Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- Laboratory: Serum amylase and/or lipase at least three times the upper reference limits;
- Clinical: Pain suggestive of acute pancreatitis (AP); and
- Radiologic: Characteristic imaging findings of AP.
2.2. MR-Derived Variables
2.3. Laboratory Variables
2.4. Other Variables
2.5. Statistical Analyses
3. Results
3.1. Study Characteristics
3.2. Psoas Muscle Volume in the Study Groups
3.3. Associations between Psoas Muscle Volume and Insulin Traits
3.4. Associations between Psoas Muscle Volume and Ectopic Fat
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 2712–2781. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; Alarcon Ramos, G.C.; Ko, J.; Stuart, C.E.; Modesto, A.E.; Singh, R.G.; Petrov, M.S. Trajectories of glycaemia following acute pancreatitis: A prospective longitudinal cohort study with 24 months follow-up. J. Gastroenterol. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.; Mathew, J.; Petrov, M.S. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig. Liver Dis. 2017, 49, 5405–5444. [Google Scholar] [CrossRef] [PubMed]
- Woodmansey, C.; McGovern, A.; McCullough, K.; Whyte, M.B.; Munro, N.M.; Correa, A.C.; Gatenby, P.A.; Jones, S.A.; de Lusignan, S. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): A retrospective cohort study. Diabetes Care 2019, 40, 1486–1493. [Google Scholar]
- Cho, J.; Scragg, R.; Petrov, M.S. Risk of mortality and hospitalization after post-pancreatitis diabetes mellitus vs type 2 diabetes mellitus: A population-based matched cohort study. Am. J. Gastroenterol. 2019, 114, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Scragg, R.; Petrov, M.S. Use of insulin and the risk of progression of pancreatitis: A population-based cohort study. Clin. Pharmacol. Ther. 2020, 107, 5805–5887. [Google Scholar] [CrossRef]
- Cho, J.; Scragg, R.; Pandol, S.J.; Goodarzi, M.O.; Petrov, M.S. Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: A nationwide cohort study. Diabetes Care 2019, 42, 1675–1683. [Google Scholar] [CrossRef]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 1751–1784. [Google Scholar] [CrossRef]
- Gillies, N.A.; Pendharkar, S.A.; Singh, R.G.; Windsor, J.A.; Bhatia, M.; Petrov, M.S. Fasting levels of insulin and amylin after acute pancreatitis are associated with pro-inflammatory cytokines. Arch. Physiol. Biochem. 2017, 123, 238–248. [Google Scholar] [CrossRef]
- Pendharkar, S.A.; Asrani, V.M.; Xiao, A.Y.; Yoon, H.D.; Murphy, R.; Windsor, J.A.; Petrov, M.S. Relationship between pancreatic hormones and glucose metabolism: A cross-sectional study in patients after acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G50–G58. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.G.; Nguyen, N.; DeSouza, S.V.; Pendharkar, S.A.; Petrov, M.S. Comprehensive analysis of body composition and insulin traits associated with intra-pancreatic fat deposition in healthy individuals and people with new-onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes. Metab. 2019, 21, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Pendharkar, S.A.; Cervantes, A.; Cho, J.; Miranda-Soberanis, V.; Petrov, M.S. Abdominal obesity and insulin resistance after an episode of acute pancreatitis. Dig. Liver Dis. 2018, 50, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S. Skeletal muscle: A new piece in the pancreatitis puzzle. United Eur. Gastroenterol. J. 2019, 7, 283–1284. [Google Scholar] [CrossRef] [Green Version]
- Cadore, E.; Izquierdo, M. Exercise interventions in polypathological aging patients that coexist with diabetes mellitus: Improving functional status and quality of life. AGE 2015, 37, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miljkovic, I.; Cauley, J.; Wang, P.; Holton, K.F.; Lee, C.G.; Sheu, Y.; Barrett-Connor, E.; Hoffman, A.R.; Lewis, C.B.; Orwoll, E.S.; et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 2013, 21, 2118–2125. [Google Scholar] [CrossRef] [Green Version]
- Levelt, E.; Pavlides, M.; Banerjee, R.; Mahmod, M.; Kelly, C.; Sellwood, J.; Ariga, R.; Thomas, S.; Francis, J.; Rodgers, C.; et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J. Am. Coll. Cardiol. 2016, 68, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.G.; Cervantes, A.; Kim, J.; Nguyen, N.N.; DeSouza, S.V.; Dokpuang, D.; Lu, J.; Petrov, M.S. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G806–G815. [Google Scholar] [CrossRef]
- Singh, R.G.; Nguyen, N.N.; Cervantes, A.; Alarcon Ramos, G.C.; Cho, J.; Petrov, M.S. Associations between intra-pancreatic fat deposition and circulating levels of cytokines. Cytokine 2019, 120, 107–114. [Google Scholar] [CrossRef]
- Petrov, M.S. Diabetes of the exocrine pancreas: American Diabetes Association-compliant lexicon. Pancreatology 2017, 17, 523–526. [Google Scholar] [CrossRef]
- Singh, R.G.; Nguyen, N.N.; Cervantes, A.; Cho, J.; Petrov, M.S. Serum lipid profile as a biomarker of intra-pancreatic fat deposition: A nested cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Modesto, A.E.; Stuart, C.E.; Cho, J.; Ko, J.; Singh, R.G.; Petrov, M.S. Psoas muscle size as a biomarker of progression of pancreatitis: A magnetic resonance imaging study. Eur. Radiol. 2020, 30, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.E.; Ko, J.; Alarcon Ramos, G.C.; Modesto, A.E.; Cho, J.; Petrov, M.S. Associations between cannabis use, abdominal fat phenotypes and insulin traits. J. Clin. Med. Res. 2020, 12, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Singh, R.G.; Kim, J.U.; DeSouza, S.V.; Petrov, M.S. Relationship of anthropometric indices to abdominal body composition: A multi-ethnic New Zealand magnetic resonance imaging study. J. Clin. Med. Res. 2019, 11, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.G.; Nguyen, N.N.; Cervantes, A.; Kim, J.U.; Stuart, C.E.; Petrov, M.S. Circulating levels of lipocalin-2 are associated with fatty pancreas but not fatty liver. Peptides 2019, 119, 170117. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; Stuart, C.E.; Alarcon Ramos, G.C.; Ko, J.; Petrov, M.S. Oxyntomodulin may distinguish new-onset diabetes after acute pancreatitis from type 2 diabetes. Clin. Transl. Gastroenterol. 2020, 11, e00132. [Google Scholar] [CrossRef]
- Stuart, C.E.; Ko, J.; Modesto, A.E.; Alarcon Ramos, G.C.; Bharmal, S.H.; Cho, J.; Singh, R.G.; Petrov, M.S. Implications of tobacco smoking and alcohol consumption on ectopic fat deposition in individuals after pancreatitis. Pancreas 2020. Epub ahead of print. [Google Scholar]
- Stuart, C.E.; Singh, R.G.; Alarcon Ramos, G.C.; Priya, S.; Ko, J.; DeSouza, S.V.; Cho, J.; Petrov, M.S. Relationship of pancreas volume to tobacco smoking and alcohol consumption following pancreatitis. Pancreatology 2020, 20, 60–67. [Google Scholar] [CrossRef]
- Cho, J.; Dalbeth, N.; Petrov, M.S. Bidirectional relationship between gout and diabetes mellitus in individuals after acute pancreatitis: A nationwide cohort study. J. Rheumatol. 2020, 47, 917–923. [Google Scholar] [CrossRef]
- Cho, J.; Walia, M.; Scragg, R.; Petrov, M.S. Frequency and risk factors for mental disorders following pancreatitis: A nationwide cohort study. Curr. Med. Res. Opin. 2019, 35, 1157–1164. [Google Scholar] [CrossRef]
- Sugimoto, K.; Tabara, Y.; Ikegami, H.; Takata, Y.; Kamide, K.; Ikezoe, T.; Kiyoshige, E.; Makutani, Y.; Onuma, H.; Gondo, Y. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J. Diabetes Investig. 2019, 10, 1471–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, I.; Søndergaard, E.; Sørensen, L.; Nellemann, B.; Gormsen, L.C.; Jensen, M.D.; Nielsen, S. Increased VLDL-TG fatty acid storage in skeletal muscle in men with type 2 diabetes. J. Clin. Endocrinol. Metabol. 2016, 102, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almurdhi, M.; Reeves, N.; Bowling, F.; Boulton, A.J.; Jeziorska, M.; Malik, R.A. Reduced lower-limb muscle strength and volume in patients with type 2 diabetes in relation to neuropathy, intramuscular fat, and vitamin D levels. Diabetes Care 2016, 39, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, H.; Gadeberg, P.; Brock, B.; Jakobsen, J. Muscular atrophy in diabetic neuropathy: A stereological magnetic resonance imaging study. Diabetologia 1997, 40, 1062–1069. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.; Heymsfield, S.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Tanaka, N.; Kanehisa, H. Applicability of single muscle CSA for predicting segmental muscle volume in young men. Int. J. Sports Med. 2014, 35, 608–614. [Google Scholar] [CrossRef]
- Kawanabe, S.; Nagai, Y.; Nakamura, Y.; Nishine, A.; Nakagawa, T.; Tanaka, Y. Association of the muscle/fat mass ratio with insulin resistance in gestational diabetes mellitus. Endocr. J. 2019, 66, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Srikanthan, P.; Karlamangla, A. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third national health and nutrition examination survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hwang, S.; Chung, H.; Kim, N.H.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Proportion and characteristics of the subjects with low muscle mass and abdominal obesity among the newly diagnosed and drug-naïve type 2 diabetes mellitus patients. Diabetes Metab. J. 2019, 43, 105. [Google Scholar] [CrossRef]
- Kiefer, L.; Fabian, J.; Rospleszcz, S.; Lorbeer, R.; Machann, J.; Storz, C.; Kraus, M.S.; Schlett, C.L.; Roemer, F.; Wintermeyer, E.; et al. Assessment of the degree of abdominal myosteatosis by magnetic resonance imaging in subjects with diabetes, prediabetes and healthy controls from the general population. Eur. J. Radiol. 2018, 105, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Daugaard, J.; Richter, E. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: Possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol. Scand. 2001, 171, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.; Sahebkar, A. Mitochondrial dysfunction in diabetes and the regulatory roles of antidiabetic agents on the mitochondrial function. J. Cell Physiol. 2018, 234, 8402–8410. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, K. Adipokines and myokines: A pivotal role in metabolic and cardiovascular disorders. Curr. Med. Chem. 2018, 25, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Bharmal, S.H.; Pendharkar, S.; Singh, R.; Cho, J.; Petrov, M.S. Glucose counter-regulation after acute pancreatitis. Pancreas 2019, 48, 670–681. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. Metabolic abnormalities in diabetes and kidney disease: Role of uremic toxins. Curr. Diab. Rep. 2018, 18, 97. [Google Scholar] [CrossRef]
- Sénéchal, M.; Dionne, I.; Brochu, M. Dynapenic abdominal obesity and metabolic risk factors in adults 50 years of age and older. J. Aging Health 2012, 24, 812–826. [Google Scholar] [CrossRef]
- Mitchell, W.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Albers, P.; Pedersen, A.; Birk, J.; Kristensen, D.E.; Vind, B.F.; Baba, O.; Nøhr, J.; Højlund, K.; Wojtaszewski, J.F. Human muscle fiber type–specific insulin signaling: Impact of obesity and type 2 diabetes. Diabetes 2014, 64, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Ree, E.; Choi, J.; Yoo, S.; Bae, J.C.; Kim, W.J.; Choi, E.S.; Park, S.E.; Park, C.Y.; Park, S.W.; Oh, K.W. The association of unintentional changes in weight, body composition, and homeostasis model assessment index with glycemic progression in non-diabetic healthy subjects. Diabetes Metab. J. 2011, 35, 138–1488. [Google Scholar] [CrossRef] [Green Version]
- Son, J.; Lee, S.; Kim, S.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metb. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | Diabetes After AP (n = 26) | Prediabetes After AP (n = 54) | Normoglycemia After AP (n = 33) | Healthy Controls (n = 39) | p |
|---|---|---|---|---|---|
| Age (years) | 59 (49–69) | 57 (46–68) | 54 (46–62) | 49 (39–59) | 0.068 |
| Sex | 0.012 | ||||
| Men | 21 (80.8%) | 39 (53.7%) | 17 (51.5%) | 19 (48.7%) | |
| Women | 5 (19.2%) | 25 (46.3%) | 16 (48.5%) | 20 (51.3%) | |
| V/S Fat Volume Ratio | 0.95 (0.70–1.20) | 0.70 (0.35–1.05) | 0.50 (0.35–0.65) | 0.36 (0.21–51) | <0.001 |
| HbA1c (mmol/mol) | 52.0 (40.6–63.4) | 39.0 (36.8–41.2) | 34.0 (32.1–35.9) | 33 (30.5–35.5) | <0.001 |
| Fasting Plasma Glucose (mmol/L) | 7.60 (6.65–8.55) | 5.50 (5.10–5.90) | 4.90 (4.65–5.15) | 4.75 (4.30–5.20) | <0.001 |
| Physical Activity | 0.670 | ||||
| Inactive | 4 (23.5%) | 10 (22.7%) | 10 (35.7%) | 7 (26.9%) | |
| Active | 13 (76.5%) | 34 (87.3%) | 18 (64.3%) | 19 (73.1%) | |
| Smoking status | 0.093 | ||||
| Never | 12 (50.0%) | 20 (37.8%) | 13 (39.4%) | 22 (57.9%) | |
| Former | 9 (37.5%) | 20 (37.8%) | 13 (39.4%) | 11 (28.9%) | |
| Light | 2 (8.3%) | 6 (11.3%) | 3 (9.1%) | 5 (13.2%) | |
| Moderate/ heavy | 1 (4.2%) | 7 (13.2%) | 4 (12.1%) | 0 (0%) | |
| Alcohol consumption (U/week) | 21 (0–102) | 24 (0–93) | 96 (0–258) | 0 (0–2) | 0.152 |
| ACCI | 2.0 (0.5–3.5) | 2.0 (0.5–3.5) | 1.0 (0–1) | 0.0 (0.0–0.5) | 0.001 |
| Pancreatic amylase (U/L) | 25.0 (15.3–32.7) | 14.1 (7.6–20.6) | 14.1 (9.1–19.1) | 20.6 (12.1–29.1) | 0.073 |
| Study Groups | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| Normoglycemia | −24.135 | 16.626 | 0.149 | −24.264 | 11.095 | 0.030 | −23.420 | 12.64 | 0.066 | −23.254 | 12.940 | 0.075 | −21.626 | 11.397 | 0.060 |
| Prediabetes | 3.097 | 14.739 | 0.834 | −16.824 | 10.092 | 0.098 | −20.266 | 12.125 | 0.098 | −16.587 | 12.705 | 0.195 | −12.179 | 10.799 | 0.261 |
| Diabetes | −13.546 | 17.783 | 0.447 | −42.619 | 12.368 | 0.001 | −35.860 | 15.494 | 0.023 | −36.667 | 16.089 | 0.025 | −30.015 | 14.038 | 0.034 |
| Insulin Traits | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| Indices of insulin secretion | |||||||||||||||
| HOMA-ß | 0.026 | 0.109 | 0.816 | 0.020 | 0.077 | 0.795 | 0.035 | 0.079 | 0.663 | 0.068 | 0.073 | 0.355 | 0.039 | 0.075 | 0.605 |
| Stumvoll index | 0.000 | 0.001 | 0.540 | 0.000 | 0.000 | 0.486 | 0.000 | 0.000 | 0.606 | 0.000 | 0.000 | 0.993 | 0.000 | 0.000 | 0.923 |
| Insulinogenic index 30′ | 1.731 | 8.883 | 0.846 | −1.613 | 5.893 | 0.785 | −4.068 | 5.574 | 0.469 | −3.810 | 5.562 | 0.497 | −2.840 | 6.268 | 0.653 |
| Insulinogenic index 60′ | 11.858 | 8.787 | 0.183 | 5.398 | 6.427 | 0.405 | 4.670 | 6.084 | 0.446 | −2.496 | 5.883 | 0.674 | −3.227 | 6.025 | 0.595 |
| Indices of insulin sensitivity | |||||||||||||||
| HOMA-IS | −9.835 | 16.762 | 0.559 | −9.260 | 11.869 | 0.438 | −8.596 | 11.957 | 0.475 | −8.370 | 10.850 | 0.444 | −5.824 | 13.911 | 0.677 |
| Raynaud index | 7.307 | 7.116 | 0.307 | 5.247 | 4.718 | 0.269 | 3.877 | 4.763 | 0.418 | 1.947 | 4.477 | 0.665 | 2.133 | 4.527 | 0.639 |
| 1/fasting insulin | 42.089 | 40.987 | 0.307 | 30.225 | 27.174 | 0.269 | 22.332 | 27.437 | 0.418 | 11.216 | 25.786 | 0.665 | 12.283 | 26.075 | 0.639 |
| Matsuda index | 0.959 | 0.971 | 0.327 | 1.801 | 0.619 | 0.005 | 1.560 | 0.630 | 0.016 | 1.359 | 0.581 | 0.022 | 1.633 | 0.612 | 0.010 |
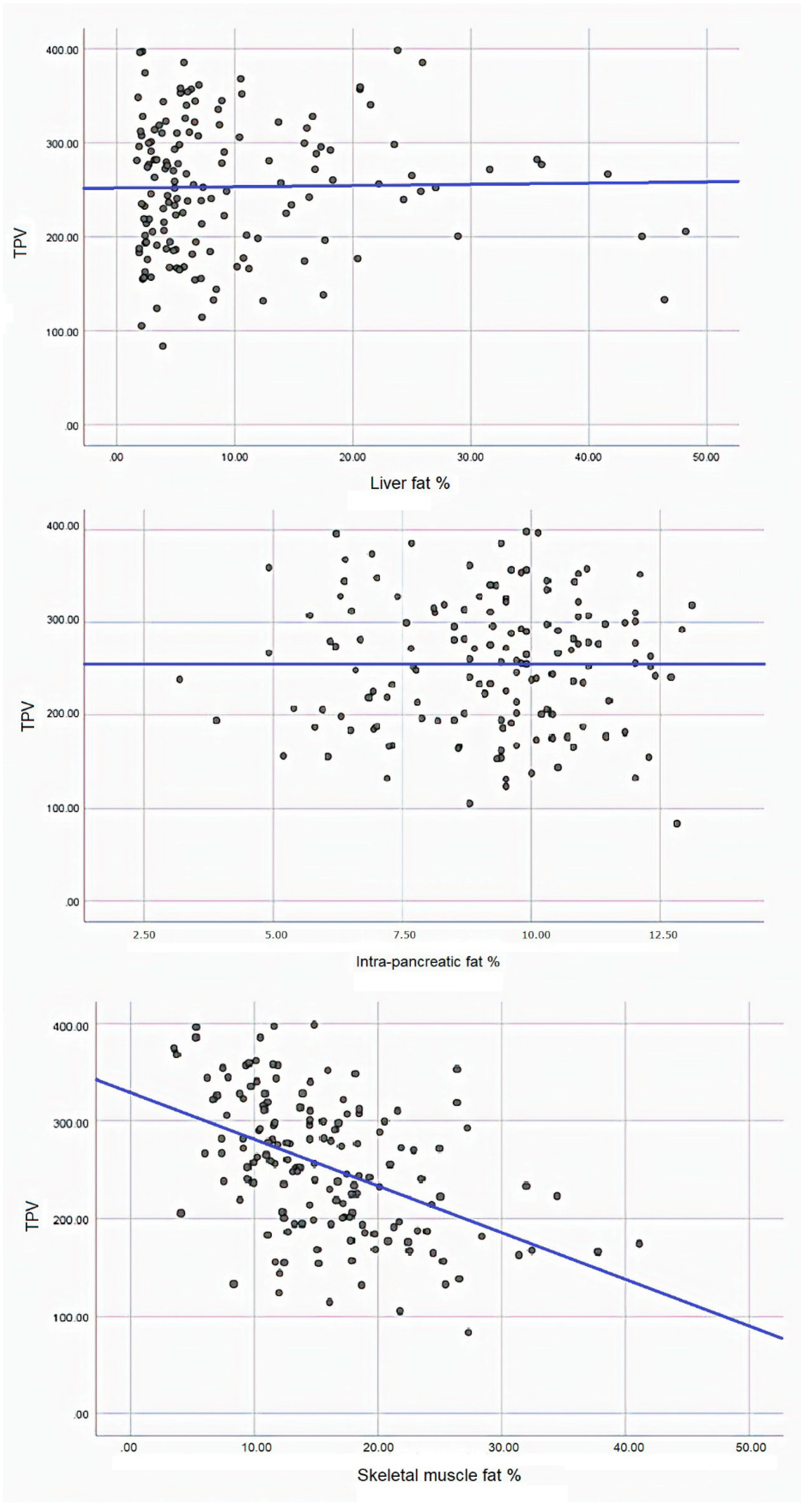
| Liver Fat | Intra-Pancreatic Fat | Skeletal Muscle Fat | ||
|---|---|---|---|---|
| Model 1 | β | 0.133 | 0.259 | −4.794 |
| SE | 0.609 | 2.891 | 0.749 | |
| p | 0.827 | 0.929 | <0.001 | |
| Model 2 | β | −0.518 | −1.725 | −2.321 |
| SE | 0.418 | 2.093 | 0.764 | |
| p | 0.218 | 0.411 | 0.003 | |
| Model 3 | β | 0.005 | 2.430 | −2.514 |
| SE | 0.537 | 2.379 | 0.841 | |
| p | 0.397 | 0.304 | 0.003 | |
| Model 4 | β | 0.322 | 2.675 | −2.590 |
| SE | 0.563 | 2.422 | 0.833 | |
| p | 0.569 | 0.272 | 0.002 | |
| Model 5 | β | −0.001 | 1.518 | −3.080 |
| SE | 0.480 | 2.257 | 0.761 | |
| p | 0.999 | 0.503 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Modesto, A.; Ko, J.; E. Stuart, C.; H. Bharmal, S.; Cho, J.; Petrov, M.S. Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study. Diseases 2020, 8, 25. https://doi.org/10.3390/diseases8030025
E. Modesto A, Ko J, E. Stuart C, H. Bharmal S, Cho J, Petrov MS. Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study. Diseases. 2020; 8(3):25. https://doi.org/10.3390/diseases8030025
Chicago/Turabian StyleE. Modesto, Andre, Juyeon Ko, Charlotte E. Stuart, Sakina H. Bharmal, Jaelim Cho, and Maxim S. Petrov. 2020. "Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study" Diseases 8, no. 3: 25. https://doi.org/10.3390/diseases8030025
APA StyleE. Modesto, A., Ko, J., E. Stuart, C., H. Bharmal, S., Cho, J., & Petrov, M. S. (2020). Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study. Diseases, 8(3), 25. https://doi.org/10.3390/diseases8030025





