Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study
Abstract
:1. Introduction
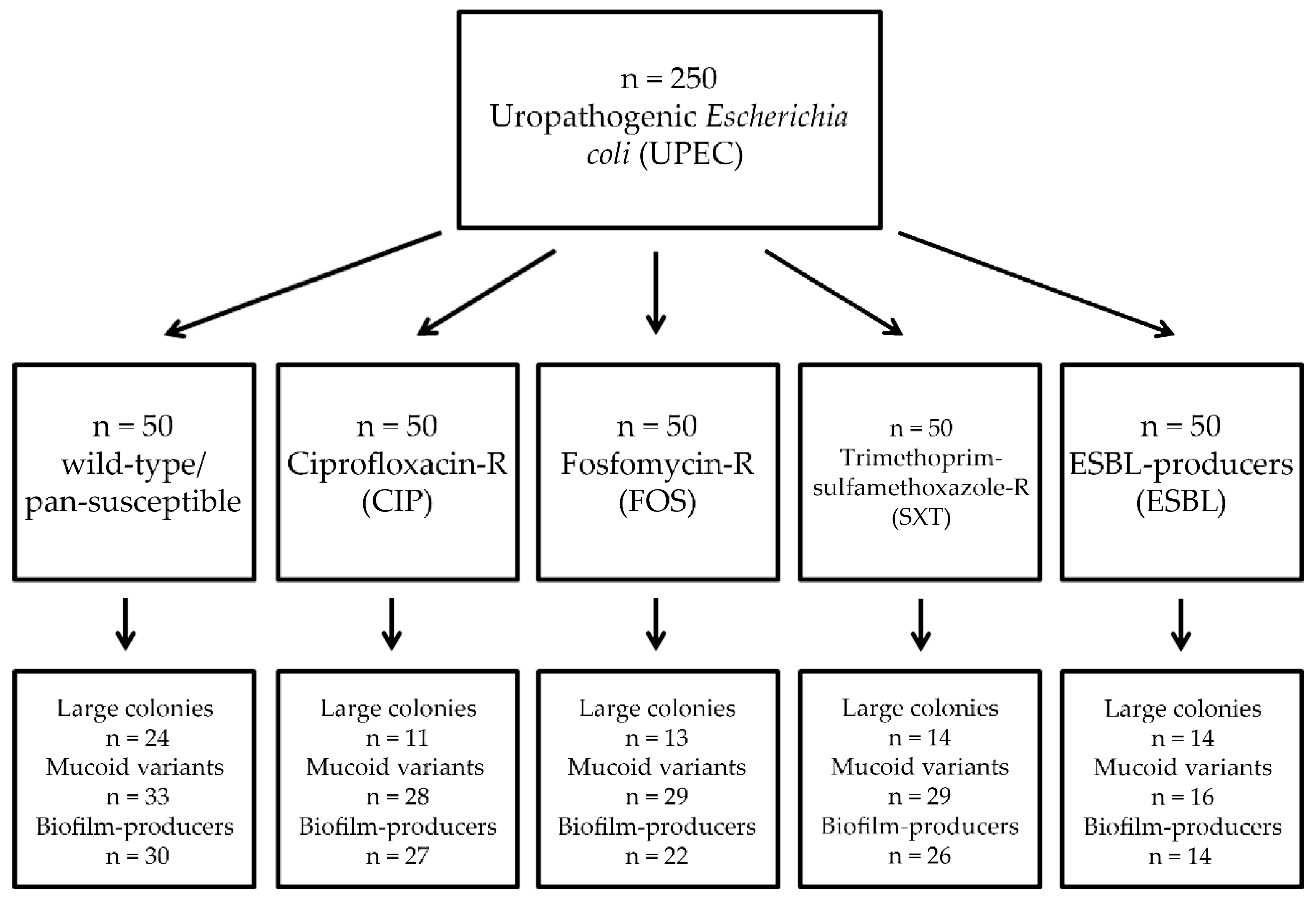
2. Materials and Methods
2.1. Collection of Isolates
2.2. Bacterial Identification
2.3. Colony Characteristics
2.4. Antimicrobial Susceptibility Testing, Resistance Detection
2.5. Assessment of Biofilm-Production by the Tube-Adherence Method
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Association of Antibiotic Resistance with Colony Characteristics in Uropathogenic E. coli (UPEC)
3.2. Association of Antibiotic Resistance with Biofilm-Formation in UPEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michaud, C. Global burden of infectious diseases. Encycl. Microbiol. 2009, 444–454. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Genet. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Jahandeh, N.; Ranjbar, R.; Behzadi, P.; Behzadi, E. Uropathogenic Escherichia coli virulence genes: Invaluable approaches for designing DNA microarray probes. Central Eur. J. Urol. 2015, 68, 452–458. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, B.; Heisig, A.; Heisig, P. Uncomplicated Urinary Tract Infections and Antibiotic Resistance—Epidemiological and Mechanistic Aspects. Antibiotics 2014, 3, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Topics Spin. Cord. Rehab. 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-year survey. Medicina 2019, 55, 285. [Google Scholar] [CrossRef] [Green Version]
- Gajdács, M.; Urbán, E. Comparative Epidemiology and Resistance Trends of Proteae in Urinary Tract Infections of Inpatients and Outpatients: A 10-Year Retrospective Study. Antibiotics 2019, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Ciani, O.; Grassi, D.; Tarricone, R. An economic perspective on urinary tract infection: The “Costs of Resignation”. Clin. Drug Investig. 2013, 33, 255–261. [Google Scholar] [CrossRef]
- Flower, A.; Bishop, F.L.; Lewith, G. How women manage recurrent urinary tract infections: An analysis of postings on a popular web forum. BMC Fam. Pract. 2014, 15, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.N.; Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 2015, 15, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxman, B.; Brown, P. Epidemiology of urinary tract infections. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [Google Scholar] [CrossRef]
- Dason, S.; Dason, J.T.; Kapoor, A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can. Urol. Assoc. J. 2011, 5, 316–322. [Google Scholar] [CrossRef]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.G.; Schmiemann, G.; et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients: Part 1. Urol. Int. 2018, 100, 263–270. [Google Scholar] [CrossRef]
- Melia, M.; DeMaio, J. Urinary Tract Infection, Complicated (UTI) John Hopkins Antibiotic Guide. 2017. Available online: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540573/all/Urinary_Tract_Infection_Complicated__UTI_?q=complicated (accessed on 9 May 2020).
- Negus, M.; Phillips, C.; Hindley, R. Recurrent urinary tract infections: A critical review of the currently available treatment options. Obstet. Gynaecol. 2020, 22, 115–121. [Google Scholar] [CrossRef]
- Anne, J.H. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008, 337, 135. [Google Scholar] [CrossRef] [Green Version]
- Kuula, L.S.M.; Viljemaa, K.M.; Backman, J.T.; Blom, M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS ONE 2019, 14, e0216029. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- Gajdács, M.; Batori, Z.; Ábrók, M.; Lázár, A.; Burián, K. Characterization of resistance in gram-negative urinary isolates using existing and novel indicators of clinical relevance: A 10-year data analysis. Life 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzanlou, M.; Chai, W.C. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar] [PubMed]
- Garau, J. Impact of antibiotic restrictions: The ethical perspective. Clin. Microbiol. Infect. 2006, 12, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Boil. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazăr, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Boil. 2014, 2014, 543974. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.; Corcoran, D.; Finn, K.; Lucey, B. Uropathogenic Escherichia coli Biofilm-Forming Capabilities are not Predictable from Clinical Details or from Colonial Morphology. Diseases 2020, 8, 11. [Google Scholar] [CrossRef]
- Naves, P.L.F.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Dahbi, G.; Blanco, M.; Ponte, M.D.C.; Soriano, F. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Jakielaszek, C.J.; Miller, L.A.; Poupard, J.A. Escherichia coli ATCC 35218 as a Quality Control Isolate for Susceptibility Testing of Haemophilus influenzae with Haemophilus Test Medium. Antimicrob. Agents Chemother. 1999, 43, 283–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajdács, M.; Urbán, E. The relevance of anaerobic bacteria in brain abscesses: A ten-year retrospective analysis (2008-2017). Infect. Dis. 2019, 51, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.W.; Oakton, K.J.; Warner, M.; Livermore, D.M. Detection of extended-spectrum b-lactamases in klebsiellae with the Oxoid combination disk method. J. Clin. Microbiol. 2000, 38, 4228–4232. [Google Scholar] [CrossRef] [Green Version]
- Dumaru, R.; Baral, R.; Shrestha, L.B. Study of biofilm formation and antibiotic resistance pattern of gram-negative Bacilli among the clinical isolates at BPKIHS, Dharan. BMC Res. Notes 2019, 12, 38. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Yazdanbod, H.; Aghapour, R.; Cheshmeh, M.A.; Omran, D.S. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica 2010, 5, 111–115. [Google Scholar]
- Alizade, H. Escherichia coli in Iran: An overview of antibiotic resistance: A review article. Iran. J. Public Heal. 2018, 47, 1–12. [Google Scholar]
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; E Youngerman, B.; A Green, R.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the risk factors for catheter-associated urinary tract infections: A large cross-sectional study of six hospitals. BMJ Open 2019, 9, e022137. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Navidifar, T.; Salehshooshtari, F.; Rashno, M.; Savari, M.; Jahangirmehr, F.; Arshadi, M. Association between biofilm formation, structure, and the expression levels of genes related to biofilm formation and biofilm-specific resistance of acinetobacter baumannii strains isolated from burn infection in ahvaz, iran. Infect. Drug Resist. 2019, 12, 3867–3881. [Google Scholar] [CrossRef] [Green Version]
- Seifi, K.; Kazemian, H.; Heidari, H.; Rezagholizadeh, F.; Saee, Y.; Shirvani, F.; Houri, H. Evaluation of biofilm formation among klebsiella pneumoniae isolates and molecular characterization by ERIC-PCR. Jundishapur. J. Microbiol. 2016, 9, 30682. [Google Scholar] [CrossRef] [Green Version]
- Vishwanath, S.; Munim, F.C.; Chawla, K. Nonfermenting gram-negative bacilli other than pseudomonas aeruginosa and acinetobacter spp. causing respiratory tract infections in a tertiary care center. J. Glob. Infect. Dis. 2013, 5, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E. A 10-year single-center experience on Stenotrophomonas maltophilia resistotyping in Szeged, Hungary. Eur. J. Microbiol. Immunol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadell, C.D.; Bassler, B.L. A fitness trade-off between local competition and dispersal in vibrio cholerae biofilms. Proc. Nat. Atac. Sci. USA 2011, 108, 14181–14185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederdorfer, R.; Besemer, K.; Battin, T.J.; Peter, H. Ecological strategies and metabolic trade-offs of complex environmental biofilms. Biofilms Microbes 2017, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Lajhar, S.; Brownlie, J.C.; Barlow, R. Characterization of biofilm-forming capacity and resistance to sanitizers of a range of E. coli O26 pathotypes from clinical cases and cattle in Australia. BMC Microbiol. 2018, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Yaratha, G.; Perloff, S.; Changala, K. Lactose vs Non-Lactose Fermenting E. coli: Epidemiology, Clinical Outcomes, and Resistance. Open Forum Infect. Dis. 2017, 4, S589–S590. [Google Scholar] [CrossRef] [Green Version]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Differential Epidemiology and Antibiotic-Resistance of Lactose-Fermenting and Non-Fermenting Escherichia Coli: Is It Just a Matter of Taste. Biol. Futura 2020. [Google Scholar] [CrossRef]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 20–28. [Google Scholar] [CrossRef]
- Avila-Novoa, M.-G.; Solís-Velázquez, O.-A.; Rangel-López, D.-E.; González-Gómez, J.-P.; Guerrero-Medina, P.-J.; Gutiérrez-Lomelí, M. Biofilm Formation and Detection of Fluoroquinolone- and Carbapenem-Resistant Genes in Multidrug-Resistant Acinetobacter baumannii. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, e3454907. [Google Scholar] [CrossRef] [Green Version]
- Cepas, V.; Lopez, V.C.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship Between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, L.R.R. Acinetobacter baumannii displays inverse relationship between meropenem resistance and biofilm production. J. Chemother. 2014, 27, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Musafer, H.; Kuchma, S.L.; Naimie, A.A.; Schwartzman, J.D.; Al-Mathkhury, H.; O’Toole, G.A. Investigating the Link Between Imipenem Resistance and Biofilm Formation by Pseudomonas aeruginosa. Microb. Ecol. 2014, 68, 111–120. [Google Scholar] [CrossRef]
- Fabrega, A.; Soto, S.M.; Ballesté-Delpierre, C.; Fernández-Orth, D.; De Anta, M.T.J.; Vila, J.; Fàbrega, A. Impact of quinolone-resistance acquisition on biofilm production and fitness in Salmonella enterica. J. Antimicrob. Chemother. 2014, 69, 1815–1824. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, P.; Gurung, J.; Khyriem, A.B.; Banik, A.; Lyngdoh, W.V.; Choudhury, B. Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Indian J. Crit. Care Med. 2013, 17, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control. 2016, 5, 5. [Google Scholar]
- Soto, S.M.; Smithson, A.; Martinez, J.; Horcajada, J.P.; Mensa, J.; Vila, J. Biofilm Formation in Uropathogenic Escherichia coli Strains: Relationship With Prostatitis, Urovirulence Factors and Antimicrobial Resistance. J. Urol. 2007, 177, 365–368. [Google Scholar] [CrossRef]
- Bartoletti, R.; Cai, T.; Nesi, G.; Albanese, S.; Meacci, F.; Mazzoli, S.; Naber, K. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: Results from a longitudinal cohort study. World J. Urol. 2013, 32, 737–742. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Drenkard, E.; Ausubel, F. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behzadi, P.; Urbán, E.; Gajdács, M. Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases 2020, 8, 17. https://doi.org/10.3390/diseases8020017
Behzadi P, Urbán E, Gajdács M. Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases. 2020; 8(2):17. https://doi.org/10.3390/diseases8020017
Chicago/Turabian StyleBehzadi, Payam, Edit Urbán, and Márió Gajdács. 2020. "Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study" Diseases 8, no. 2: 17. https://doi.org/10.3390/diseases8020017
APA StyleBehzadi, P., Urbán, E., & Gajdács, M. (2020). Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases, 8(2), 17. https://doi.org/10.3390/diseases8020017






