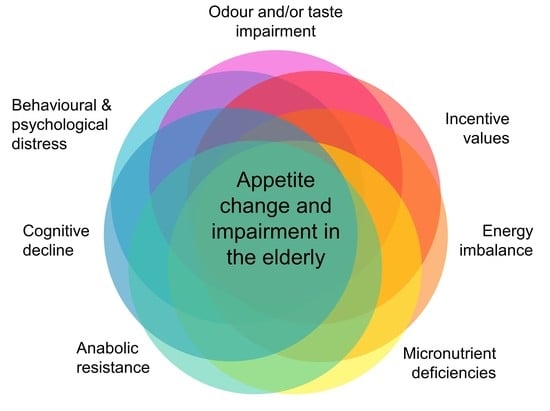
Appetite, Metabolism and Hormonal Regulation in Normal Ageing and Dementia
Abstract
:1. Introduction
2. Can We Maintain Sensitivity against Food Modalities over Age?
3. Is Food Still Perceived as Alluring and Delicious with Age?
4. The Importance of Affect in the Restitution of Appetite Impairment
5. Is Energy and Nutrient Imbalance a Hallmark of Ageing?
6. To Grow or Not to Grow till Late Age?
7. Energy Balance, Micronutrient Deficiencies and Adipokines in Dementia
8. Behavioural and Neurochemical Correlates of Appetite Impairment in Dementia
9. The Emerging Role of Salience Networks in Appetite Regulation
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Linde, R.; Stephan, B.C.; Matthews, F.E.; Brayne, C.; Savva, G.M.; Medical Research Council Cognitive Function and Ageing Study. Behavioural and psychological symptoms in the older population without dementia-relationship with socio-demographics, health and cognition. BMC Geriatr. 2010, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.; Watchman, K.; Janicki, M.P.; Coppus, A.; Gaertner, C.; Fortea, J.; Santos, F.H.; Keller, S.M.; Strydom, A. Consensus statement of the international summit on intellectual disability and Dementia related to post-diagnostic support. Aging Ment. Health 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewczuk, P.; Riederer, P.; O’Bryant, S.E.; Verbeek, M.M.; Dubois, B.; Visser, P.J.; Jellinger, K.A.; Engelborghs, S.; Ramirez, A.; Parnetti, L.; et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 2018, 19, 244–328. [Google Scholar] [CrossRef] [PubMed]
- Papaliagkas, V.; Papantoniou, G.; Tsolaki, M.; Moraitou, D. Self-report instruments of cognitive failures as screening tools for Subjective Cognitive Impairment in older adults. Hell. J. Nucl. Med. 2017, 20, 58–70. [Google Scholar] [PubMed]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Kluger, A.; Franssen, E.; Borenstein, J.; Alba, R.C. The stage specific temporal course of Alzheimer’s disease: Functional and behavioral concomitants based upon cross-sectional and longitudinal observation. Prog. Clin. Biol. Res. 1989, 317, 23–41. [Google Scholar] [PubMed]
- Hays, N.P.; Roberts, S.B. The anorexia of aging in humans. Physiol. Behav. 2006, 88, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Kellerhals, M.B.; Nichols, T.E. Age differences in the brain mechanisms of good taste. Neuroimage 2015, 113, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Fauth, E.B.; Gibbons, A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int. J. Geriatr. Psychiatry 2014, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, M.; Castro, J.; Molano, A.; Zarranz, J.J.; Rodrigo, R.M.; Ortega, R. Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr. Alzheimer Res. 2008, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Brown, J.; Holland, A.J.; Fukuhara, R.; Hodges, J.R. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Hashimoto, M.; Amano, K.; Tanaka, H.; Fukuhara, R.; Ikeda, M. Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS ONE 2015, 10, e0133666. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, C.E.; Tam, C.; Chan, M.; Young, K.W.; Binns, M.A.; van Reekum, R. Behavioral disturbances, not cognitive deterioration, are associated with altered food selection in seniors with Alzheimer’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 499–505. [Google Scholar] [CrossRef]
- Nagata, T.; Shinagawa, S.; Nakajima, S.; Plitman, E.; Mihashi, Y.; Hayashi, S.; Mimura, M.; Nakayama, K. Classification of Neuropsychiatric Symptoms Requiring Antipsychotic Treatment in Patients with Alzheimer’s Disease: Analysis of the CATIE-AD Study. J. Alzheimers Dis. 2016, 50, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, P.; Hamshere, M.L.; Moskvina, V.; Dowzell, K.; Moore, P.J.; Foy, C.; Archer, N.; Lynch, A.; Lovestone, S.; Brayne, C.; et al. Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer’s disease. J. Am. Geriatr. Soc. 2006, 54, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Pink, A.; Stokin, G.B.; Bartley, M.M.; Roberts, R.O.; Sochor, O.; Machulda, M.M.; Krell-Roesch, J.; Knopman, D.S.; Acosta, J.I.; Christianson, T.J.; et al. Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: A population-based study. Neurology 2015, 84, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Chemerinski, E.; Petracca, G.; Sabe, L.; Kremer, J.; Starkstein, S.E. The specificity of depressive symptoms in patients with Alzheimer’s disease. Am. J. Psychiatry 2001, 158, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Wennberg, A.; Berger, S.; Polotsky, V.Y.; Spira, A.P. Disturbed sleep and diabetes: A potential nexus of dementia risk. Metabolism 2018, 84, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S.; Abrams, R.C.; Young, R.C.; Shamoian, C.A. Cornell Scale for Depression in Dementia. Biol. Psychiatry 1988, 23, 271–284. [Google Scholar] [CrossRef]
- Harwood, D.G.; Ownby, R.L.; Barker, W.W.; Duara, R. The behavioral pathology in Alzheimer’s Disease Scale (BEHAVE-AD): Factor structure among community-dwelling Alzheimer’s disease patients. Int. J. Geriatr. Psychiatry 1998, 13, 793–800. [Google Scholar] [CrossRef]
- Harwood, D.G.; Ownby, R.L.; Barker, W.W.; Duara, R. The factor structure of the Cornell Scale for Depression in Dementia among probable Alzheimer’s disease patients. Am. J. Geriatr. Psychiatry 1998, 6, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.N.; Mack, J.L.; Patterson, M.B.; Edland, S.D.; Weiner, M.F.; Fillenbaum, G.; Blazina, L.; Teri, L.; Rubin, E.; Mortimer, J.A.; et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am. J. Psychiatry 1995, 152, 1349–1357. [Google Scholar] [PubMed]
- Mbodj, E.B.; Ngom, P.I.; Seck, M.T.; Aidara, A.W.; Ndiaye, C.; Dieng, L.; Toure, S.N.; Faye, D.; Diallo, P.D. Study of the characteristics of the food bolus in elderly subjects with complete dentures. Odontostomatol. Trop 2007, 30, 11–16. [Google Scholar] [PubMed]
- Woda, A.; Mishellany, A.; Peyron, M.A. The regulation of masticatory function and food bolus formation. J. Oral Rehabil. 2006, 33, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. Taste. Robust across the age span? Ann. N. Y. Acad. Sci. 1989, 561, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Horwath, C.C. Chewing difficulty and dietary intake in the elderly. J. Nutr. Elder. 1989, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Ahn, Y.S.; Lim, D.S. Association between Chewing Difficulty and Symptoms of Depression in Adults: Results from the Korea National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc. 2016, 64, e270–e278. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, C.M. Aging effects on anatomy and neurophysiology of taste and smell. Gerodontology 1984, 3, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Gouveris, H.; Anogeianaki, A.; Koutsonikolas, D.; Anogianakis, G.; Kekes, G. Age-related changes in electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae in humans. Chem. Senses 2013, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Daimon, C.M.; Cong, W.N.; Wang, R.; Chirdon, P.; de Cabo, R.; Sevigny, J.; Maudsley, S.; Martin, B. Longitudinal analysis of calorie restriction on rat taste bud morphology and expression of sweet taste modulators. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Cong, W.N.; Cai, H.; Kim, W.; Maudsley, S.; Egan, J.M.; Martin, B. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C. Nutrition and chemosensory perception in the elderly. Crit. Rev. Food Sci. Nutr. 1993, 33, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Guido, D.; Perna, S.; Carrai, M.; Barale, R.; Grassi, M.; Rondanelli, M. Multidimensional evaluation of endogenous and health factors affecting food preferences, taste and smell perception. J. Nutr. Health Aging 2016, 20, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Bobowski, N.K. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, N.; Satoh-Ku Riwada, S.; Sasano, T. Clinical Significance of Umami Taste and Umami-Related Gene Expression Analysis for the Objective Assessment of Umami Taste Loss. Curr. Pharm. Des. 2016, 22, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S. Taste and smell losses in normal aging and disease. JAMA 1997, 278, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Chau, P.H.; Ngai, H.H.; Leung, A.Y.; Li, S.F.; Yeung, L.O.; Tan-Un, K.C. Preference of Food Saltiness and Willingness to Consume Low-Sodium Content Food in a Chinese Population. J. Nutr. Health Aging 2017, 21, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Sattely-Miller, E.A.; Zimmerman, I.A.; Graham, B.G.; Erickson, R.P. Taste perception of monosodium glutamate (MSG) in foods in young and elderly subjects. Physiol. Behav. 1994, 56, 265–275. [Google Scholar] [CrossRef]
- Mojet, J.; Christ-Hazelhof, E.; Heidema, J. Taste perception with age: Pleasantness and its relationships with threshold sensitivity and supra-threshold intensity of five taste qualities. Food Qual. Pref. 2005, 16, 413–423. [Google Scholar] [CrossRef]
- Mojet, J.; Heidema, J.; Christ-Hazelhof, E. Effect of concentration on taste-taste interactions in foods for elderly and young subjects. Chem. Senses 2004, 29, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F. Experimental studies of food choices and palatability responses in European subjects exposed to the Umami taste. Asia Pac. J. Clin. Nutr. 2008, 17, 376–379. [Google Scholar] [PubMed]
- Essed, N.H.; Oerlemans, P.; Hoek, M.; Van Staveren, W.A.; Kok, F.J.; De Graaf, C. Optimal preferred MSG concentration in potatoes, spinach and beef and their effect on intake in institutionalized elderly people. J. Nutr. Health Aging 2009, 13, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F.; Monneuse, M.O.; Chabert, M.; Larue-Achagiotis, C.; Lanteaume, M.T.; Louis-Sylvestre, J. Monosodium glutamate as a palatability enhancer in the European diet. Physiol. Behav. 1991, 49, 869–873. [Google Scholar] [CrossRef]
- Tuorila, H.; Niskanen, N.; Maunuksela, E. Perception and pleasantness of a food with varying odor and flavor among the elderly and young. J. Nutr. Health Aging 2001, 5, 266–268. [Google Scholar] [PubMed]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet. Assoc. 1995, 95, 879–884. [Google Scholar] [CrossRef]
- De Graaf, C.; Polet, P.; van Staveren, W.A. Sensory perception and pleasantness of food flavors in elderly subjects. J. Gerontol. 1994, 49, P93–P99. [Google Scholar] [CrossRef] [PubMed]
- Flohr, E.L.; Erwin, E.; Croy, I.; Hummel, T. Sad man’s nose: Emotion induction and olfactory perception. Emotion 2017, 17, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mungas, D.; Cooper, J.K.; Weiler, P.G.; Gietzen, D.; Franzi, C.; Bernick, C. Dietary preference for sweet foods in patients with dementia. J. Am. Geriatr. Soc. 1990, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sanke, H.; Mita, T.; Yoshii, H.; Yokota, A.; Yamashiro, K.; Ingaki, N.; Onuma, T.; Someya, Y.; Komiya, K.; Tamura, Y.; et al. Relationship between olfactory dysfunction and cognitive impairment in elderly patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 106, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lietzau, G.; Davidsson, W.; Ostenson, C.G.; Chiazza, F.; Nathanson, D.; Pintana, H.; Skogsberg, J.; Klein, T.; Nystrom, T.; Darsalia, V.; et al. Type 2 diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor Linagliptin. Acta Neuropathol. Commun. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Reijs, B.L.R.; Ramakers, I.; Elias-Sonnenschein, L.; Teunissen, C.E.; Koel-Simmelink, M.; Tsolaki, M.; Wahlund, L.O.; Waldemar, G.; Hausner, L.; Johannsen, P.; et al. Relation of Odor Identification with Alzheimer’s Disease Markers in Cerebrospinal Fluid and Cognition. J. Alzheimers Dis. 2017, 60, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- DeVere, R. Disorders of Taste and Smell. Continuum 2017, 23, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Malaty, J.; Malaty, I.A. Smell and taste disorders in primary care. Am. Fam. Physician 2013, 88, 852–859. [Google Scholar] [PubMed]
- Landis, B.N.; Stow, N.W.; Lacroix, J.S.; Hugentobler, M.; Hummel, T. Olfactory disorders: The patients’ view. Rhinology 2009, 47, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Smell, taste, texture, and temperature multimodal representations in the brain, and their relevance to the control of appetite. Nutr. Rev. 2004, 62, S193–S204. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.H.; Lee, S.M.; Kim, S.S.; Kim, K.O. Importance of Applying Condiments in a Commonly Consumed Food System for Understanding the Association Between Familiarity and Sensory Drivers of Liking: A Study Focused on Doenjang. J. Food Sci. 2018, 83, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Cantau, B.; Delahunty, C.M.; Elsner, R.J. Interactions between texture and trigeminal stimulus in a liquid food system: Effects on elderly consumers preferences. J. Nutr. Health Aging 2002, 6, 130–133. [Google Scholar] [PubMed]
- Forde, C.G.; Delahunty, C.M. Understanding the role cross-modal sensory interactions play in food acceptability in younger and older consumers. Food Qual. Pref. 2004, 15, 715–727. [Google Scholar] [CrossRef]
- Narchi, I.; Walrand, S.; Boirie, Y.; Rousset, S. Emotions generated by food in elderly French people. J. Nutr. Health Aging 2008, 12, 626–633. [Google Scholar] [PubMed]
- Saha, S.; Hatch, D.J.; Hayden, K.M.; Steffens, D.C.; Potter, G.G. Appetite and Weight Loss Symptoms in Late-Life Depression Predict Dementia Outcomes. Am. J. Geriatr. Psychiatry 2016, 24, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Van Wymelbeke, V.; Jiang, T.; Pfitzenmeyer, P. Change in taste preference in undernourished elderly hospitalized subjects during periods of infection and convalescence. J. Nutr. Health Aging 2009, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.I. Taste and odor recognition memory: The emotional flavor of life. Rev. Neurosci. 2012, 23, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, I.; Kuven, B.M. Moments of joy and delight: The meaning of traditional food in dementia care. J. Clin. Nurs. 2016, 25, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Malnutrition. Available online: http://www.who.int/en/news-room/fact-sheets/detail/malnutrition (accessed on 1 May 2018).
- Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2018).
- Obesity Update. 2017. Available online: www.oecd.org/health/obesity-update.htm (accessed on 1 May 2018).
- Troesch, B.; Biesalski, H.K.; Bos, R.; Buskens, E.; Calder, P.C.; Saris, W.H.; Spieldenner, J.; Verkade, H.J.; Weber, P.; Eggersdorfer, M. Increased Intake of Foods with High Nutrient Density Can Help to Break the Intergenerational Cycle of Malnutrition and Obesity. Nutrients 2015, 7, 6016–6037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C. Obesity as malnutrition: The dimensions beyond energy balance. Eur. J. Clin. Nutr. 2013, 67, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, M.B.; Perez, V.; Alexander, D.D.; Thomas, D.R. A self-completed nutrition screening tool for community-dwelling older adults with high reliability: A comparison study. J. Nutr. Health Aging 2013, 17, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, M.; Ansorena, D.; Garcia, A.; Gonzalez Martinez, M.A.; Astiasaran, I.; Martinez, J.A. Assessment of calf circumference as an indicator of the risk for hyponutrition in the elderly. Nutr. Hosp. 2009, 24, 63–67. [Google Scholar] [PubMed]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, V.; Santos, K.F.D.; Malaquias, S.G.; Bachion, M.M.; Silveira, E.A. Calf circumference: Clinical validation for evaluation of muscle mass in the elderly. Rev. Bras. Enferm. 2018, 71, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Woods, A.J.; Anton, S.; Cohen, R.; Pahor, M. Frailty Clinical Phenotype: A Physical and Cognitive Point of View. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 55–63. [Google Scholar] [PubMed]
- Laur, C.V.; McNicholl, T.; Valaitis, R.; Keller, H.H. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl. Physiol. Nutr. Metab. 2017, 42, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research, Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garrido, J.; Ruiz-Ros, V.; Buigues, C.; Navarro-Martinez, R.; Cauli, O. Clinical features of prefrail older individuals and emerging peripheral biomarkers: A systematic review. Arch. Gerontol. Geriatr. 2014, 59, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.; Roberts, S.B. Effects of energy imbalance on energy expenditure and respiratory quotient in young and older men: A summary of data from two metabolic studies. Aging 1996, 8, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Fuss, P.; Heyman, M.B.; Evans, W.J.; Tsay, R.; Rasmussen, H.; Fiatarone, M.; Cortiella, J.; Dallal, G.E.; Young, V.R. Control of food intake in older men. JAMA 1994, 272, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Hajduk, C.L.; Howarth, N.C.; Russell, R.; McCrory, M.A. Dietary variety predicts low body mass index and inadequate macronutrient and micronutrient intakes in community-dwelling older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 613–621. [Google Scholar] [CrossRef]
- Wernette, C.M.; White, B.D.; Zizza, C.A. Signaling proteins that influence energy intake may affect unintentional weight loss in elderly persons. J. Am. Diet. Assoc. 2011, 111, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Machon, M.; Mateo-Abad, M.; Vrotsou, K.; Zupiria, X.; Guell, C.; Rico, L.; Vergara, I. Dietary Patterns and Their Relationship with Frailty in Functionally Independent Older Adults. Nutrients 2018, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Russell, J.; Kifley, A.; Flood, V.M.; Mitchell, P. Adherence to Dietary Guidelines and Successful Aging Over 10 Years. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.H.; Jyvakorpi, S.K.; Pitkala, K.H.; Finne-Soveri, H.; Hakala, P.; Mannisto, S.; Soini, H.; Sarlio-Lahteenkorva, S. Nutritional guidelines for older people in Finland. J. Nutr. Health Aging 2014, 18, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Yu, S.; Medicine, A. Australian and New Zealand Society for Geriatric Medicine Position Statement (2015): Undernutrition and the older person. Australas. J. Ageing 2017, 36, 75. [Google Scholar] [CrossRef]
- Conley, K.E.; Esselman, P.C.; Jubrias, S.A.; Cress, M.E.; Inglin, B.; Mogadam, C.; Schoene, R.B. Ageing, muscle properties and maximal O2 uptake rate in humans. J. Physiol. 2000, 526, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J. Exercise training guidelines for the elderly. Med. Sci. Sports Exerc. 1999, 31, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian and New Zealand Society for Geriatric Medicine. Australian and New Zealand Society for Geriatric Medicine: Position Statement-Exercise guidelines for older adults. Australas. J. Ageing 2014, 33, 287–294. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Hua Poi, P.J.; et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.; Ng, K. Australian and New Zealand Society for Geriatric Medicine: Position statement—Frailty in older people. Australas. J. Ageing 2015, 34, 68–73. [Google Scholar] [PubMed]
- American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. American Geriatrics Society feeding tubes in advanced dementia position statement. J. Am. Geriatr. Soc. 2014, 62, 1590–1593. [Google Scholar]
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontology 2000 2016, 72, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.; Dunning, T.; Izquierdo, M.; Rodriguez Manas, L.; Bourdel-Marchasson, I.; Morley, J.E.; Munshi, M.; Woo, J.; Vellas, B. An International Position Statement on the Management of Frailty in Diabetes Mellitus: Summary of Recommendations 2017. J. Frailty Aging 2018, 7, 10–20. [Google Scholar] [PubMed]
- Koyama, A.; Hashimoto, M.; Tanaka, H.; Fujise, N.; Matsushita, M.; Miyagawa, Y.; Hatada, Y.; Fukuhara, R.; Hasegawa, N.; Todani, S.; et al. Malnutrition in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Lobar Degeneration: Comparison Using Serum Albumin, Total Protein, and Hemoglobin Level. PLoS ONE 2016, 11, e0157053. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.N.; Yang, C.L.; Lin, K.N.; Chen, W.T.; Chwang, L.C.; Liu, H.C. Weight loss, nutritional status and physical activity in patients with Alzheimer’s disease. A controlled study. J. Neurol. 2004, 251, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Rullier, L.; Lagarde, A.; Bouisson, J.; Bergua, V.; Barberger-Gateau, P. Nutritional status of community-dwelling older people with dementia: Associations with individual and family caregivers’ characteristics. Int. J. Geriatr. Psychiatry 2013, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Recent Approaches to Assessing Nutritional Adequacy and Exploring Chronic Disease. In Redesigning the Process for Establishing the Dietary Guidelines for Americans; The National Academies Press: Washington, DC, USA, 2017; pp. 189–210. [Google Scholar]
- Combs, G.F., Jr.; Trumbo, P.R.; McKinley, M.C.; Milner, J.; Studenski, S.; Kimura, T.; Watkins, S.M.; Raiten, D.J. Biomarkers in nutrition: New frontiers in research and application. Ann. N. Y. Acad. Sci. 2013, 1278, 1–10. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.K.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 11–18. [Google Scholar] [PubMed]
- Yanai, H.; Fraifeld, V.E. The role of cellular senescence in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2018, 41, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A. Human growth hormone. Dis. Mon. 1968, 14, 1–33. [Google Scholar] [CrossRef]
- Press, M. Growth hormone and metabolism. Diabetes Metab. Rev. 1988, 4, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Raiti, S.; Blizzard, R.M. Human growth hormone: Current knowledge regarding its role in normal and abnormal metabolic states. Adv. Pediatr. 1970, 17, 99–123. [Google Scholar] [PubMed]
- GHR. Available online: https://www.proteinatlas.org/ENSG00000112964-GHR/tissue (accessed on 1 May 2018).
- IGF1R. Available online: https://www.proteinatlas.org/ENSG00000140443-IGF1R/tissue (accessed on 1 May 2018).
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chen, J.A.; Garcia, J.M. Clinical development of ghrelin axis-derived molecules for cancer cachexia treatment. Curr. Opin. Support. Palliat. Care 2013, 7, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The Homeostatic Force of Ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sevigny, J.; Tschop, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef] [PubMed]
- Donahue, L.R.; Hunter, S.J.; Sherblom, A.P.; Rosen, C. Age-related changes in serum insulin-like growth factor-binding proteins in women. J. Clin. Endocrinol. Metab. 1990, 71, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Nedic, O.; Sunderic, M.; Gligorijevic, N.; Malenkovic, V.; Miljus, G. Analysis of Four Circulating Complexes of Insulin-Like Growth Factor Binding Proteins in Human Blood during Aging. Biochemistry 2017, 82, 1200–1206. [Google Scholar] [PubMed]
- Meites, J. Role of hypothalamic catecholamines in aging processes. Acta Endocrinol. 1991, 125, 98–103. [Google Scholar] [PubMed]
- Bando, H.; Zhang, C.; Takada, Y.; Yamasaki, R.; Saito, S. Impaired secretion of growth hormone-releasing hormone, growth hormone and IGF-I in elderly men. Acta Endocrinol. 1991, 124, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Lenham, J.E.; Ingram, R.L. Effects of aging and dietary restriction on tissue protein synthesis: Relationship to plasma insulin-like growth factor-1. J. Gerontol. 1992, 47, B159–B163. [Google Scholar] [CrossRef] [PubMed]
- Crace, C.J.; Swenne, I.; Hill, D.J.; Milner, R.D. Tissue and serum insulin-like growth factor I (IGF I) concentrations in rats subjected to temporary protein-energy malnutrition early in life. Upsala J. Med. Sci. 1991, 96, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.C.; Colletti, R.B.; Devlin, J.T.; McAuliffe, T.L. The relationship between insulin-like growth factor-I, adiposity, and aging. Metabolism 1990, 39, 584–587. [Google Scholar] [CrossRef]
- Allensworth-James, M.L.; Odle, A.; Haney, A.; Childs, G. Sex Differences in Somatotrope Dependency on Leptin Receptors in Young Mice: Ablation of LEPR Causes Severe Growth Hormone Deficiency and Abdominal Obesity in Males. Endocrinology 2015, 156, 3253–3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennberg, A.M.V.; Hagen, C.E.; Petersen, R.C.; Mielke, M.M. Trajectories of plasma IGF-1, IGFBP-3, and their ratio in the Mayo Clinic Study of Aging. Exp. Gerontol. 2018, 106, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.M.V.; Hagen, C.E.; Machulda, M.M.; Hollman, J.H.; Roberts, R.O.; Knopman, D.S.; Petersen, R.C.; Mielke, M.M. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol. Aging 2018, 66, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Di Somma, C.; Rota, F.; Colao, A. Associated hormonal decline in aging: Is there a role for GH therapy in aging men? J. Endocrinol. Investig. 2005, 28, 99–108. [Google Scholar]
- Short, K.R.; Moller, N.; Bigelow, M.L.; Coenen-Schimke, J.; Nair, K.S. Enhancement of muscle mitochondrial function by growth hormone. J. Clin. Endocrinol. Metab. 2008, 93, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.E.; Silver, A.J.; Morley, J.E. The effect of recombinant human growth hormone on malnourished older individuals. J. Am. Geriatr. Soc. 1991, 39, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Sanders, J.L.; Kizer, J.R.; Boudreau, R.M.; Odden, M.C.; Zeki Al Hazzouri, A.; Arnold, A.M. Trajectories of function and biomarkers with age: The CHS All Stars Study. Int. J. Epidemiol. 2016, 45, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association between Insulin-Like Growth Factor-1 and Frailty among Older Adults. J. Nutr. Health Aging 2018, 22, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, M.; Nygaard, M.; Debrabant, B.; Mengel-From, J.; Dato, S.; Thinggaard, M.; Christensen, K.; Christiansen, L. No Association between Variation in Longevity Candidate Genes and Aging-related Phenotypes in Oldest-old Danes. Exp. Gerontol. 2016, 78, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartke, A.; Darcy, J. GH and ageing: Pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podshivalova, K.; Kerr, R.A.; Kenyon, C. How a Mutation that Slows Aging Can also Disproportionately Extend End-of-Life Decrepitude. Cell Rep. 2017, 19, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Carlesi, C.; Lucetti, C.; Danti, S.; Nuti, A. Eating Behaviors and Dietary Changes in Patients With Dementia. Am. J. Alzheimers Dis. Other Dement. 2016, 31, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, Y.; Feng, Z.; Liu, M.; Li, Y.; Yang, H.; Wang, D.; Zheng, L.; Lou, D.; Cheng, L.; et al. Nutritional Deficiency in Early Life Facilitates Aging-Associated Cognitive Decline. Curr. Alzheimer Res. 2017, 14, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D. A life course of adiposity and dementia. Eur. J. Pharmacol. 2008, 585, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pedditzi, E.; Peters, R.; Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Diehl-Wiesenecker, E.; von Armin, C.A.; Dupuis, L.; Muller, H.P.; Ludolph, A.C.; Kassubek, J. Adipose Tissue Distribution in Patients with Alzheimer’s Disease: A Whole Body MRI Case-Control Study. J. Alzheimers Dis. 2015, 48, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Kamogawa, K.; Kohara, K.; Tabara, Y.; Uetani, E.; Nagai, T.; Yamamoto, M.; Igase, M.; Miki, T. Abdominal fat, adipose-derived hormones and mild cognitive impairment: The J-SHIPP study. Dement. Geriatr. Cogn. Disord. 2010, 30, 432–439. [Google Scholar] [CrossRef] [PubMed]
- West, N.A.; Haan, M.N. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Edland, S.D.; Cha, R.H.; Petersen, R.C.; Rocca, W.A. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007, 69, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Associations between Homocysteine, Folic Acid, Vitamin B12 and Alzheimer’s Disease: Insights from Meta-Analyses. J. Alzheimers Dis. 2015, 46, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangialasche, F.; Westman, E.; Kivipelto, M.; Muehlboeck, J.S.; Cecchetti, R.; Baglioni, M.; Tarducci, R.; Gobbi, G.; Floridi, P.; Soininen, H.; et al. Classification and prediction of clinical diagnosis of Alzheimer’s disease based on MRI and plasma measures of α-/γ-tocotrienols and gamma-tocopherol. J. Intern. Med. 2013, 273, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Haley, A.P.; Oleson, S.; Pasha, E.; Birdsill, A.; Kaur, S.; Thompson, J.; Tanaka, H. Phenotypic heterogeneity of obesity-related brain vulnerability: One-size interventions will not fit all. Ann. N. Y. Acad. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Banati, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Une, K.; Takei, Y.A.; Tomita, N.; Asamura, T.; Ohrui, T.; Furukawa, K.; Arai, H. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur. J. Neurol. 2011, 18, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Makaruk, M.; Graban, A.; Wisniewska, A.; Lojkowska, W.; Bochynska, A.; Gugala-Iwaniuk, M.; Slawinska, K.; Lugowska, A.; Ryglewicz, D.; Wehr, H. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology 2017, 18, 561–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Yamashita, T.; Fukui, Y.; Song, D.; Li, X.; Zhai, Y.; Nakano, Y.; Morihara, R.; Hishikawa, N.; Ohta, Y.; et al. Different Associations of Plasma Biomarkers in Alzheimer’s Disease, Mild Cognitive Impairment, Vascular Dementia, and Ischemic Stroke. J. Clin. Neurol. 2018, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Van Himbergen, T.M.; Beiser, A.S.; Ai, M.; Seshadri, S.; Otokozawa, S.; Au, R.; Thongtang, N.; Wolf, P.A.; Schaefer, E.J. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: Results from the Framingham Heart Study. Arch. Neurol. 2012, 69, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Dukic, L.; Simundic, A.M.; Martinic-Popovic, I.; Kackov, S.; Diamandis, A.; Begcevic, I.; Diamandis, E.P. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin. Biochem. 2016, 49, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Miwa, K.; Okazaki, S.; Sakaguchi, M.; Mochizuki, H. Serum high-molecular-weight adiponectin level and incident dementia in patients with vascular risk factors. Eur. J. Neurol. 2016, 23, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. Neuromol. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Borkowska, A.; Ciebiada, M.; Loba, J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab. Brain Dis. 2016, 31, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kawashima, S.; Satake, S.; Miura, H.; Tokuda, H.; Toba, K. Differential subtypes of diabetic older adults diagnosed with Alzheimer’s disease. Geriatr. Gerontol. Int. 2014, 14, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bahari, T.; Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Nakamoto, M.; Miki, K.; Sawachika, F.; Arisawa, K. Association between dietary patterns and serum adiponectin: A cross-sectional study in a Japanese population. Int. J. Food Sci. Nutr. 2018, 69, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, X.; Yang, C.; Fang, D.; Wang, X.; An, J.; Zhang, J.; Wang, L.; Lu, T.; Ruan, H.B.; et al. Brown adipose tissue activation in a rat model of Parkinson’s disease. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E731–E736. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Dharmavaram, S.; Wadhawan, S.; Sethi, A.; Bhadoria, P. Donepezil: A cause of inadequate muscle relaxation and delayed neuromuscular recovery. J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Kakinuma, Y.; Arikawa, M.; Okazaki, K.; Hoshino, E.; Iiyama, T.; Kubo, T.; Kitaoka, H.; Doi, Y.; Sato, T. Donepezil can improve ischemic muscle atrophy by activating angiomyogenic properties of satellite cells. Circ. J. 2014, 78, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Park, M.; Jeong, J.; Kim, H.; Lee, S.K.; Lee, E.; Oh, B.H.; Namkoong, K. Cholinesterase Inhibitor Donepezil Increases Mitochondrial Biogenesis through AMP-Activated Protein Kinase in the Hippocampus. Neuropsychobiology 2016, 73, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pakaski, M.; Feher, A.; Juhasz, A.; Drotos, G.; Fazekas, O.C.; Kovacs, J.; Janka, Z.; Kalman, J. Serum adipokine levels modified by donepezil treatment in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Consolim-Colombo, F.M.; Sangaleti, C.T.; Costa, F.O.; Morais, T.L.; Lopes, H.F.; Motta, J.M.; Irigoyen, M.C.; Bortoloto, L.A.; Rochitte, C.E.; Harris, Y.T.; et al. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight 2017, 2, 93340. [Google Scholar] [CrossRef] [PubMed]
- Furiya, Y.; Tomiyama, T.; Izumi, T.; Ohba, N.; Ueno, S. Rivastigmine Improves Appetite by Increasing the Plasma Acyl/Des-Acyl Ghrelin Ratio and Cortisol in Alzheimer Disease. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, A.; Setoguchi, M.; Uchino, Y.; Nagata, K.; Hokonohara, D. Effect of rivastigmine on plasma butyrylcholine esterase activity and plasma ghrelin levels in patients with dementia in Alzheimer’s disease. Geriatr. Gerontol. Int. 2018, 18, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, K.C.; Ryu, W.I.; Amirault, K.M.; Healy, R.A.; Siegel, A.J.; McPhie, D.L.; Forester, B.; Cohen, B.M. Late-onset Alzheimer’s disease is associated with inherent changes in bioenergetics profiles. Sci. Rep. 2017, 7, 14038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, G.A.; Redman, L.M.; de Jonge, L.; Covington, J.; Rood, J.; Brock, C.; Mancuso, S.; Martin, C.K.; Smith, S.R. Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. Am. J. Clin. Nutr. 2015, 101, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, N.Y.; Kim, H.J.; Kim, Y.J.; Kim, S.; Seo, S.W.; Kim, E.J.; Na, D.L. Neuropsychiatric characteristics of PiB-negative subcortical vascular dementia versus behavioral variant frontotemporal dementia. Arch. Gerontol. Geriatr. 2016, 67, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Irish, M.; Henning, E.; Dermody, N.; Bartley, L.; Kiernan, M.C.; Piguet, O.; Farooqi, S.; Hodges, J.R. Assessment of Eating Behavior Disturbance and Associated Neural Networks in Frontotemporal Dementia. JAMA Neurol. 2016, 73, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.; Rothenberg, E.; Blennow, K.; Steen, B.; Skoog, I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003, 163, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Piguet, O.; Petersen, A.; Yin Ka Lam, B.; Gabery, S.; Murphy, K.; Hodges, J.R.; Halliday, G.M. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann. Neurol. 2011, 69, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Konofal, E.; Lecendreux, M. Alertness and feeding behaviors in ADHD: Does the hypocretin/orexin system play a role? Med. Hypotheses 2008, 71, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Chen, J.; Pink, R.; Carter, D.; Saunders, N.; Sotiriadis, G.; Bai, B.; Pan, Y.; Howlett, D.; Payne, A.; et al. Orexin receptors exert a neuroprotective effect in Alzheimer’s disease (AD) via heterodimerization with GPR103. Sci. Rep. 2015, 5, 12584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fronczek, R.; van Geest, S.; Frolich, M.; Overeem, S.; Roelandse, F.W.; Lammers, G.J.; Swaab, D.F. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C. Orexin and Alzheimer’s Disease. Curr. Top. Behav. Neurosci. 2017, 33, 305–322. [Google Scholar] [PubMed]
- Yasui, K.; Inoue, Y.; Kanbayashi, T.; Nomura, T.; Kusumi, M.; Nakashima, K. CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 2006, 250, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Finlayson, G.; Dando, R. Sleep, food cravings and taste. Appetite 2018, 125, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.L.; Park, D.; Zhu, L.; Palmer, M.R.; Broadhurst, R.Y.; Arrigoni, E. Regulation of Lateral Hypothalamic Orexin Activity by Local GABAergic Neurons. J. Neurosci. 2018, 38, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Hofer, E.; Bouwman, F.H.; Buerger, K.; Cordonnier, C.; Fladby, T.; Galimberti, D.; Georges, J.; Heneka, M.T.; Hort, J.; et al. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2012, 366, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Hermanussen, M.; Tresguerres, J.A. A new anti-obesity drug treatment: First clinical evidence that, antagonising glutamate-gated Ca2+ ion channels with memantine normalises binge-eating disorders. Econ. Hum. Biol. 2005, 3, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Suma, S.; Watanabe, Y.; Hirano, H.; Kimura, A.; Edahiro, A.; Awata, S.; Yamashita, Y.; Matsushita, K.; Arai, H.; Sakurai, T. Factors affecting the appetites of persons with Alzheimer’s disease and mild cognitive impairment. Geriatr. Gerontol. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Connors, M.H.; Xu, J.; Woodward, M.; Ames, D.; PRIME Study Group. The course of neuropsychiatric symptoms in dementia: A 3-year longitudinal study. J. Am. Med. Dir. Assoc. 2015, 16, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Orsitto, G. Different components of nutritional status in older inpatients with cognitive impairment. J. Nutr. Health Aging 2012, 16, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Campbell, N.; Khan, B.; Callahan, C.; Boustani, M. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013, 9, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, D.M. Flavor is in the brain. Physiol. Behav. 2012, 107, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Miller, L.A.; McGrillen, K. Perception of odor-induced tastes following insular cortex lesion. Neurocase 2015, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Namkung, H.; Kim, S.H.; Sawa, A. The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017, 40, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010, 214, 495–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenfeld, C.; Werner, C.J.; Reetz, K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? Neuroimage Clin. 2018, 18, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.F.; Englund, E. Greater loss of von Economo neurons than loss of layer II and III neurons in behavioral variant frontotemporal dementia. Am. J. Neurodegener. Dis. 2014, 3, 64–71. [Google Scholar] [PubMed]
- Seeley, W.W.; Carlin, D.A.; Allman, J.M.; Macedo, M.N.; Bush, C.; Miller, B.L.; Dearmond, S.J. Early frontotemporal dementia targets neurons unique to apes and humans. Ann. Neurol. 2006, 60, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gefen, T.; Papastefan, S.T.; Rezvanian, A.; Bigio, E.H.; Weintraub, S.; Rogalski, E.; Mesulam, M.M.; Geula, C. Von Economo neurons of the anterior cingulate across the lifespan and in Alzheimer’s disease. Cortex 2018, 99, 69–77. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifli, A.-P. Appetite, Metabolism and Hormonal Regulation in Normal Ageing and Dementia. Diseases 2018, 6, 66. https://doi.org/10.3390/diseases6030066
Nifli A-P. Appetite, Metabolism and Hormonal Regulation in Normal Ageing and Dementia. Diseases. 2018; 6(3):66. https://doi.org/10.3390/diseases6030066
Chicago/Turabian StyleNifli, Artemissia-Phoebe. 2018. "Appetite, Metabolism and Hormonal Regulation in Normal Ageing and Dementia" Diseases 6, no. 3: 66. https://doi.org/10.3390/diseases6030066
APA StyleNifli, A. -P. (2018). Appetite, Metabolism and Hormonal Regulation in Normal Ageing and Dementia. Diseases, 6(3), 66. https://doi.org/10.3390/diseases6030066