Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients?
Abstract
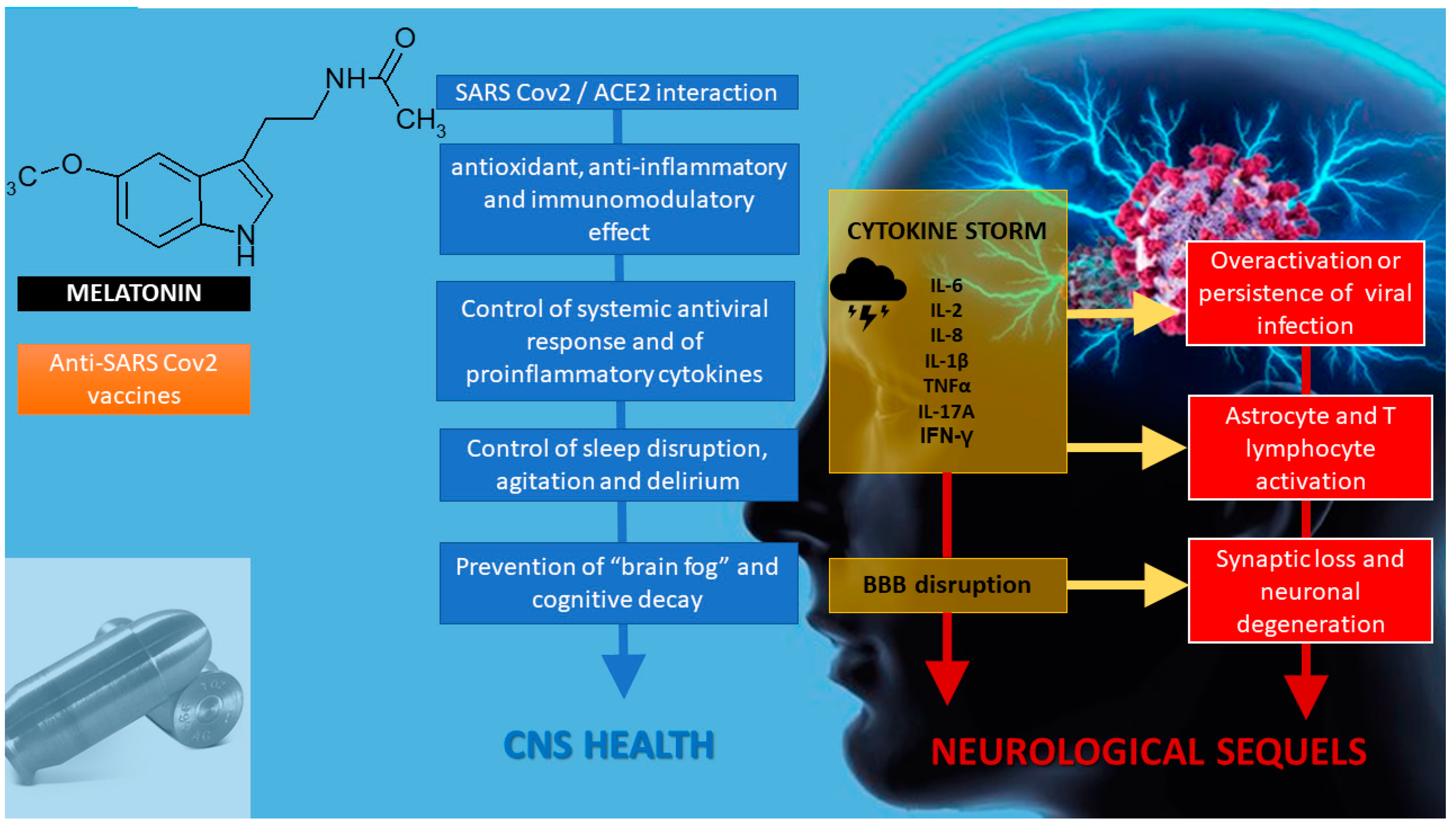
:1. Introduction
2. Melatonin in SARS-CoV-2 Infection
3. Melatonin as an Anti-Inflammatory/Immunoregulatory and Antioxidant Treatment
3.1. Anti-Inflammatory/Immunoregulatory Activity of Melatonin
3.2. Antioxidant Properties of Melatonin
4. Melatonin as a Chronobiotic Agent
5. Melatonin and Cytoprotection
6. Melatonin and Neuroprotection
7. Melatonin as an Adjuvant in Anti-SARS-CoV-2 Vaccination
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Roby, C.A. Werewolves (Creatures of Fantasy); Cavendish Square: New York, NY, USA, 2005. [Google Scholar]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Slominski, A.T.; Steinbrink, K.; Reiter, R.J. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients 2020, 12, 2561. [Google Scholar] [CrossRef]
- Wong, S.K.; Li, W.; Moore, M.J.; Choe, H.; Farzan, M. A 193-Amino Acid Fragment of the SARS Coronavirus S Protein Efficiently Binds Angiotensin-converting Enzyme. J. Biol. Chem. 2004, 279, 3197–3201. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Kallianpur, A.; Zein, J.; Culver, D.A.; Farha, S.; Comhair, S.; Fiocchi, C.; Gack, M.U.; et al. A Network Medicine Approach to Investigation and Population-based Validation of Disease Manifestations and Drug Repurposing for COVID-19. PLoS Biol. 2020, 18, e3000970. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Pooventhiran, T.; Alsalme, A.; Warad, I.; John, A.M.; Thomas, R. Structural and physico-chemical evaluation of melatonin and its solution-state excited properties, with emphasis on its binding with novel coronavirus proteins. J. Mol. Liq. 2020, 318, 114082. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Júnior, F.T.D.S.S.; Neto, J.A.D.O.N.; Matos, L.F.L.; Moura, M.H.D.S.; Rosales, T.O.; De Freitas, G.B.L. Covid-19: Rational discovery of the therapeutic potential of melatonin as a sars-cov-2 main protease inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef]
- Benítez-King, G.; Ríos, A.; Martínez, A.; Antón-Tay, F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim. Biophys. Acta Gen. Subj. 1996, 1290, 191–196. [Google Scholar] [CrossRef]
- Benítez-King, G.; Huerto-Delgadillo, L.; Antón-Tay, F. Binding of 3H-melatonin to calmodulin. Life Sci. 1993, 53, 201–207. [Google Scholar] [CrossRef]
- Lambert, D.W.; Clarke, N.E.; Hooper, N.M.; Turner, A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008, 582, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Jehi, L.; Ji, X.; Milinovich, A.; Erzurum, S.; Rubin, B.; Gordon, S.; Young, J.; Kattan, M.W. Individualizing risk prediction for positive COVID-19 testing: Results from 11,672 patients. Chest 2020, 158, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Arendse, L.B.; Jan Danser, A.H.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.A.S.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The renin-angiotensin system: Going beyond the classical paradigms. Am. J. Physiol. Hear. Circ. Physiol. 2019, 316, H958–H970. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Yang, R.C.; Lu, T.S. Two hits to the renin-angiotensin system may play a key role in severe COVID-19. Kaohsiung J. Med. Sci. 2020, 36, 389–392. [Google Scholar] [CrossRef]
- Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Maleki, V.; Moludi, J.; Alizadeh, M. The effects of melatonin on neurohormonal regulation in cardiac cachexia: A mechanistic review. J. Cell. Biochem. 2019, 120, 16340–16351. [Google Scholar] [CrossRef]
- Campos, L.A.; Cipolla-Neto, J.; Amaral, F.G.; Michelini, L.C.; Bader, M.; Baltatu, O.C. The angiotensin-melatonin axis. Int. J. Hypertens. 2013, 2013. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, A.U.; Kobori, H. Melatonin in chronic kidney disease: A promising chronotherapy targeting the intrarenal renin–angiotensin system. Hypertens. Res. 2019, 42, 920–923. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. Therapeutic potential of melatonin in immunodeficiency states, viral diseases, and cancer. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2000; Volume 467, pp. 217–226. [Google Scholar]
- Anderson, G.; Maes, M.; Markus, R.P.; Rodriguez, M. Ebola virus: Melatonin as a readily available treatment option. J. Med. Virol. 2015, 87, 537–543. [Google Scholar] [CrossRef]
- Abbas, A.; Lichman, A.; Pillai, S. Basic Immunology, 6th ed.; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Antony, P.A.; Restifo, N.P. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin. J. Immunother. 2005, 28, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryczek, I.; Wei, S.; Vatan, L.; Escara-Wilke, J.; Szeliga, W.; Keller, E.T.; Zou, W. Cutting Edge: Opposite Effects of IL-1 and IL-2 on the Regulation of IL-17 + T Cell Pool IL-1 Subverts IL-2-Mediated Suppression. J. Immunol. 2007, 179, 1423–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Grimm, E.A.; Mazumder, A.; Zhang, H.Z.; Rosenberg, S.A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 1982, 155, 1823–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Liu, Y. Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front. Immunol. 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Damoiseaux, J. The IL-2–IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin. Immunol. 2020, 218, 108515. [Google Scholar] [CrossRef]
- Brivio, F.; Fumagalli, L.; Parolini, D.; Messina, G.; Rovelli, F.; Rescaldani, R.; Vigore, L.; Vezzo, R.; Vaghi, M.; Di Bella, S.; et al. T-helper/T-regulator lymphocyte ratio as a new immunobiological index to quantify the anticancer immune status in cancer patients. In Vivo 2008, 22, 647–650. [Google Scholar]
- Yu, Z.X.; Ji, M.S.; Yan, J.; Cai, Y.; Liu, J.; Yang, H.F.; Li, Y.; Jin, Z.C.; Zheng, J.X. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care 2015, 19, 82. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, F.; Corbi, G.; Mazzeo, G.; Boccia, M.; Aronne, L.; D’Agnano, V.; Komici, K.; Mazzarella, G.; Parrella, R.; Bianco, A. COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 2020, 32, 1599–1608. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cunha Pedrosa, A.M.; Weinlich, R.; Mognol, G.P.; Robbs, B.K.; de Biaso Viola, J.P.; Campa, A.; Amarante-Mendes, G.P. Melatonin Protects CD4 + T Cells from Activation-Induced Cell Death by Blocking NFAT-Mediated CD95 Ligand Upregulation. J. Immunol. 2010, 184, 3487–3494. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Xu, S.P.; Wu, Y.; Jiang, Y.X.; Wu, Z.Y.; Yuan, S.Y.; Yao, S.L. Melatonin reduces acute lung injury in endotoxemic rats. Chin. Med. J. 2009, 122, 1388–1393. [Google Scholar]
- Deng, W.G.; Tang, S.T.; Tseng, H.P.; Wu, K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108, 518–524. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.B.; Souza, G.; Muraro, S.; Carregari, V.; Biagi, C.; Crunfli, F.; Restrepo, J.; Vendramini, P.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Liu, C.; Manucha, W.; Abreu-Gonzalez, P.; Dominguez-Rodriguez, A. Plasticity of glucose metabolism in activated immune cells: Advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 2020, 3, 362–379. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.J.; Acuna-Castroviejo, D. Melatonin mitigates mitochondrial meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Zare Javid, A. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: A double-blind, placebo-controlled trial. Inflammopharmacology 2019, 27, 67–76. [Google Scholar] [PubMed]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kücükakin, B.; Lykkesfeldt, J.; Nielsen, H.J.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. Utility of melatonin to treat surgical stress after major vascular surgery—A safety study. J. Pineal Res. 2008, 44, 426–431. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, C.; Li, T.; Wang, W.; Ye, W.; Zeng, R.; Ni, L.; Lai, Z.; Wang, X.; Liu, C. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In vivo assessment and a randomized controlled trial. J. Pineal Res. 2018, 65, e12521. [Google Scholar] [CrossRef]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T.; et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P. Are melatonin doses employed clinically adequate for melatonin-induced cytoprotection? Melatonin Res. 2019, 2, 106–132. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Volt, H.; García, J.A.; Doerrier, C.; Díaz-Casado, M.E.; Guerra-Librero, A.; Lõpez, L.C.; Escames, G.; Tresguerres, J.A.; Acuña-Castroviejo, D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60, 193–205. [Google Scholar] [CrossRef]
- Dai, W.; Huang, H.; Si, L.; Hu, S.; Zhou, L.; Xu, L.; Deng, Y. Melatonin prevents sepsis-induced renal injury via the PINK1/Parkin1 signaling pathway. Int. J. Mol. Med. 2019, 44, 1197–1204. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xie, W.; Hu, S.; Zhou, H.; Zhu, P.; Zhu, H. Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell. Physiol. 2020, 235, 2847–2856. [Google Scholar] [CrossRef]
- Chen, J.; Xia, H.; Zhang, L.; Zhang, H.; Wang, D.; Tao, X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019, 117, 109150. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Amodio, A.; Romeo, C.; Cuzzocrea, E.; Sabatino, G.; Buonocore, G.; Cordaro, V.; Trimarchi, G.; Barberi, I. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J. Pineal Res. 2004, 36, 250–255. [Google Scholar] [CrossRef]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Xian Tan, D.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of melatonin treatment in septic newborns. Pediatr. Res. 2001, 50, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I.; et al. Melatonin Reduces Oxidative Stress in Surgical Neonates. J. Pediatric Surg. 2004, 39, 184–189. [Google Scholar] [CrossRef]
- El-Gendy, F.; El-Hawy, M.; Hassan, M.G. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern Fetal Neonatal Med. 2018, 31, 2299–2303. [Google Scholar] [CrossRef]
- Lewandowska, K.; Małkiewicz, M.A.; Siemiński, M.; Cubała, W.J.; Winklewski, P.J.; Mędrzycka-Dąbrowska, W.A. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit—A clinical review. Sleep Med. 2020, 69, 127–134. [Google Scholar] [CrossRef]
- Cardinali, D.P. High doses of melatonin as a potential therapeutic tool for the neurologic sequels of covid-19 infection. Melatonin Res. 2020, 3, 311–317. [Google Scholar] [CrossRef]
- Castillo, R.R.; Quizon, G.R.A.; Juco, M.J.M.; Roman, A.D.E.; De Leon, D.G.; Punzalan, F.E.R.; Guingon, R.B.L.; Morales, D.D.; Tan, D.-X.; Reiter, R.J. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): A case series. Melatonin Res. 2020, 3, 297–310. [Google Scholar] [CrossRef]
- Ramlall, V.; Zucker, J.; Tatonetti, N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Dawson, D.; Armstrong, S.M. Chronobiotics—Drugs that shift rhythms. Pharmacol. Ther. 1996, 69, 15–36. [Google Scholar] [CrossRef]
- Lewy, A.; Emens, J.; Jackman, A.; Yuhas, K. Circadian uses of melatonin in humans. Chronobiol. Int. 2006, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef]
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef] [Green Version]
- Gulia, K.K.; Kumar, V.M. Importance of Sleep for Health and Wellbeing Amidst COVID-19 Pandemic. Sleep Vigil. 2020, 4, 49–50. [Google Scholar] [CrossRef]
- Jawaid, A. Protecting older adults during social distancing. Science 2020, 368, 145. [Google Scholar]
- Duffy, J.F.; Zitting, K.M.; Chinoy, E.D. Aging and circadian rhythms. Sleep Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P. Melatonin as a chronobiotic/cytoprotector: Its role in healthy aging. Biol. Rhythm Res. 2019, 50, 28–45. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, G.; Otsuka, K. Chronobiology of Aging: A Mini-Review. Gerontology 2017, 63, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [Green Version]
- Zaki, N.F.W.; Spence, D.W.; BaHammam, A.S.; Pandi-Perumal, S.R.; Cardinali, D.P.; Brown, G.M. Chronobiological theories of mood disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 107–118. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Brown, G.M.; Reiter, R.J.; Pandi-Perumal, S.R. Elderly as a High-risk Group during COVID-19 Pandemic: Effect of Circadian Misalignment, Sleep Dysregulation and Melatonin Administration. Sleep Vigil. 2020, 1–7. [Google Scholar] [CrossRef]
- Haspel, J.A.; Anafi, R.; Brown, M.K.; Cermakian, N.; Depner, C.; Desplats, P.; Gelman, A.E.; Haack, M.; Jelic, S.; Kim, B.S.; et al. Perfect timing: Circadian rhythms, sleep, and immunity—An NIH workshop summary. JCI Insight 2020, 5, e131487. [Google Scholar] [CrossRef] [Green Version]
- Al-Waeli, H.; Nicolau, B.; Stone, L.; Abu Nada, L.; Gao, Q.; Abdallah, M.; Abdulkader, E.; Suzuki, M.; Mansour, A.; Al Subaie, A.; et al. Chronotherapy of Non-Steroidal Anti-Inflammatory Drugs May Enhance Postoperative Recovery. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Tang, S.Y.; Devine, J.C.; Anderson, S.T.; Nayak, S.; Zhang, S.L.; Valenzuela, A.; Fisher, D.G.; Grant, G.R.; López, C.B.; et al. Circadian control of lung inflammation in influenza infection. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020, NEJMoa2021436. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of delirium in critically ill patients: Systematic review and meta-analysis. BMJ 2015, 350, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Gao, F.; Zhang, S.; Sun, W.; Li, Z. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: A systematic review and meta-analysis of randomized controlled trials. Sleep Breath. 2019, 23, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrelli, E.; Canevini, M.; Gambini, O.; D’Agostino, A. Delirium and sleep disturbances in COVID–19: A possible role for melatonin in hospitalized patients? Sleep Med. 2020, 70, 111. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryckman, K.K. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 295–302. [Google Scholar]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, N.; Fleming-Dutra, K.; Gierke, R.; Hall, A.; Hughes, M.; Pilishvili, T.; Ritchey, M.; Roguski, K.; Skoff, T.; Ussery, E. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12–March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 382–386. [Google Scholar]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [Green Version]
- Simko, F.; Hrenak, J.; Dominguez-Rodriguez, A.; Reiter, R.J. Melatonin as a putative protection against myocardial injury in COVID-19 infection. Expert Rev. Clin. Pharmacol. 2020, 13, 921–924. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; García, J.A.; Doerrier, C.; Volt, H.; Escames, G.; Lõpez, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Analysis of the daily changes of melatonin receptors in the rat liver. J. Pineal Res. 2013, 54, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; López-Muñoz, F.; Gil-Martín, E.; Escames, G.; Reiter, R.J. Coronavirus Disease 2019 (COVID-19) and Its Neuroinvasive Capacity: Is It Time for Melatonin? Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, H.; Magira, E.; Bitzogli, K.; Kafasi, N.; Vlachoyiannopoulos, P.; Tzioufas, A.; Kotanidou, A.; Dalakas, M.C. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Kremer, S.; Lersy, F.; de Sèze, J.; Ferré, J.-C.; Maamar, A.; Carsin-Nicol, B.; Collange, O.; Bonneville, F.; Adam, G.; Martin-Blondel, G.; et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology 2020, 297, 202222. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N = 84,285 online study. medRxiv 2020. [Google Scholar] [CrossRef]
- Raj, V.; Opie, M.; Arnold, A.C. Cognitive and psychological issues in postural tachycardia syndrome. Auton. Neurosci. Basic Clin. 2018, 215, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.; Paterson, F.; Bacchi, S.; Page, A.; Baumert, M.; Lau, D.H. Brain fog in postural tachycardia syndrome: An objective cerebral blood flow and neurocognitive analysis. J. Arrhythmia 2020, 36, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P. Melatonin: Clinical perspectives in neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Brusco, L.I. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants 2014, 3, 245–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Furio, A.M.; Brusco, L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012, 1, 280. [Google Scholar]
- Eraslan, M.; Cerman, E.; Yildiz Balci, S.; Celiker, H.; Sahin, O.; Temel, A.; Suer, D.; Tuncer Elmaci, N. The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: Enhanced depth imaging optical coherence tomography study. Acta Ophthalmol. 2016, 94, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, H.; Sun, H.; Hu, B.; Geng, Y. Dietary melatonin therapy alleviates the lamina cribrosa damages in patients with mild cognitive impairments: A double-blinded, randomized controlled study. Med. Sci. Monit. 2020, 26, e923232-1. [Google Scholar]
- Maestroni, G. Exogenous melatonin as potential adjuvant in anti-SarsCov2 vaccines. J. Neuroimmune Pharmacol. 2020, 1–2. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. The immunoneuroendocrine role of melatonin. J. Pineal Res. 1993, 14, 1–10. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Brusco, L.I.; Selgas, L.; Esquifino, A.I. Diurnal rhythms in ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats during Freund’s adjuvant-induced arthritis. Brain Res. 1998, 789, 283–292. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Castrillón, P.O.; Esquifino, A.I.; Varas, A.; Zapata, A.; Cutrera, R.A.; Cardinali, D.P. Effect of melatonin treatment on 24-h variations in responses to mitogens and lymphocyte subset populations in rat submaxillary lymph nodes. J. Neuroendocrinol. 2000, 12, 758–765. [Google Scholar] [CrossRef]
- Moreno, A.C.; Porchia, B.F.; Pagni, R.L.; Souza, P.D.C.; Pegoraro, R.; Rodrigues, K.B.; Barros, T.B.; Aps, L.R.; de Araújo, E.F.; Calich, V.L.; et al. The Combined Use of Melatonin and an Indoleamine 2,3-Dioxygenase-1 Inhibitor Enhances Vaccine-Induced Protective Cellular Immunity to HPV16-Associated Tumors. Front. Immunol. 2018, 9, 1914. [Google Scholar] [CrossRef]
- Baghban Rahimi, S.; Mohebbi, A.; Vakilzadeh, G.; Biglari, P.; Razeghi Jahromi, S.; Mohebi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018, 163, 587–597. [Google Scholar] [CrossRef]
- Regodón, S.; Martín-Palomino, P.; Fernández-Montesinos, R.; Herrera, J.L.; Carrascosa-Salmoral, M.P.; Píriz, S.; Vadillo, S.; Guerrero, J.M.; Pozo, D. The use of melatonin as a vaccine agent. Vaccine 2005, 23, 5321–5327. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Papachristodoulou, E.; Kakoullis, L.; Parperis, K.; Panos, G. Long-term and herd immunity against SARS-CoV-2: Implications from current and past knowledge. Pathog. Dis. 2020, 78, ftaa025. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Śnchez, N.; Rodrĩguez-Rodrĩguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. https://doi.org/10.3390/diseases8040044
Cardinali DP, Brown GM, Pandi-Perumal SR. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases. 2020; 8(4):44. https://doi.org/10.3390/diseases8040044
Chicago/Turabian StyleCardinali, Daniel P., Gregory M. Brown, and Seithikurippu R. Pandi-Perumal. 2020. "Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients?" Diseases 8, no. 4: 44. https://doi.org/10.3390/diseases8040044
APA StyleCardinali, D. P., Brown, G. M., & Pandi-Perumal, S. R. (2020). Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases, 8(4), 44. https://doi.org/10.3390/diseases8040044





