatively associated with fat-free mass |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Bilici [105] | 82 | SLE | 12.9 | EWGSOP 2019 | SMI ₩, HGS | 0.823 (kg/[kg/m2]) for women | BIA |
| Pardali [71] | 28 | SLE | 25 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Pena [106] | 49 | SLE | 16.3 | EWGSOP 2019 | FFMI ¤, HGS, chair rise test | <15 kg/m2 for women | DXA |
| Santos [72] | 92 | SLE | 10.9 | FFMI | FFMI ¤ | ≤2 SD below the mean of a reference Caucasian sample | BIA |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Kulyk [111] | 70 | JIA | 59 | EWGSOP 2019 | SMI §, HGS, 4MWS | 7.0 kg/m2 for men and 5.7 kg/m2 for women | DXA |
| Slitine [112] | 34 | JIA | 20.6/11.7 | EWGSOP 2019 | SMI §, HGS, SARC-F | EWGSOP: 7.0 kg/m2 for men and 5.5 kg/m2 for women and Alternative: SMI-2SD compared with age- and sex-matched reference values from a Danish cohort | DXA |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Alunno [118] | 144 | pSS | 27 | SARC-F | SARC-F | N/A | N/A |
| Colak [117] | 44 | pSS | 25 | EWGSOP 2019 | SMMI Ø, HGS, 6MWS | 9.2 kg/m2 for men and 7.4 kg/m2 for women | BIA |
| Öztürk [116] | 49 | pSS | 28.5 | EWGSOP 2019 | SMMI Ø, HGS, 4MWS, sit-to-stand | 9.2 kg/m2 for men and 7.4 kg/m2 for women | BIA |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Giannini [122] | 29 | myositis | 13.8 | EWGSOP 2019 | SMI §, HGS, 6MWT, number of squats | NR | DXA |
| Giannini [121] | 34 | myositis | 20.6 | EWGSOP 2019 | SMI §, HGS, 6MWT, number of squats | NR | DXA |
| Giannini [120] | 40 | myositis | 17.5 | EWGSOP 2019 | SMI §, HGS, 6MWT | NR | DXA |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Kapuczinski [133] | 45 | FMS | 0 | EWGSOP 2010 | SMMI œ, HGS, SPPB, SARC-F | 6.42 kg/m2 for women | ΒΙA |
| Koca [131] | 150 | FMS | 8.7 | EWGSOP 2010 | SMI §, HGS, MWS | 6.75 kg/m2 for women | BIA |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnosis | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Henriquez [135] | 120 | vasculitis | 0 | EWGSOP 2010 | SMI §, HGS | 7.23 kg/m2 for men and 5.67 kg/m2 for women | DXA |
| Pardali [71] | 32 | vasculitis | 15.6 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| First Author | Sample | Sarcopenia | Muscle Mass | ||||
|---|---|---|---|---|---|---|---|
| N | Diagnoses | Prevalence (%) | Diagnostic Criteria | Methods of Assessment | Threshold Values | Method of Assessment | |
| Hanaoka [138] | 49 | RDs | 83.7 | AWGS 2019 | SMI § | 7.0 kg/m2 for men and 5.4 kg/m2 for women | DXA |
| Pardali [71] | 220 | RDs | 15.9 | EWGSOP 2010 | FFMI ¤, HGS | 18 kg/m2 for men and 15 kg/m2 for women | Skinfold thickness |
| Ureña [139] | 46 | RDs | 26 | NR | NR | NR | DXA |
| First Author | Sample | Sarcopenic Obesity Prevalence (%) | |
|---|---|---|---|
| N | Diagnoses | ||
| Ajdynan [89] | 43 | SSc | 4.7 |
| Baker [142] | 444 | RA | 12.6 |
| Dobrovolskaya [65] | 91 | RA | 18.7 |
| Guzmán-Guzmán [54] | 223 | RA | 44 |
| Mena-Vázquez [52] | 94 | RA | NR |
| Santos [72] | 92/89 | SLE/RA | 6.5/5.6 |
| Pardali [71] | 220 | Mixed RDs | 0.05 |
| Vlietstra [76] | 82 | RA | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardali, E.C.; Klonizakis, M.; Goulis, D.G.; Papadopoulou, S.K.; Cholevas, C.; Giaginis, C.; Tsigalou, C.; Bogdanos, D.P.; Grammatikopoulou, M.G. Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern. Diseases 2025, 13, 134. https://doi.org/10.3390/diseases13050134
Pardali EC, Klonizakis M, Goulis DG, Papadopoulou SK, Cholevas C, Giaginis C, Tsigalou C, Bogdanos DP, Grammatikopoulou MG. Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern. Diseases. 2025; 13(5):134. https://doi.org/10.3390/diseases13050134
Chicago/Turabian StylePardali, Eleni C., Markos Klonizakis, Dimitrios G. Goulis, Sousana K. Papadopoulou, Christos Cholevas, Constantinos Giaginis, Christina Tsigalou, Dimitrios P. Bogdanos, and Maria G. Grammatikopoulou. 2025. "Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern" Diseases 13, no. 5: 134. https://doi.org/10.3390/diseases13050134
APA StylePardali, E. C., Klonizakis, M., Goulis, D. G., Papadopoulou, S. K., Cholevas, C., Giaginis, C., Tsigalou, C., Bogdanos, D. P., & Grammatikopoulou, M. G. (2025). Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern. Diseases, 13(5), 134. https://doi.org/10.3390/diseases13050134










