Celiac Disease: Diagnostic Standards and Dilemmas
Abstract
:1. Introduction
2. Who Should be Tested for Celiac Disease

| High Risk Patients Routinely Test for CD: Consider Endoscopy even if Serology Negative | Medium Risk Patients Consider CD Serologic Testing: CD Sufficiently Excluded if Serology Negative | Low Risk Patients Consider testing if refractory to standard therapy or other clinically unusual features: CD sufficiently excluded if serology negative |
|---|---|---|
| (1) Chronic gastrointestinal symptoms with a family history of celiac disease or a personal history of autoimmune disease or IgA deficiency (2) Biopsy proven dermatitis herpetiformis (3) Chronic diarrhea (4) Failure to thrive in children (5) Iron deficiency anemia refractory to oral supplementation | (1) Irritable bowel syndrome (2) Elevated liver function tests (3) Iron deficiency anemia (4) Fatigue/lethargy (5) Chronic gastrointestinal symptoms without a family history of celiac disease or a personal history of autoimmune disease (6) Peripheral neuropathy (7) Ataxia (8) Dental enamel defects (9) Recurrent aphthous ulcerations (10) Hyposplenism (11) Fertility abnormalities (12) Down’s or Turner’s syndrome (13) Known IgA deficiency (14) Microscopic colitis | (1) Osteopenia/osteoporosis (2) Fibromyalgia (3) Chronic Fatigue Syndrome (4) Heartburn/GERD (5) Acute or chronic pancreatitis (6) Alopecia (7) Myalgias/Arthralgias (8) Autoimmune liver disease (9) Personal history of autoimmune disease or connective tissue disease without ongoing unexplained symptoms (10) Skin lesions other than dermatitis herpetiformis (11) Headaches including migraines (12) Mood disorders (13) Attention deficit disorder/cognitive impairment (14) Epilepsy (15) Restless leg syndrome |
3. Current Diagnostic Guidelines for Celiac Disease
4. Serologic Tests
5. Genetic Testing
6. Endoscopy and Histology
| Histologic Findings | Marsh 0 | Marsh I | Marsh II | Marsh IIIa | Marsh IIIb | Marsh IIIc |
|---|---|---|---|---|---|---|
| IEL/100 Enterocytes(EC) | <40/100EC | >40/100EC | >40/100EC | >40/100EC | >40/100EC | >40/100EC |
| Villous atrophy | None | None | None | PVA | STVA | TVA |
| Crypt Hyperplasia | None | None | Hyperplastic | Hyperplastic | Hyperplastic | Hyperplastic |
| Villanacci Classification | Type 0 | Type A | Type B | |||
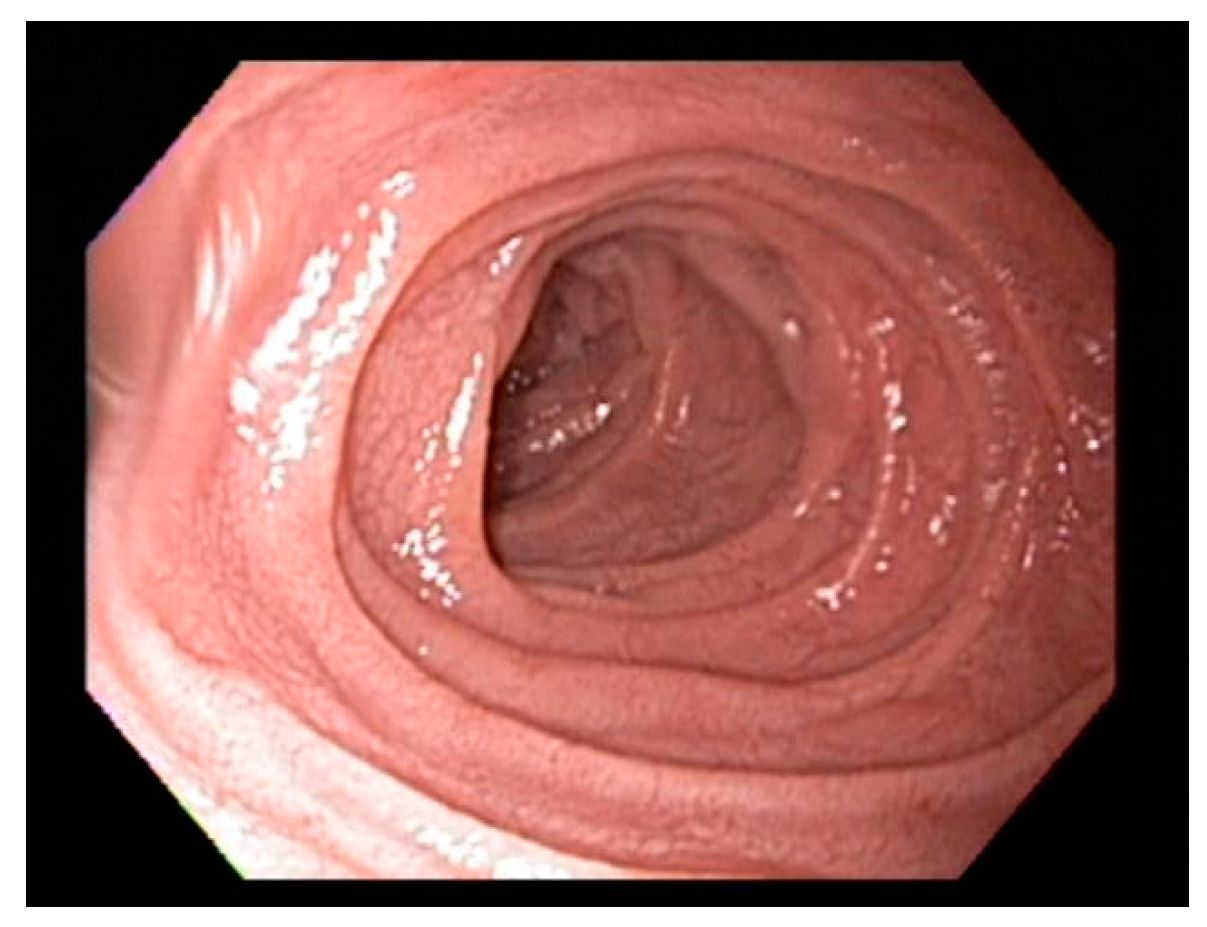
7. Endoscopic Markers of Celiac Disease
8. Dilemmas in Diagnosis of Celiac Disease
8.1. Positive Serology with Normal Biopsy
8.2. Normal Villous Architecture with Duodenal Lymphocytosis
8.3. Negative Serology with Duodenal Biopsy Consistent with Celiac Disease
8.4. Patients on a GFD Prior to Testing Challenge

8.5. Diagnosis in Infants and Young Children
9. Conclusions
Conflicts of Interest
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Murray, J.A.; Kagnoff, M.F. American Gastroenterological Association (AGA) Institute Technical Review on the Diagnosis and Management of Celiac Disease. Gastroenterology 2006, 131, 1981–2002. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Jabri, B. Coeliac disease. Lancet 2003, 362, 383–391. [Google Scholar] [CrossRef]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Rapacciuolo, L.; Zanzi, D.; Franzese, A.; Sblattero, D.; et al. Majority of children with type 1 diabetes produce and deposit anti-tissue transglutaminase antibodies in the small intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Jamma, S.; Rubio-Tapia, A.; Kelly, C.P.; Murray, J.; Najarian, R.; Sheth, S.; Schuppan, D.; Dennis, M.; Leffler, D.A. Celiac Crisis Is a Rare but Serious Complication of Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2010, 8, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Ludvigsson, J.F.; Brantner, T.L.; Murray, J.A; Everhart, J.E. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [PubMed]
- Leffler, D. Celiac disease diagnosis and management: A 46-year-old woman with anemia. JAMA 2011, 306, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Hadjivassiliou, M.; Green, P.H.R.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zeballos, E.; Fried, M.; Corraza, G. World Gastroenterology Organisation Practice Guidelines: Celiac Disease; World Gastroenterology Organisation: Milwaukee, WI, USA, 2013; Volume 48, pp. 1–18. [Google Scholar]
- O’Farrelly, C.; Kelly, J.; Hekkens, W.; Bradley, B.; Thompson, A.; Feighery, C.; Weir, D.G. Alpha gliadin antibody levels: A serological test for coeliac disease. Br. Med. J. Clin. Res. Ed. 1983, 286, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Schuppan, D. Update on serologic testing in celiac disease. Am. J. Gastroenterol. 2010, 105, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Miele, L.; Corazza, G.R.; Gasbarrini, A. When was celiac disease born?: The Italian case from the archeologic site of Cosa. J. Clin. Gastroenterol. 2010, 44, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, M.; Colizzi, V. Tissue transglutaminase: Apoptosis versus autoimmunity. Immunol. Today 1999, 20, 130–134. [Google Scholar] [CrossRef]
- Salmi, T.T.; Collin, P.; Korponay-Szabó, I.R.; Laurila, K.; Partanen, J.; Huhtala, H.; Király, R.; Lorand, L.; Reunala, T.; Mäki, M.; et al. Endomysial antibody-negative coeliac disease: Clinical characteristics and intestinal autoantibody deposits. Gut 2006, 55, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Tosco, A.; Paparo, F.; Auricchio, R.; Granata, V.; Colicchio, B.; Indolfi, V.; Miele, E.; Troncone, R. Serum and intestinal celiac disease-associated antibodies in children with celiac disease younger than 2 years of age. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rashtak, S.; Ettore, M.W.; Homburger, H.A.; Murray, J.A. Combination testing for antibodies in the diagnosis of coeliac disease: Comparison of multiplex immunoassay and ELISA methods. Aliment. Pharmacol. Ther. 2008, 28, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Sugai, E.; Vázquez, H.; Nachman, F.; Moreno, M.L.; Mazure, R.; Smecuol, E.; Niveloni, S.; Cabanne, A.; Kogan, Z.; Gómez, J.C.; et al. Accuracy of Testing for Antibodies to Synthetic Gliadin-Related Peptides in Celiac Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Rostami, K.; Kerckhaert, J.; Tiemessen, R.; von Blomberg, B.M.E.; Meijer, J.W.R.; Mulder, C.J.J. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: Disappointing in clinical practice. Am. J. Gastroenterol. 1999, 94, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E. Evaluation of the INOVA diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin G (IgG) and IgA to deamidated gliadin peptides. Clin. Vaccine Immunol. 2006, 13, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, I.; Olsson, M.; Forooz, N.K.; Sjöholm, A.G.; Truedsson, L.; Hansson, T. Immunoglobulin G (IgG) anti-tissue transglutaminase antibodies used as markers for IgA-deficient celiac disease patients. Clin. Diagn. Lab. Immunol. 2005, 12, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.; Karczewska, K.; Lukasik, M.; Kasner, J.; Dyduch, A.; Zabka, A.; Ronczkowski, S.; Sulej, J. Negative results of antiendomysial antibodies: Long term follow up. Arch. Dis. Child. 2005, 90, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Amarri, S.; Alvisi, P.; de Giorgio, R.; Gelli, M.C.; Cicola, R.; Tovoli, F.; Sassatelli, R.; Caio, G.; Volta, U. Antibodies to deamidated gliadin peptides: An accurate predictor of coeliac disease in infancy. J. Clin. Immunol. 2013, 33, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Lagerqvist, C.; Dahlbom, I.; Hansson, T.; Jidell, E.; Juto, P.; Olcén, P.; Stenlund, H.; Hernell, O.; Ivarsson, A. Antigliadin immunoglobulin A best in finding celiac disease in children younger than 18 months of age. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Jinga, M.; Jurcut, C.; Balaban, V.; Bardas, C.; Laurila, K.; Vasilescu, F.; Ene, A.; Anca, I.; Mäki, M. Fingertip rapid point-of-care test in adult case-finding in coeliac disease. BMC Gastroenterol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Mooney, P.D.; Kurien, M.; Evans, K.E.; Chalkiadakis, I.; Hale, M.F.; Kannan, M.Z.; Courtice, V.; Johnston, A.J.; Irvine, A.J.; Hadjivassiliou, M.; et al. Point-of-care testing for celiac disease has a low sensitivity in endoscopy. Gastrointest. Endosc. 2014, 80, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Dube, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The diagnostic accuracy of serologic tests for celiac disease: A systematic review. Gastroenterology 2005, 128, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, F.; Besson Duvanel, C.; Seignovert, C.; Rouzaire, P.; Lachaux, A.; Bienvenu, J. Evaluation of a point-of-care test based on deamidated gliadin peptides for celiac disease screening in a large pediatric population. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Benkebil, F.; Combescure, C.; Anghel, S.I.; Besson Duvanel, C.; Schäppi, M.G. Diagnostic accuracy of a new point-of-care screening assay for celiac disease. World J. Gastroenterol. 2013, 19, 5111–5117. [Google Scholar] [CrossRef] [PubMed]
- Cronk, D.R.; Houseworth, T.P.; Cuadrado, D.G.; Herbert, G.S.; McNutt, P.M.; Azarow, K.S. Intestinal Fatty Acid Binding Protein (I-FABP) for the Detection of Strangulated Mechanical Small Bowel Obstruction. Curr. Surg. 2006, 63, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, A.C.; Wolters, V.M.; Adriaanse, M.P.; van den Neucker, A.M.; van Bijnen, A.A.; Houwen, R.; Buurman, W.A. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand. J. Gastroenterol. 2011, 46, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Suchy, F.J.; Brannon, P.M.; Carpenter, T.O.; Fernandez, J.R.; Gilsanz, V.; Gould, J.B.; Hall, K.; Hui, S.L.; Lupton, J.; Mennella, J.; et al. National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28–30, 2004. Gastroenterology 2005, 128, S1–S9. [Google Scholar]
- Pallav, K.; Kabbani, T.; Tariq, S.; Vanga, R.; Kelly, C.P.; Leffler, D.A. Clinical Utility of Celiac Disease-Associated HLA Testing. Dig. Dis. Sci. 2014, 59, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Partanen, J.; Mäki, M.; Collin, P. HLA-DQ typing in the diagnosis of celiac disease. Am. J. Gastroenterol. 2002, 97, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Lee, H.-S.; Aronsson, C.A; Hagopian, W.A; Koletzko, S.; Rewers, M.J.; Eisenbarth, G.S.; Bingley, P.J.; Bonifacio, E.; Simell, V.; et al. Risk of pediatric celiac disease according to HLA haplotype and country. N. Engl. J. Med. 2014, 371, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Bourgey, M.; Calcagno, G.; Tinto, N.; Gennarelli, D.; Margaritte-Jeannin, P.; Limongelli, M.G.; Greco, L.; Esposito, O.; Marano, C.; Troncone, R.; et al. HLA related genetic risk for coeliac disease. Gut 2007, 56, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Karell, K.; Louka, A.S.; Moodie, S.J.; Ascher, H.; Clot, F.; Greco, L.; Ciclitira, P.J.; Sollid, L.M.; Partanen, J. HLA types in celiac disease patients not carrying the DQA1 *05-DQB1 *02 (DQ2) heterodimer: Results from the European genetics cluster on celiac disease. Hum. Immunol. 2003, 64, 469–477. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Robins, G.; Howdle, P.D. Advances in coeliac disease. Curr. Opin. Gastroenterol. 2008, 24, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Tonutti, E.; Amarri, S.; Barbato, M.; Barbera, C.; Barera, G.; Bellantoni, A.; et al. Introduction of Gluten, HLA Status, and the Risk of Celiac Disease in Children. N. Engl. J. Med. 2014, 371, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Meini, A.; Pillan, N.M.; Villanacci, V.; Monafo, V.; Ugazio, A.G.; Plebani, A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann. Allergy Asthma Immunol. 1996, 77, 333–336. [Google Scholar] [CrossRef]
- Bao, F.; Green, P.H.R.; Bhagat, G. An update on celiac disease histopathology and the road ahead. Arch. Pathol. Lab. Med. 2012, 136, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.S.; Smith, J.; Rosty, C. Gastrointestinal pathology in celiac disease: A case series of 150 consecutive newly diagnosed patients. Am. J. Clin. Pathol. 2012, 138, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Weir, D.C.; Glickman, J.N.; Roiff, T.; Valim, C.; Leichtner, A.M. Variability of histopathological changes in childhood celiac disease. Am. J. Gastroenterol. 2010, 105, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Pontone, S.; Pontone, P.; Petrarca, L.; Mennini, M.; Standoli, M.; Mastrogiorgio, G.; Bonamico, M.; Magliocca, F.M. Duodenal Bulb in Celiac Adults. J. Clin. Gastroenterol. 2012, 46, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Kapel, R.C.; Neugut, A.I.; Green, P.H.R.; Genta, R.M. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest. Endosc. 2011, 74, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Niveloni, S.; Fiorini, A.; Dezi, R.; Pedreira, S.; Smecuol, E.; Vazquez, H.; Cabanne, A.; Boerr, L.A.; Valero, J.; Kogan, Z.; et al. Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: Assessment of interobserver agreement. Gastrointest. Endosc. 1998, 47, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Pallav, K.; Leffler, D.A.; Tariq, S.; Kabbani, T.; Hansen, J.; Peer, A.; Bhansali, A.; Najarian, R.; Kelly, C.P. Noncoeliac enteropathy: The differential diagnosis of villous atrophy in contemporary clinical practice. Aliment. Pharmacol. Ther. 2012, 35, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V. The histological classification of biopsy in celiac disease: Time for a change? Dig. Liver Dis. 2015, 47, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Brocchi, E.; Tomassetti, P.; Misitano, B.; Epifanio, G.; Corinaldesi, R.; Bonvicini, F.; Gasbarrini, G.; Corazza, G. Endoscopic markers in adult coeliac disease. Dig. Liver Dis. 2002, 34, 177–182. [Google Scholar] [CrossRef]
- Smith, A.D.; Graham, I.; Dudfield, J.; Rose, J.D.R. A prospective endoscopic study of scalloped folds and grooves in the mucosa of the duodenum as signs of villous atrophy. Gastrointest. Endosc. 1998, 47, 461–465. [Google Scholar] [CrossRef]
- Rokkas, T.; Niv, Y. The role of video capsule endoscopy in the diagnosis of celiac disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Nind, G.; Tucker, G.; Nguyen, N.; Holloway, R.; Bate, J.; Shetti, M.; George, B.; Tam, W. Narrow-band imaging in the evaluation of villous morphology: A feasibility study assessing a simplified classification and observer agreement. Endoscopy 2010, 42, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Castellaneta, S.; Pulvirenti, A.; Tonutti, E.; Francavilla, R.; Fasano, A.; Catassi, C. Prevalence and Natural History of Potential Celiac Disease in At-Family-Risk Infants Prospectively Investigated from Birth. J. Pediatr. 2015, 161, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Waisbourd-Zinman, O.; Hojsak, I.; Rosenbach, Y.; Mozer-Glassberg, Y.; Shalitin, S.; Phillip, M.; Shamir, R. Spontaneous Normalization of Anti-Tissue Transglutaminase Antibody Levels Is Common in Children with Type 1 Diabetes Mellitus. Dig. Dis. Sci. 2012, 57, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.T.G.; Greenson, J.K. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch. Pathol. Lab. Med. 2013, 137, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Mino-Kenudson, M.; Deshpande, V.; Lauwers, G.Y. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: An increasing diagnostic problem with a wide differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1020–1025. [Google Scholar] [PubMed]
- Kakar, S.; Nehra, V.; Murray, J.A.; Dayharsh, G.A.; Burgart, L.J. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am. J. Gastroenterol. 2003, 98, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Caselani, F.; Magni, A.; Turini, D.; Ferraresi, A.; Lanzarotto, F.; Villanacci, V.; Carabellese, N.; Ricci, C.; Lanzini, A. Celiac disease with mild enteropathy is not mild disease. Clin. Gastroenterol. Hepatol. 2013, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.-T.; Murray, J.A. Mucosal Recovery and Mortality in Adults with Celiac Disease after Treatment with a Gluten-Free Diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fasano, A. Celiac disease diagnosis: Simple rules are better than complicated algorithms. Am. J. Med. 2010, 123, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Dahill, S.; Nakshabendi, I.; Lee, F.D.; Sturrock, R.D.; Russell, R.I. Duodenal histology, ulceration, and Helicobacter pylori in the presence or absence of non-steroidal anti-inflammatory drugs. Gut 1993, 34, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Verkarre, V.; Suarez, F.; Viallard, J.-F.; Lascaux, A.-S.; Cosnes, J.; Bouhnik, Y.; Lambotte, O.; Bechade, D.; Ziol, M.; et al. The Enteropathy Associated With Common Variable Immunodeficiency: The Delineated Frontiers With Celiac Disease. Am. J. Gastroenterol. 2010, 105, 2262–2275. [Google Scholar] [CrossRef] [PubMed]
- Babbin, B.A.; Crawford, K.; Sitaraman, S.V. Malabsorption Work-up: Utility of Small Bowel Biopsy. Clin. Gastroenterol. Hepatol. 2015, 4, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Brandt, L.; Montgomery, S.M.; Granath, F.; Ekbom, A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2012, 62, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Vanga, R.R.; Kelly, C.P.; Chen, X.; Xu, H.; Schuppan, D.; Theethira, T.G.; Pallav, K.; Najarian, R.M.; Kabbani, T.A.; Dennis, M.; et al. Su1447 Ex Vivo Gluten Challenge Differentiates Patients With Celiac Disease on a Gluten Free Diet From Healthy Individuals. Gastroenterology 2015, 146, S-471. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaswala, D.H.; Veeraraghavan, G.; Kelly, C.P.; Leffler, D.A. Celiac Disease: Diagnostic Standards and Dilemmas. Diseases 2015, 3, 86-101. https://doi.org/10.3390/diseases3020086
Kaswala DH, Veeraraghavan G, Kelly CP, Leffler DA. Celiac Disease: Diagnostic Standards and Dilemmas. Diseases. 2015; 3(2):86-101. https://doi.org/10.3390/diseases3020086
Chicago/Turabian StyleKaswala, Dharmesh H., Gopal Veeraraghavan, Ciaran P. Kelly, and Daniel A. Leffler. 2015. "Celiac Disease: Diagnostic Standards and Dilemmas" Diseases 3, no. 2: 86-101. https://doi.org/10.3390/diseases3020086
APA StyleKaswala, D. H., Veeraraghavan, G., Kelly, C. P., & Leffler, D. A. (2015). Celiac Disease: Diagnostic Standards and Dilemmas. Diseases, 3(2), 86-101. https://doi.org/10.3390/diseases3020086





