Right Ventricular Geometry and Function in Pulmonary Hypertension: Non-Invasive Evaluation
Abstract
:1. Introduction
2. The Right Ventricle
3. Echocardiography
3.1. Right Ventricular Myocardial Deformation by Speckle Tracking Echocardiography


3.2. Three-Dimensional Echocardiography

| Author | Three-dimensional echocardiography | ||
|---|---|---|---|
| End-diastolic volume (mL) | End-systolic volume (mL) | Ejection fraction (%) | |
| Grapsa et al. | −4 (−11, 4) | 0 (−6, 6) | −1 (−3, 0) |
| Sugeng et al. | −14 (−28, 0) | −9 (−19, 1) | −2 (−4, 0) |
| van der Zwaan et al. | −34 (−43, −25) | −11 (−19, 3) | −4 (−6, −2) |
| Leibundgut et al. [66] | −10 (−15, −6) | −5 (−8, −1) | 0 (−2, 1) |
| Shimada et al. [67] | −14 (−18, −10) | −6 (−8, −3) | −1 (−2, 0) |
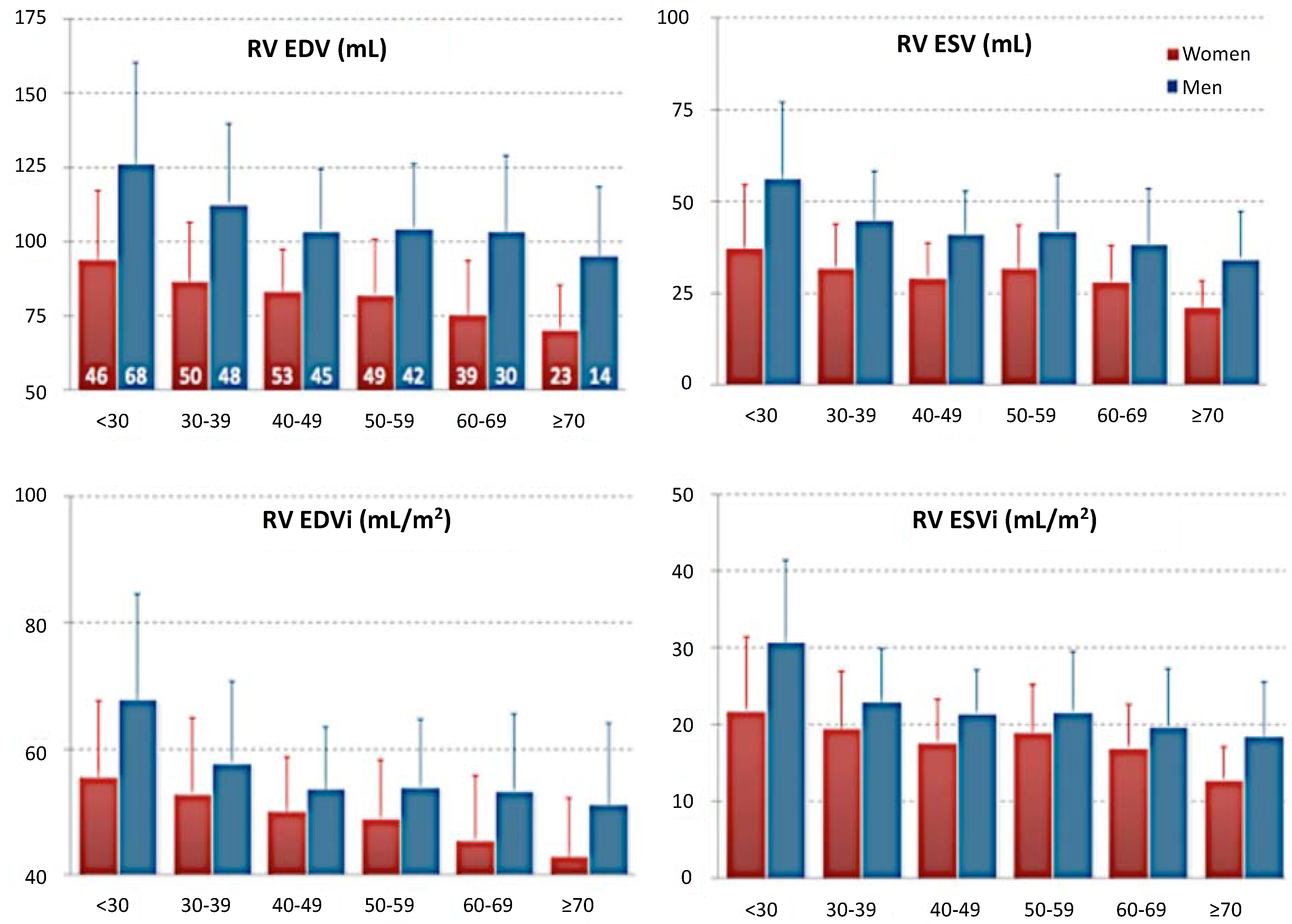

4. Cardiac Magnetic Resonance

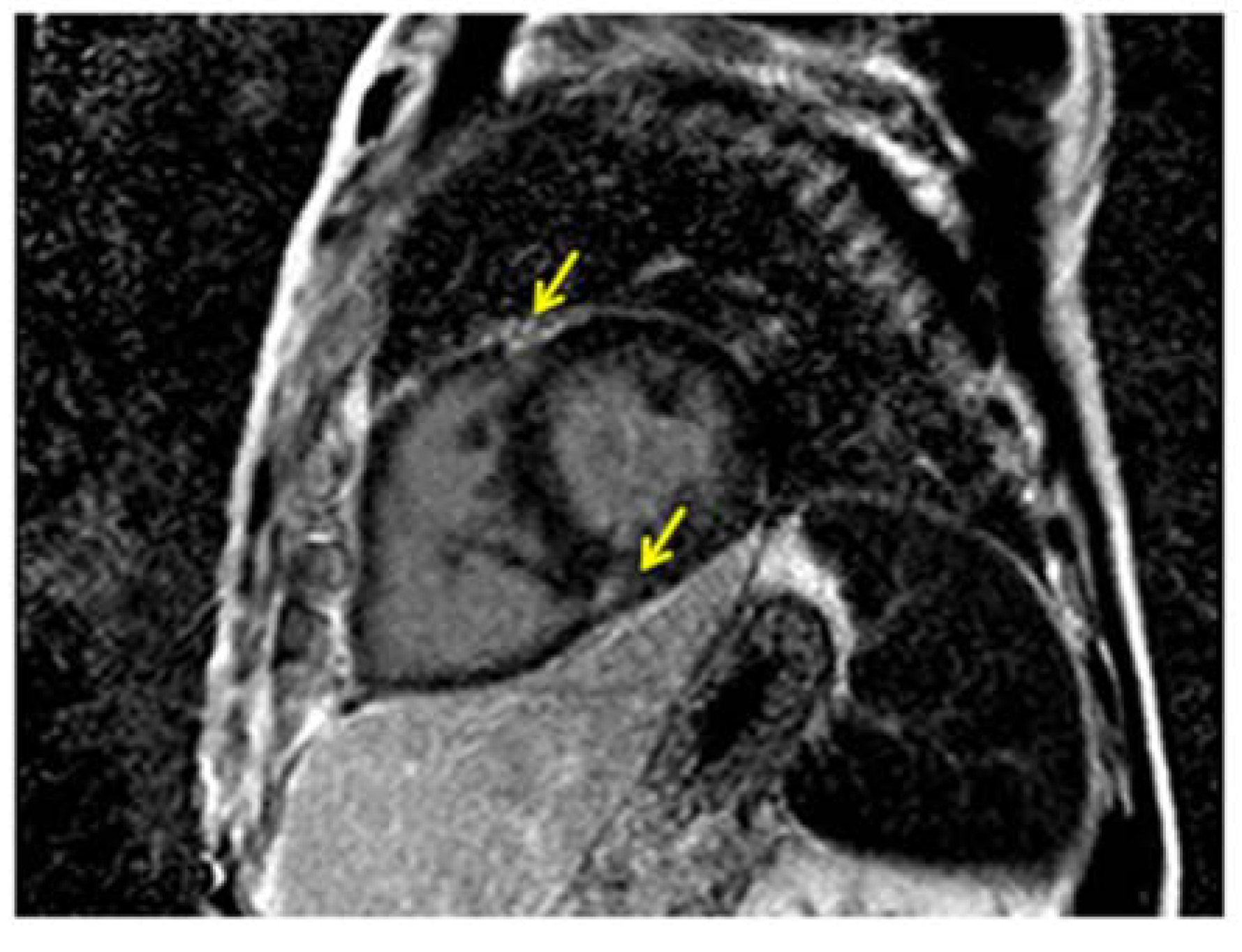
5. Multi Detector Cardiac Tomography
6. Nuclear Imaging
| Advantages | Disadvantages | |
|---|---|---|
| Echocardiography | widely available; easily repeatable; independent from cardiac rhythm; complete and accurate RV function assessment by 3D echo and STE; no exposure to radiation | operator-dependent; acoustic window-dependent |
| Cardiac Magnetic Resonance | I independent from acoustic window; no exposure to radiation; quantification of RV mass; evaluation of myocardial tissue by Late Gadolinium Enhancement | presence of contraindications such as arrhytmias, claustrophobia, end-stage renal disease, implanted methallic devices |
| Multi Detector Computed Tomography | independent from acoustic window; provides information on the adjacent lung parenchyma | exposure to radiation; use of iodinated contrast agent injection |
| Nuclear Imaging | independent from acoustic window; opportunity to assess myocardial perfusion and metabolism. | exposure to radiation; lack of validated automatic measurements algorithms |
7. Conclusions
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| CMR | cardiac magnetic resonance |
| FPRNA | first pass planar equilibrium radionuclide angiocardiography |
| LGE | late gadolinium enhancement |
| MDCT | multi detector cardiac tomography |
| PH | pulmonary hypertension |
| RHC | right heart catheterization |
| RV | right ventricle/ventricular |
| SPECT | single photon emission computed tomography |
| STE | speckle tracking echocardiography |
| TAPSE | tricuspid annular plane systolic excursion |
Author Contributions
Conflicts of Interest
References
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar]
- Humbert, M.; Sitbon, O.; Yaïci, A.; Montani, D.; O’Callaghan, D.S.; Jaïs, X.; Parent, F.; Savale, L.; Natali, D.; Günther, S.; et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur. Respir. J. 2010, 36, 549–555. [Google Scholar]
- van Wolferen, S.A.; Marcus, J.T.; Boonstra, A.; Marques, K.M.J.; Spreeuwenberg, M.D.; Postmus, P.E.; Bronzwaer, J.G.F.; Vonk-Noordegraaf, A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur. Heart J. 2007, 28, 1250–1257. [Google Scholar]
- Zafrir, N.; Zingerman, B.; Solodky, A.; Ben-Dayan, D.; Sagie, A.; Sulkes, J.; Mats, I.; Kramer, M.R. Use of noninvasive tools in primary pulmonary hypertension to assess the correlation of right ventricular function with functional capacity and to predict outcome. Int. J. Cardiovasc. Imaging 2007, 23, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kawut, S.M.; Horn, E.M.; Berekashvili, K.K.; Garofano, R.P.; Goldsmith, R.L.; Widlitz, A.C.; Rosenzweig, E.B.; Kerstein, D.; Barst, R.J. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am. J. Cardiol. 2005, 95, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Raymond, R.J.; Hinderliter, A.L.; Willis, P.W.; Ralph, D.; Caldwell, E.J.; Williams, W.; Ettinger, N.A.; Hill, N.S.; Summer, W.R.; de Boisblanc, B.; et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2002, 39, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Klersy, C.; Magrini, G.; D'Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Vigano, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Badesch, D.B.; Champion, H.C.; Sanchez, M.A.; Hoeper, M.M.; Loyd, J.E.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and assessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. 1), S55–S66. [Google Scholar]
- Thenappan, T.; Shah, S.J.; Rich, S.; Tian, L.; Archer, S.L.; Gomberg-Maitland, M. Survival in pulmonary arterial hypertension: A reappraisal of the NIH risk stratification equation. Eur. Respir. J. 2010, 35, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Farber, H.W.; Loscalzo, J. Pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Presberg, K.W.; Doyle, R.L.; Abman, S.H.; McCrory, D.C.; Fortin, T.; Ahearn, G. Prognosis of pulmonary arterial hypertension: Accp evidence-based clinical practice guidelines. Chest 2004, 126, 78S–92S. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.I.; Mogulkoc, N.; Bright-Thomas, R.J.; Bishop, P.; Egan, J.J.; Ray, S.G. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J. Am. Soc. Echocardiogr. 2002, 15, 633–639. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, M.C.; Kind, T.; Marcus, J.T.; Mauritz, G.-J.; Heymans, M.W.; Bogaard, H.-J.; Boonstra, A.; Marques, K.M.J.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J. Am. Coll. Cardiol. 2011, 58, 2511–2519. [Google Scholar]
- Badano, L.P.; Ginghina, C.; Easaw, J.; Muraru, D.; Grillo, M.T.; Lancellotti, P.; Pinamonti, B.; Coghlan, G.; Perazzolo Marra, M.; Popescu, B.A.; et al. Right ventricle in pulmonary hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur. J. Echoc. 2010, 11, 27–37. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92 (Suppl. 1), i2–i13. [Google Scholar]
- Valsangiacomo Buechel, E.R.; Mertens, L.M. Imaging the right heart: The use of integrated multimodality imaging. Eur. Heart J. 2012, 33, 949–960. [Google Scholar]
- Kukulski, T.; Hübbert, L.; Arnold, M.; Wranne, B.; Hatle, L.; Sutherland, G.R. Normal regional right ventricular function and its change with age: A Doppler myocardial imaging study. J. Am. Soc. Echocardiogr. 2000, 13, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Haber, I.; Metaxas, D.N.; Geva, T.; Axel, L. Three-dimensional systolic kinematics of the right ventricle. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1826–H1833. [Google Scholar] [CrossRef] [PubMed]
- Selton-Suty, C.; Juilliere, Y. Non-invasive investigations of the right heart: How and why? Arch. Cardiovasc. Dis. 2009, 102, 219–232. [Google Scholar]
- Geva, T.; Powell, A.J.; Crawford, E.C.; Chung, T.; Colan, S.D. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation 1998, 98, 339–345. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Come, P.C.; Goldhaber, M.S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quintana, D.; Anderson, R.H.; Ho, S.Y. Ventricular myoarchitecture in tetralogy of Fallot. Heart 1996, 76, 280–286. [Google Scholar]
- Pettersen, E.; Helle-Valle, T.; Edvardsen, T.; Lindberg, H.; Smith, H.J.; Smevik, B.; Smiseth, O.A.; Andersen, K. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J. Am. Coll. Cardiol. 2007, 49, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Rushmer, R.F.; Crystal, D.K.; Wagner, C. The functional anatomy of ventricular contraction. Circ. Res. 1953, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Mauritz, G.J.; Marcus, J.T.; van de Veerdonk, M.; Westerhof, N.; Vonk-Noordegraaf, A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J. Cardiovasc. Magn. Res. 2010, 12. [Google Scholar] [CrossRef]
- Mauritz, G.-J.; Kind, T.; Marcus, J.T.; Bogaard, H.-J.; van de Veerdonk, M.; Postmus, P.E.; Boonstra, A.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012, 141, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; De Boeck, B.W.; Olimulder, M.; Prakken, N.H.; Doevendans, P.A.; Cramer, M.J. Echocardiographic assessment of regional right ventricular function: A head-to-head comparison between 2-dimensional and tissue Doppler-derived strain analysis. J. Am. Soc. Echocardiogr. 2008, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Scherptong, R.W.; Mollema, S.A.; Blom, N.A.; Kroft, L.J.; de Roos, A.; Vliegen, H.W.; van der Wall, E.E.; Bax, J.J.; Holman, E.R. Right ventricular peak systolic longitudinal strain is a sensitive marker for right ventricular deterioration in adult patients with tetralogy of Fallot. Int. J. Cardiovasc. Imaging 2009, 25, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Dragulescu, A.; Mertens, L.L. Developments in echocardiographic techniques for the evaluation of ventricular function in children. Arch. Cardiovasc. Dis. 2010, 103, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.L.; Friedberg, M.K. Imaging the right ventricle—Current state of the art. Nat. Rev. Cardiol. 2010, 7, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Pirat, B.; McCulloch, M.L.; Zoghbi, W.A. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am. J. Cardiol. 2006, 98, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Tanaka, H.; Sugiyama, D.; Ryo, K.; Onishi, T.; Fukuya, H.; Nogami, M.; Ohno, Y.; Emoto, N.; Kawai, H.; et al. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2011, 24, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McGoon, M.D.; Hsiao, J.F.; Maalouf, J.F.; Ammash, N.M.; McCully, R.B.; Miller, F.A.; Pellikka, P.A.; et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011, 139, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Haeck, M.L.; Scherptong, R.W.; Antoni, M.L.; Marsan, N.A.; Vliegen, H.W.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V. Right ventricular longitudinal peak systolic strain measurements from the subcostal view in patients with suspected pulmonary hypertension: A feasibility study. J. Am. Soc. Echocardiogr. 2012, 25, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Haeck, M.L.; Scherptong, R.W.; Ajmone Marsan, N.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Vliegen, H.W.; Delgado, V. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Meris, A.; Faletra, F.; Conca, C.; Klersy, C.; Regoli, F.; Klimusina, J.; Penco, M.; Pasotti, E.; Pedrazzini, G.B.; Moccetti, T.; et al. Timing and Magnitude of Regional Right Ventricular Function: A Speckle Tracking-Derived Strain Study of Normal Subjects and Patients with Right Ventricular Dysfunction. J. Am. Soc. Echocardiogr. 2010, 23, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Grover, S.; Molaee, P.; Chakrabarty, A.; Shirazi, M.; Cheng, Y.H.; Penhall, A.; Perry, R.; Greville, H.; Joseph, M.X.; et al. Nonvolumetric Echocardiographic Indices of Right Ventricular Systolic Function: Validation with Cardiovascular Magnetic Resonance and Relationship with Functional Capacity. Echocardiography 2012, 29, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Puwanant, S.; Park, M.; Popovic, Z.B.; Tang, W.H.; Farha, S.; George, D.; Sharp, J.; Puntawangkoon, J.; Loyd, J.E.; Erzurum, S.C.; et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010, 121, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Dambrauskaite, V.; Delcroix, M.; Claus, P.; Herbots, L.; D’Hooge, J.; Bijnens, B.; Rademakers, F.; Sutherland, G.R. Regional right ventricular dysfunction in chronic pulmonary hypertension. J. Am. Soc. Echocardiogr. 2007, 20, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Motoji, Y.; Tanaka, H.; Fukuda, Y.; Ryo, K.; Emoto, N.; Kawai, H.; Hirata, K. Efficacy of right ventricular free-wall longitudinal speckle-tracking strain for predicting long-term outcome in patients with pulmonary hypertension. Circ. J. 2013, 77, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.; Oh, J.K.; Kane, G.C. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imag. 2013, 6, 711–721. [Google Scholar] [CrossRef]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar]
- Tedford, R.J.; Hassoun, P.M.; Mathai, S.C.; Girgis, R.E.; Russell, S.D.; Thiemann, D.R.; Cingolani, O.H.; Mudd, J.O.; Borlaug, B.A.; Redfield, M.M.; et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012, 125, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Rajagopalan, N.; Mathier, M.A.; Shroff, S.G.; Pinsky, M.R.; López-Candales, A. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest. Heart Fail. 2009, 15, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Reichek, N. Right ventricular strain in pulmonary hypertension. Flavor du jour enduring prognostic index? Circul. Cardiovasc. Imag. 2013, 6, 611–613. [Google Scholar]
- La Gerche, A.; Ruxandra, J.; Voigt, J.-U. Right ventricular function by strain echocardiography. Curr. Opin. Cardiol. 2010, 25, 430–436. [Google Scholar]
- Hardegree, E.L.; Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McCoon, M.D.; Kushwaha, S.S.; Hsiao, J.-F.; McCully, R.B.; Oh, J.K.; Pellikka, P.A.; et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am. J. Cardiol. 2013, 111, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, A.; Ishizu, T.; Kameda, Y.; Yamamoto, M.; Harimura, Y.; Machino-Ohtsuka, T.; Kawamura, R.; Enomoto, M.; Seo, Y.; Aonuma, K. Application of 3-dimensional speckle tracking imaging to the assessment of right ventricular regional deformation. Circul. J. 2013, 77, 1760–1768. [Google Scholar] [CrossRef]
- Di Salvo, G.; Pacileo, G.; Rea, A.; Limongelli, G.; Baldini, L.; D’Andrea, A.; D’Alto, M.; Sarubbi, B.; Russo, M.G.; Calabrò, R. Transverse strain predicts exercise capacity in systemic right ventricle patients. Int. J. Cardiol. 2010, 145, 193–196. [Google Scholar]
- Schindera, S.T.; Mehwald, P.S.; Sahn, D.J.; Kececioglu, D. Accuracy of real time three-dimensional echocardiography for quantifying right ventricular volume. J. Ultrasound Med. 2002, 21, 1069–1075. [Google Scholar] [PubMed]
- Nesser, H.J.; Tkalec, W.; Patel, A.R.; Masani, N.D.; Niel, J.; Markt, B.; Pandian, N.G. Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: Comparison with magnetic resonance imaging and radionuclide ventriculography. Echocardiography 2006, 23, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, K.; Huang, G. In vitro validation of right ventricular volume and mass measurement by real- time three dimensional echocardiography. Echocardiography 2006, 23, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Angelini, E.D.; Homma, S.; Pearson, G.; Holmes, J.W.; Laine, A.F. Segmentation of real time three dimensional ultrasound for quantification of ventricular function: A clinical study on right and left ventricles. Ultrasound Med. Biol. 2005, 31, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Chan, J.; Bricknell, K.; Strudwick, M.; Marwick, T.H. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: comparison with cardiac MRI. Chest 2007, 131, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Maffesanti, F.; Muraru, D.; Esposito, R.; Gripari, P.; Ermacora, D.; Santoro, C.; Tamborini, G.; Galderisi, M.; Pepi, M.; Badano, L.P. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography. Circ. Cardiovasc. Imag 2013, 6, 700–710. [Google Scholar] [CrossRef]
- Chua, S.; Levine, R.A.; Yosefy, C.; Handschumacher, M.D.; Chu, J.; Qureshi, A.; Neary, J.; Ton-Nu, T.-T.; Fu, M.; Jen Wu, C.; et al. Assessment of right ventricular function by real-time three-dimensional echocardiography improves accuracy and decreases interobserver variability compared with conventional two-dimensional views. Eur. J. Echocardiogr. 2009, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Niemann, P.S.; Pinho, L.; Balbach, T.; Galuschky, C.; Blankenhagen, M.; Silberbach, M.; Broberg, C.; Jerosch-Herold, M.; Sahn, D.J. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-Tesla magnetic resonance imaging. J. Am. Coll. Cardiol. 2007, 50, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Boccalini, F.; Muraru, D.; Dal Bianco, L.; Peluso, D.; Bellu, R.; Zoppellaro, G.; Iliceto, S. Current Clinical Applications of Transthoracic Three-Dimensional Echocardiography. J. Cardiovasc. Ultrasound 2012, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nadvoretskiy, B.L.; Stolpen, A.; Ayres, N.; Pignatelli, R.H.; Kovalchin, J.P.; Grenier, M.; Klas, B.; Ge, S. Accuracy and reproducibility of real-time three-dimensional echocardiography for assessment of right ventricular volumes and ejection fraction in children. J. Am. Soc. Echocardiogr. 2008, 21, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Grison, A.; Maschietto, N.; Reffo, E.; Stellin, G.; Padalino, M.; Vida, V.; Milanesi, O. Three-dimensional echocardiographic evaluation of right ventricular volume and function in pediatric patients: validation of the technique. J. Am. Soc. Echocardiogr. 2007, 20, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Leibundgut, G.; Rohner, A.; Grize, L.; Bernheim, A.; Kessel-Schaefer, A.; Bremerich, J.; Zellweger, M.; Buser, P.; Handke, M. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: A comparison study with magnetic resonance imaging in 100 adult patients. J. Am. Soc. Echocardiogr. 2010, 23, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.J.; Shiota, M.; Siegel, R.J.; Shiota, T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: A meta-analysis study. J. Am. Soc. Echocardiogr. 2010, 23, 943–995. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.J.; Rajaram, S.; Condliffe, R.; Capener, D.; Hurdman, J.; Elliot, C.A.; Wild, J.M.; Kiely, D.G. Diagnostic accuracy of cardiovascular magnetic resonance imaging of right ventricular morphology and function in the assessment of suspected pulmonary hypertension results from the ASPIRE registry. J. Cardiovasc. Magn. Res. 2012, 14. [Google Scholar] [CrossRef]
- Swift, A.J.; Rajaram, S.; Hurdman, J.; Hill, C.; Davies, C.; Sproson, T.W.; Morton, A.C.; Capener, D.; Elliot, C.; Condliffe, R.; et al. Noninvasive Estimation of PA Pressure, Flow, and Resistance With CMR Imaging. Derivation and Prospective Validation Study From the ASPIRE Registry. JACC Cardiovasc. Imag. 2013, 6, 1036–1047. [Google Scholar]
- Morikawa, T.; Murata, M.; Okuda, S.; Tsuruta, H.; Iwanaga, S.; Murata, M.; Satoh, T.; Ogawa, S.; Fukuda, K. Quantitative Analysis of Right Ventricular Function in Patients With Pulmonary Hypertension Using Three-Dimensional Echocardiography and a Two-Dimensional Summation Method Compared to Magnetic Resonance Imaging. Am. J. Cardiol. 2011, 107, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Majdalany, D.; Syed, I.; Pellikka, P.; Warnes, C.A. Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: Comparison with magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2010, 23, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.B.; Sun, J.P.; Gao, R.F.; Lee, A.P.-W.; Feng, Y.L.; Liu, X.R.; Sheng, W.; Liu, F.; Yang, X.S.; Fang, F.; et al. Feasibility of single-beat full-volume capture real-time three-dimensional echocardiography for quantification of right ventricular volume: validation by cardiac magnetic resonance imaging. Int. J. Cardiol. 2013, 168, 3991–3995. [Google Scholar] [CrossRef] [PubMed]
- Calcutteea, A.; Chung, R.; Lindqvist, P.; Hodson, M.; Henein, M.Y. Differential right ventricular regional function and the effect of pulmonary hypertension: Three-dimensional echo study. Heart 2011, 97, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Shu, X.; Pan, C.; Cheng, L.; Dong, L.; Yao, H.; Zhou, D. Evaluation of right ventricular regional volume and systolic function in patients with pulmonary arterial hypertension using three-dimensional echocardiography. Echocardiography 2012, 29, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, V.; Conte, L.; Delle Donne, M.G.; Giannini, C.; Barletta, V.; Fabiani, I.; Palagi, C.; Nardi, C.; Dini, F.L.; Marconi, L.; et al. Advantages of real time three-dimensional echocardiography in the assessment of right ventricular volumes and function in patients with pulmonary hypertension compared with conventional two-dimensional echocardiography. Echocardiography 2013, 30, 820–828. [Google Scholar]
- Vitarelli, A.; Barillà, F.; Capotosto, L.; D’Angeli, I.; Truscelli, G.; De Maio, M.; Ashurov, R. Right ventricular function in acute pulmonary embolism: A combined assesment by three-dimensional and speckle tracking echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, W.M.; Gibbs, J.S.R.; Mohiaddin, RH. Cardiovascular magnetic resonance in pulmonary hypertension. J. Cardiovasc. Magn. Res. 2012, 14. [Google Scholar] [CrossRef]
- Mogelvang, J.; Stokholm, K.H.; Stubgaard, M. Assessment of right ventricular volumes by magnetic resonance imaging and by radionuclide angiography. Am. J. Noninvasive Cardiol. 1991, 5, 321. [Google Scholar]
- Katz, J.; Whang, J.; Boxt, L.M.; Barst, R.J. Estimation of right ventricular mass in normal subjects and in patients with primary pulmonary hypertension by nuclear magnetic resonance imaging. J. Am. Coll. Cardiol. 1993, 21, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Alfakih, K.; Plein, S.; Thiele, H.; Jones, T.; Ridgway, J.P.; Sivananthan, M.U. Normal human left and right ven- tricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession im- aging sequences. J. Magn. Reson. Imaging 2003, 17, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J.; Geva, T.; Kaemmerer, H.; Trindade, P.T.; Schwitter, J.; Webb, G.D. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur. Heart J. 2010, 31, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.; Prasad, S.; Khan, M.; Pennell, D. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur. Heart J. 2006, 27, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Buechel, E.; Kaiser, T.; Jackson, C.; Schmitz, A.; Kellenberger, C. Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2009, 11. [Google Scholar] [CrossRef]
- Saba, T.S.; Foster, J.; Cockburn, M.; Cowan, M.; Peacock, A.J. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur. Respir. J. 2002, 20, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Hagger, D.; Condliffe, R.; Woodhouse, N.; Elliot, C.A.; Armstrong, I.J.; Davies, C.; Hill, C.; Akil, M.; Wild, J.M.; Kiely, D.G. Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology 2009, 48, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hudsmith, L.E.; Petersen, S.E.; Francis, J.M.; Robson, M.D.; Neubauer, S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.H.; Walker, E.S.; Morgan, V.L.; Klein, S.S.; Graham, T.P., Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 1999, 1, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Hardziyenka, M.; Campian, M.E.; Reesink, H.J.; Surie, S.; Bouma, B.J.; Groenink, M.; Klemens, C.A.; Beekman, L.; Remme, C.A.; Bresser, P.; et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass evidence for atrophic remodeling. J. Am. Coll. Cardiol. 2011, 57, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.T.; Lima, J.A.; Bluemke, D.A.; Chirinos, J.A.; Chahal, H.; Bristow, M.R.; Kronmal, R.A.; Barr, R.G.; Ferrari, V.A.; Propert, K.J.; et al. Regional left ventricular systolic function and the right ventricle: The multi-ethnic study. Chest 2011, 140, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sandstede, J.; Lipke, C.; Beer, M.; Hofmann, S.; Pabst, T.; Kenn, W.; Neubauer, S.; Hahn, D. Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine mag- netic resonance imaging. Eur. Radiol. 2000, 10, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Doherty, N.E., III.; Fujita, N.; Caputo, G.R.; Higgins, C.B. Measurement of right ventricular mass in normal and dilated car- diomyopathic ventricles using cine magnetic resonance imaging. Am. J. Cardiol. 1992, 69, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, M.C.; Dusoswa, S.A.; Marcus, J.T.; Bogaard, H.J.; Spruijt, O.; Kind, T.; Westerhof, N.; Vonk-Noordegraaf, A. The importance of trabecular hypertrophy in right ventricular adaptation to chronic pressure overload. Int. J. Cardiovasc. Imaging 2014, 30, 357–365. [Google Scholar]
- Driessen, M.M.; Baggen, V.J.; Freling, H.G.; Pieper, P.G.; van Dijk, A.P.; Doevendans, P.A.; Snijder, R.J.; Post, M.C.; Meijboom, F.J.; Sieswerda, G.T.; et al. Pressure overloaded right ventricles: A multicenter study on the importance of trabeculae in RV function measured by CMR. Int. J. Cardiovasc. Imag. 2014, 30, 599–608. [Google Scholar] [CrossRef]
- McCann, G.P.; Beek, A.M.; Vonk-Noordegraaf, A.; van Rossum, A.C. Delayed contrast-enhanced magnetic resonance imaging in pulmonary arterial hypertension. Circulation 2005, 112. [Google Scholar] [CrossRef]
- McCann, G.P.; Gan, C.T.; Beek, A.M.; Niessen, H.W.; Vonk Noordegraaf, A.; van Rossum, A.C. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. Am. J. Roentgenol. 2007, 188, 349–355. [Google Scholar] [CrossRef]
- Blyth, K.G.; Groenning, B.A.; Martin, T.N.; Foster, J.E.; Mark, P.B.; Dargie, H.J.; Peacock, A.J. Contrast enhanced cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur. Heart J. 2005, 26, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Dellegrottaglie, S.; Kariisa, M.; Sulica, R.; Poon, M.; O’Donnell, T.P.; Mehta, D.; Fuster, V.; Rajagopalan, S. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am. J. Cardiol. 2007, 100, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, L.; Mahrholdt, H.; Wagner, A.; Choi, K.M.; Elliott, M.D.; Klocke, F.J.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.C.; McKenna, W.J.; McCrohon, J.A.; Elliott, P.M.; Smith, G.C.; Pennell, D.J. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, W.M.; Assomull, R.; Kilner, P.J.; Gibbs, S.R.; Sheppard, M.N.; Mohiaddin, R.H. Understanding late gadolinium enhancement in pulmonary hypertension. Circ. Cardiovasc. Imaging 2010, 3, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tsujino, I.; Ohira, H.; Oyama-Manabe, N.; Ito, Y.M.; Noguchi, T.; Yamada, A.; Ikeda, D.; Watanabe, T.; Nishimura, M. Paradoxical Interventricular Septal Motion as a Major Determinant of Late Gadolinium Enhancement in Ventricular Insertion Points in Pulmonary Hypertension. Plos One 2013, 8. [Google Scholar] [CrossRef]
- Freed, B.H.; Gomberg-Maitland, M.; Chandra, S.; Mor-Avi, V.; Rich, S.; Archer, S.L.; Jamison, E.B., Jr.; Lang, R.M.; Patel, A.R. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J. Cardiovasc. Magn. Res. 2012, 14. [Google Scholar] [CrossRef]
- Shehata, M.L.; Lossnitzer, D.; Skrok, J.; Boyce, D.; Lechtzin, N.; Mathai, S.C.; Girgis, R.E.; Osman, N.; Lima, J.A.; Bluemke, D.A.; et al. Myocardial delayed enhancement in pulmonary hypertension: pulmonary hemodynamics, right ventricular function, and remodeling. Am. J. Roentgenol. 2011, 196, 87–94. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Gaine, S.P.; Howard, L.S.; Leuchte, H.H.; Mathier, M.A.; Mehta, S.; Palazzini, M.; Park, M.H.; Tapson, V.F.; Sitbon, O. Treatment goals of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D73–D81. [Google Scholar] [CrossRef] [PubMed]
- Coche, E.; Vlassenbroek, A.; Roelants, V.; D'Hoore, W.; Verschuren, F.; Goncette, L.; Maldague, B. Evaluation of biventricular ejection fraction with ECG-gated 16-slice CT: Preliminary findings in acute pulmonary embolism in comparison with radionuclide ventriculography. Eur. Radiol. 2005, 15, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.; Kroft, L.J.; Bax, J.J.; Schuijf, J.D.; van der Geest, R.J.; Doornbos, J.; de Roos, A. MDCT assessment of right ventricular systolic function. Am. J. Roentgenol. 2006, 186, S366–S370. [Google Scholar]
- Guo, Y.K.; Gao, H.L.; Zhang, X.C.; Wang, Q.L.; Yang, Z.G.; Ma, E.S. Accuracy and reproducibility of as- sessing right ventricular function with 64-section multi-detector row CT: Comparison with magnetic resonance imaging. Int. J. Cardiol. 2010, 139, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Dupont, M.V.M.; Dra ̈gean, C.A.; Coche, E.E. Right ventricle function assessment by MDCT. Am. J. Roentgenol. 2011, 196, 77–86. [Google Scholar] [CrossRef]
- Lin, F.Y.; Devereux, R.B.; Roman, M.J.; Meng, J.; Jow, V.M.; Jacobs, A.; Weinsaft, J.W.; Shaw, L.J.; Berman, D.S.; Callister, T.Q.; Min, J.K. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc. Imaging 2008, 1, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Plumhans, C.D.; Muehlenbruch, G.; Rapaee, A.; Sim, K.-H.; Seyfarth, T.; Gaenther, R.W.; Mahnken, A.H. Assessment of global right ventricular function on 64-MDCT compared with MRI. Am. J. Roentgenol. 2008, 190, 1358–1361. [Google Scholar] [CrossRef]
- Rich, J.D.; Ward, R.P. Right-ventricular function by nuclear cardiology. Curr. Opin. Cardiol. 2010, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Ramani, G.V.; Gurm, G.; Dilsizian, V.; Park, M.H. Noninvasive assessment of right ventric- ular function: Will there be resurgence in radionuclide imaging techniques? Curr. Cardiol. Rep. Mar. 2010, 12, 162–169. [Google Scholar] [CrossRef]
- Daou, D.V.K.S.; Coaquila, C.; Lebtahi, R.; Fourme, T.; Sitbon, O.; Parent, F.; Slama, M.; Le Guludec, D.; Simonneau, G. Automatic quantification of right ventricular function with gated blood pool SPECT. J. Nucl. Cardiol. 2004, 11, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Prylutska, H.; Ducharme, A.; Finnerty, V.; Grègoire, J.; Marcotte, F.; Harel, F. Evaluation of the right ventricle: Comparison of gated blood-pool single photon electron computed tomography and echocardiography with cardiac magnetic resonance. Int. J. Cardiol. 2014, 171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Goto, Y.; Satoh, T.; Uematsu, S.; Hamada, S.; Kuribayashi, S.; Okano, Y.; Kyotani, S.; Shimotsu, Y.; Fukuchi, K.; et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ven-tricle. J. Nucl. Med. 1998, 39, 1676–1680. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peluso, D.; Tona, F.; Muraru, D.; Romeo, G.; Cucchini, U.; Marra, M.P.; Iliceto, S.; Badano, L.P. Right Ventricular Geometry and Function in Pulmonary Hypertension: Non-Invasive Evaluation. Diseases 2014, 2, 274-295. https://doi.org/10.3390/diseases2030274
Peluso D, Tona F, Muraru D, Romeo G, Cucchini U, Marra MP, Iliceto S, Badano LP. Right Ventricular Geometry and Function in Pulmonary Hypertension: Non-Invasive Evaluation. Diseases. 2014; 2(3):274-295. https://doi.org/10.3390/diseases2030274
Chicago/Turabian StylePeluso, Diletta, Francesco Tona, Denisa Muraru, Gabriella Romeo, Umberto Cucchini, Martina Perazzolo Marra, Sabino Iliceto, and Luigi Paolo Badano. 2014. "Right Ventricular Geometry and Function in Pulmonary Hypertension: Non-Invasive Evaluation" Diseases 2, no. 3: 274-295. https://doi.org/10.3390/diseases2030274
APA StylePeluso, D., Tona, F., Muraru, D., Romeo, G., Cucchini, U., Marra, M. P., Iliceto, S., & Badano, L. P. (2014). Right Ventricular Geometry and Function in Pulmonary Hypertension: Non-Invasive Evaluation. Diseases, 2(3), 274-295. https://doi.org/10.3390/diseases2030274




