Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management
Abstract
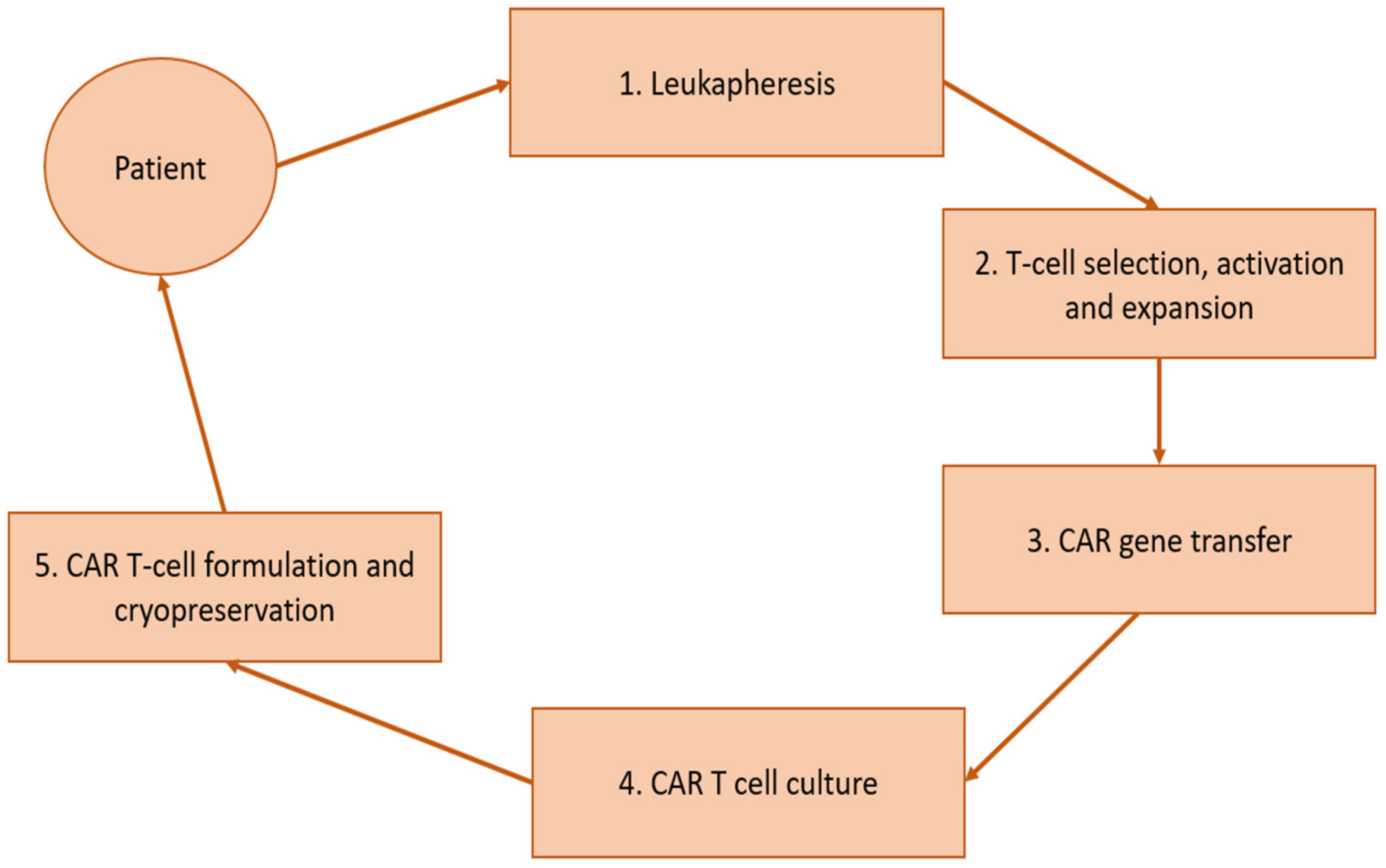
1. Introduction
2. Cardiotoxicity
2.1. Cardiotoxicity in Pediatric and Young Adult Patients
2.2. Cardiotoxicity in Adult Patients
3. Risks for Developing Cardiotoxic Events
4. Management and Treatment
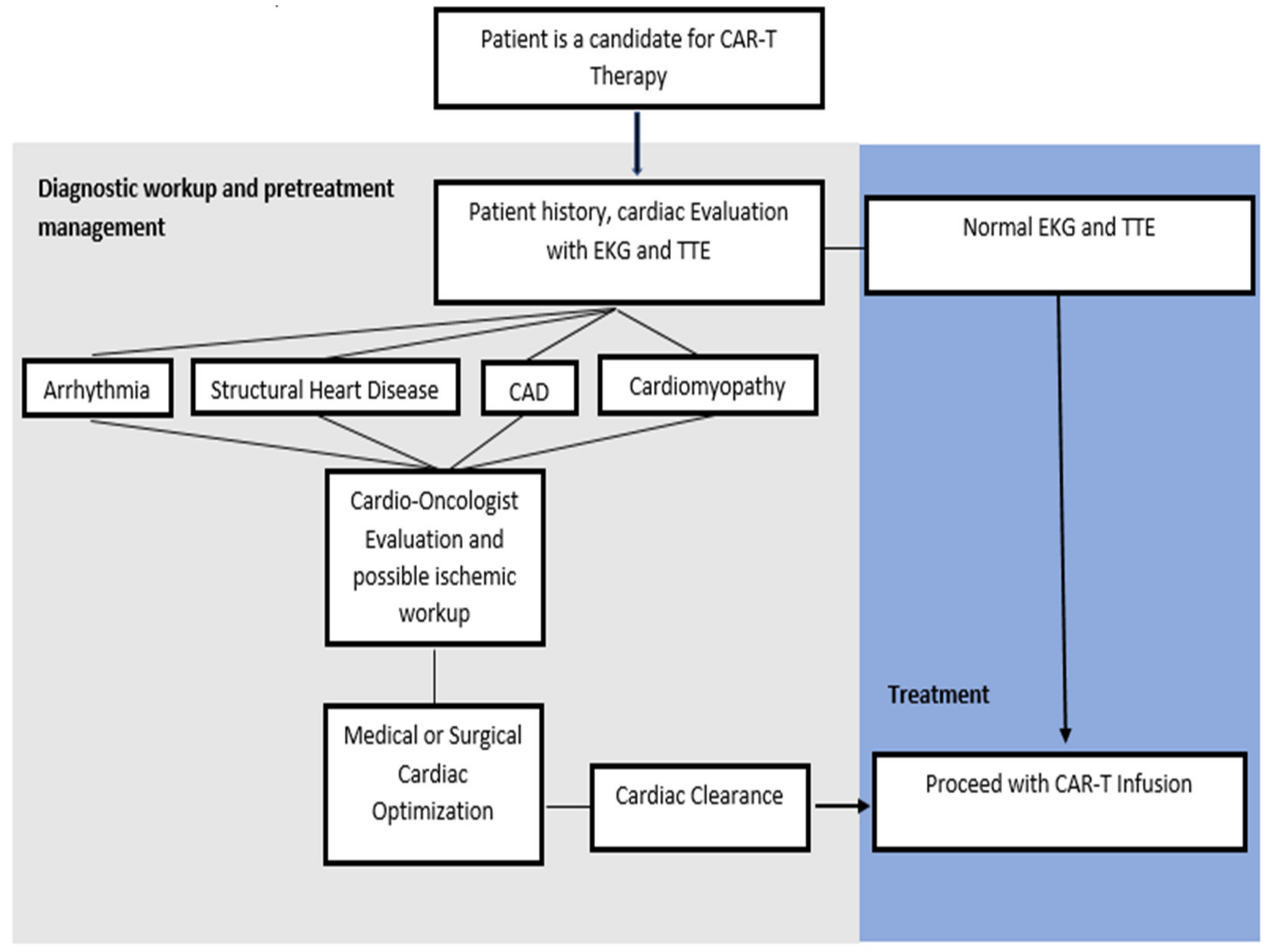
4.1. Pre-CAR-T Infusion Cardiovascular Considerations
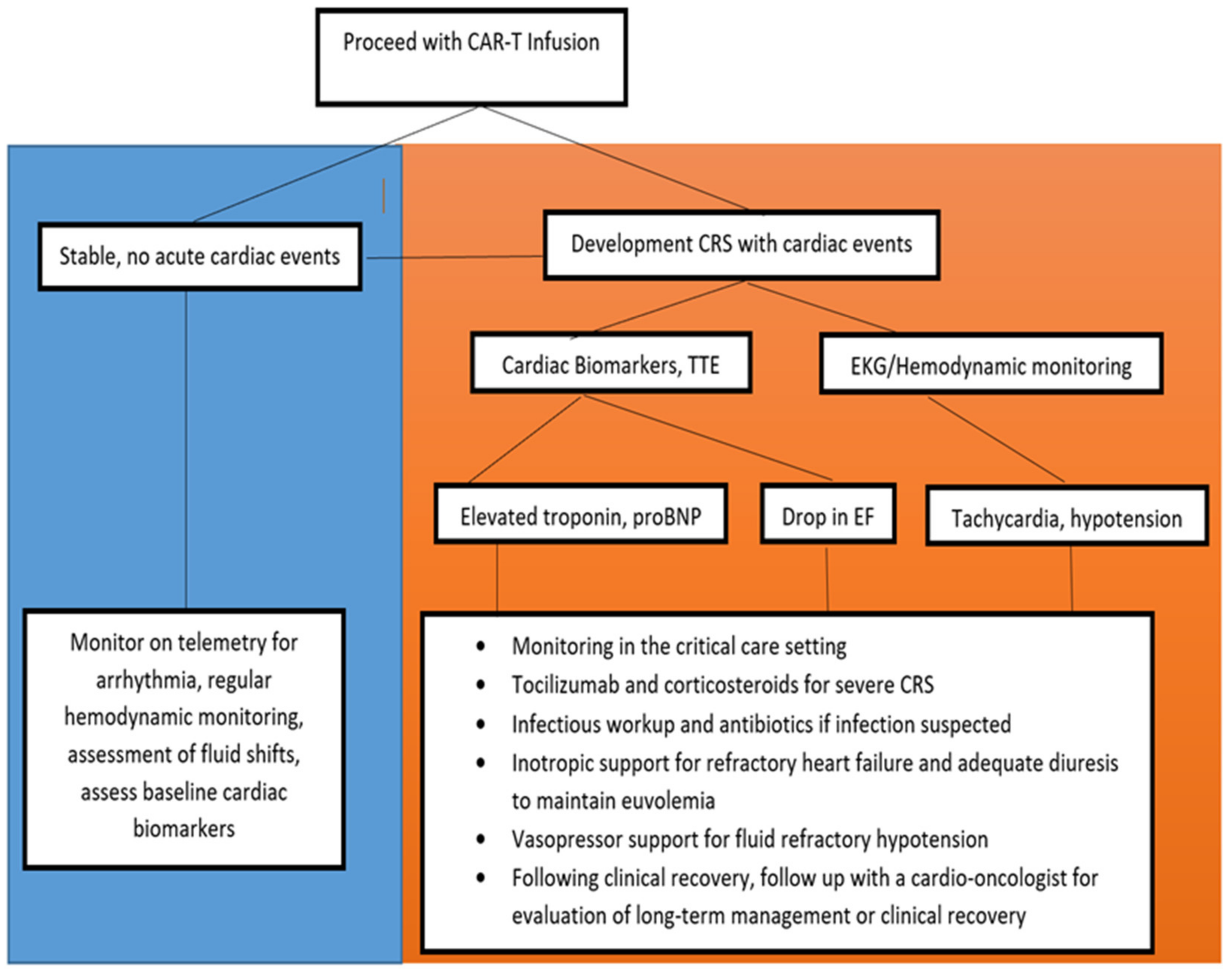
4.2. Clinical Monitoring during and after CAR-T Infusion
4.3. Management of Cardiovascular Events
4.3.1. Supportive Care
4.3.2. IL-6 Inhibitor Therapy
4.3.3. Corticosteroid Therapy
4.3.4. The Role of IL-1 Therapy
4.3.5. The Role of TNF-α Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lulla, P.D.; Hill, L.C.; Ramos, C.A.; Heslop, H.E. The use of chimeric antigen receptor T cells in patients with non-Hodgkin lym-phoma. Clin. Adv. Hematol. Oncol. 2018, 16, 375–386. [Google Scholar] [PubMed]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T Cell Therapy beyond Oncology: Autoimmune Diseases and Viral Infections. Biomedcines 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Filin, I.Y.; Solovyeva, V.V.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A. Current Trends in Cancer Immunotherapy. Biomedcines 2020, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Roberts, Z.J.; Better, M.; Bot, A.; Roberts, M.R.; Ribas, A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk. Lymphoma 2017, 59, 1785–1796. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; MacKenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy–Related Cardiovascular Outcomes and Management. JACC Cardio Oncol. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- McHayleh, W.; Bedi, P.; Sehgal, R.; Solh, M. Chimeric Antigen Receptor T-Cells: The Future is Now. J. Clin. Med. 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Penack, O.; Koenecke, C. Complications after CD19+ CAR T-Cell Therapy. Cancers 2020, 12, 3445. [Google Scholar] [CrossRef]
- Porter, D.; Frey, N.; Wood, P.A.; Weng, Y.; Grupp, S.A. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.A.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.C.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Rothberg, M.V.; Holman, P.; Yang, E.H. Immunotherapy-Associated Cardiotoxicity of Immune Checkpoint Inhibitors and Chimeric Antigen Receptor T Cell Therapy: Diagnostic and Management Challenges and Strategies. Curr. Cardiol. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oved, J.H.; Barrett, D.M.; Teachey, D.T. Cellular therapy: Immune-related complications. Immunol. Rev. 2019, 290, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Redd, R.; Hayek, S.S.; Parikh, R.; Azam, T.; Yanik, G.A.; Spendley, L.; Nikiforow, S.; Jacobson, C.; Nohria, A. Chimeric Antigen Receptor T-Cell Therapy–Associated Cardiomyopathy in Patients with Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation 2020, 142, 1687–1690. [Google Scholar] [CrossRef]
- Pathan, N.; Hemingway, C.A.; Alizadeh, A.A.; Stephens, A.C.; Boldrick, J.C.; Oragui, E.E.; McCabe, C.; Welch, S.B.; Whitney, A.; O’Gara, P.; et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 2004, 363, 203–209. [Google Scholar] [CrossRef]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef]
- Yáñez, L.; Sánchez-Escamilla, M.; Perales, M.-A. CAR T Cell Toxicity: Current Management and Future Directions. HemaSphere 2019, 3, e186. [Google Scholar] [CrossRef] [PubMed]
- Kymriah-Epar-Product-Information. Available online: https://www.ema.europa.eu/documents/product-information/kymriah-epar-product-information_en.pdf (accessed on 1 March 2021).
- Yescarta-Epar-Product-Information. Available online: https://www.ema.europa.eu/documents/product-information/yescarta-epar-product-information_en.pdf (accessed on 1 March 2021).
- Kochenderfer, J.N.; Somerville, R.P.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated with High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef]
- Shalabi, H.; Sachdev, V.; Kulshreshtha, A.; Cohen, J.W.; Yates, B.; Rosing, D.R.; Sidenko, S.; Delbrook, C.; Mackall, C.; Wiley, B.; et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J. Immunother. Cancer 2020, 8, e001159. [Google Scholar] [CrossRef]
- Burstein, D.S.; Maude, S.; Grupp, S.; Griffis, H.; Rossano, J.; Lin, K. Cardiac Profile of Chimeric Antigen Receptor T Cell Therapy in Children: A Single-Institution Experience. Biol. Blood Marrow Transpl. 2018, 24, 1590–1595. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Weiss, S.L.; Maude, S.L.; Barrett, D.M.; Lacey, S.F.; Melenhorst, J.J.; Shaw, P.; Berg, R.A.; June, C.H.; Porter, D.L.; et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit. Care Med. 2017, 45, e124–e131. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Kang, Y.; Smith, A.M.; Frey, N.V.; Carver, J.R.; Scherrer-Crosbie, M. Cardiovascular Effects of CAR T Cell Therapy. JACC Cardio Oncol. 2020, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of Immune Therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef]
- Aldoss, I.; Khaled, S.K.; Budde, E.; Stein, A.S. Cytokine Release Syndrome with the Novel Treatments of Acute Lymphoblastic Leukemia: Pathophysiology, Prevention, and Treatment. Curr. Oncol. Rep. 2019, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Carver, J.R.; Hayek, S.S.; Ky, B.; Leja, M.J.; Lenihan, D.J.; Lenneman, C.; Mousavi, N.; Park, J.H.; Perales, M.A.; et al. Chimeric Antigen Receptor T-Cell Therapy for Cancer and Heart. J. Am. Coll. Cardiol. 2019, 74, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.A.; Khaled, S.K. The Cardiovascular Complications of Chimeric Antigen Receptor T Cell Therapy. Curr. Hematol. Malign-Rep. 2020, 15, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Dal’Bo, N.; Patel, R.; Parikh, R.; Shah, S.P.; Guha, A.; Dani, S.S.; Ganatra, S. Cardiotoxicity of Contemporary Anticancer Immunotherapy. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A. Cardiotoxicity of Immunotherapy: Incidence, Diagnosis, and Management. Curr. Oncol. Rep. 2018, 20, 1–7. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Murthy, H.; Iqbal, M.; Chavez, J.C.; Kharfan-Dabaja, M.A. Cytokine Release Syndrome: Current Perspectives. ImmunoTargets Ther. 2019, 8, 43–52. [Google Scholar] [CrossRef]
- Riegler, L.L.; Jones, G.P.; Lee, D.W. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther. Clin. Risk Manag. 2019, 15, 323–335. [Google Scholar] [CrossRef]
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef]
- Mahmoudjafari, Z.; Hawks, K.G.; Hsieh, A.A.; Plesca, D.; Gatwood, K.S.; Culos, K.A. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on Chimeric Antigen Receptor T Cell Therapy Administrative, Logistic, and Toxicity Management Practices in the United States. Biol. Blood Marrow Transpl. 2019, 25, 26–33. [Google Scholar] [CrossRef]
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy—A systematic review. Int. J. Cardiol. 2021, 323, 179–187. [Google Scholar] [CrossRef]
- Annane, D.; Maxime, V.; Ibrahim, F.; Alvarez, J.C.; Abe, E.; Boudou, P. Diagnosis of Adrenal Insufficiency in Severe Sepsis and Septic Shock. Am. J. Respir. Crit. Care Med. 2006, 174, 1319–1326. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef] [PubMed]
- Bethin, K.E.; Vogt, S.K.; Muglia, L.J. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc. Natl. Acad. Sci. USA 2000, 97, 9317–9322. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.F.; Abbate, A. Interleukin-1 blockade in cardiovascular diseases: A clinical update. Eur. Hear. J. 2018, 39, 2063–2069. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Giavridis, T.; Van Der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Jatiani, S.S.; Aleman, A.; Madduri, D.; Chari, A.; Cho, H.J.; Richard, S.; Richter, J.; Brody, J.; Jagannath, S.; Parekh, S. Myeloma CAR-T CRS Management With IL-1R Antagonist Anakinra. Clin. Lymphoma Myeloma Leuk. 2020, 20, 632–636.e1. [Google Scholar] [CrossRef]
- Wang, J.; Mou, N.; Yang, Z.; Li, Q.; Jiang, Y.; Meng, J.; Liu, X.; Deng, Q. Efficacy and safety of humanized anti-CD19CAR-T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia. Br. J. Haematol. 2020, 191, 212–222. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Xu, J.; Zhang, R.; Zhu, H.; Wu, Y.; Zhu, L.; Li, J.; Chen, L. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp. Hematol. Oncol. 2021, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Otero, P.; Prósper, F.; Alfonso, A.; Paiva, B.; Miguel, J.F.S.S. CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. J. Clin. Med. 2020, 9, 3577. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
| Penn Criteria [12] | ||||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Mild reaction: Treated with supportive care, such as antipyretics, antiemetics | Moderate reaction: Some signs of organ dysfunction (grade 2 creatinine or grade 3 LFTs) related to CRS and not attributable to any other condition. Hospitalization for management of CRS-related symptoms, including neutropenic fever and need for i.v. therapies (not including fluid resuscitation for hypotension) | More severe reaction: Hospitalization required for management of symptoms related to organ dysfunction, including grade 4 LFTs or grade 3 creatinine, related to CRS and not attributable to any other condition. Hypotension treated with multiple fluid boluses or low-dose vasopressors. Coagulopathy requiring fresh frozen plasma, cryoprecipitate, or fibrinogen concentrate. Hypoxia requiring supplemental oxygen (nasal cannula oxygen, high-flow oxygen, CPAP, or BiPAP) | Life-threatening complications such as hypotension requiring high-dose vasopressors. Hypoxia requiring mechanical ventilation | |
| Lee Criteria [13] | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Symptoms are not life-threatening and require symptomatic treatment only (fever, nausea, fatigue, headache, myalgias, malaise) | Symptoms require and respond to moderate intervention: Oxygen requirement < 40% FiO2, OR hypotension responsive to i.v. fluids or low dose of one vasopressor, OR grade 2 organ toxicity * | Symptoms require and respond to aggressive intervention: Oxygen requirement ≥ 40% FiO2, OR Hypotension requiring high-dose or multiple vasopressors, OR grade 3 organ toxicity, or grade 4 transaminitis | Life-threatening symptoms: Requirement for ventilator support, OR grade 4 organ toxicity (excluding transaminitis) | |
| ASTCT Consensus Criteria [14] | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Temperature ≥ 38 °C, no hypotension, no hypoxia | Temperature ≥ 38 °C, with hypotension not requiring vasopressors, and/or hypoxia requiring low flow nasal cannula | Temperature ≥ 38 °C, with hypotension requiring vasopressors with or without vasopressin, and/or hypoxia requiring high-flow nasal cannula, facemask, nonrebreather mask, or venturi mask | Temperature ≥ 38 °C, with hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia requiring positive pressure (CPAP, BiPAP, intubation, and mechanical ventilation) | |
| Reported Cardiotoxic Events with FDA Approved CD19 CAR-T |
|---|
| Tachycardia |
| Hypotension |
| Fluid refractory hypotension |
| Pulmonary Edema |
| Depressed left ventricular function |
| Cardiac failure |
| Cardiac failure requiring inotropic support |
| Elevated troponin |
| Arrhythmia |
| ST-segment changes |
| Cardiac arrest |
| CD19-CAR-T Infusion | Tisagenlecleucel | Tisagenlecleucel | Axicabtagene Ciloleucel | Brexucabtagene Autoleucel |
|---|---|---|---|---|
| Trial | ELIANA [8] | JULIET [7] | ZUMA-1 [9] | ZUMA-2 [10] |
| Pertinent cardiovascular trial exclusion criteria | -Left Ventricular systolic function ≤ 28% confirmed by echocardiogram -Left ventricular ejection fraction ≤ 45% confirmed by echocardiogram or multigated acquisition images within 7 days of screening | -Unstable Angina or MI within 6 months of planned infusion -Uncontrolled arrhythmia | -EF < 50% determined by transthoracic echocardiogram -Evidence of pericardial effusion -Presence of clinically significant ECG findings | -Cardiac ejection fraction < 50% -Evidence of pericardial effusion -Clinically significant electrocardiogram findings -Myocardial infarction, cardiac angioplasty or stenting, unstable angina, active arrhythmias, or other clinically significant cardiac disease within 12 months of enrollment -Cardiac atrial or cardiac ventricular lymphoma involvement |
| FDA Approved CD19-CAR-T | Tisagenlecleucel | Tisagenlecleucel | Axicabtagene Ciloleucel | Brexucabtagene Autoleucel |
|---|---|---|---|---|
| Trial | JULIET [7] | ELIANA [8] | ZUMA-1 [9] | ZUMA-2 [10] |
| Disease | Adult LBCL | Pediatric B-ALL | Adult LBCL | Adult MCL |
| Study Phase | 2 | 1–2 | 2 | 2 |
| Patients Studied in Efficacy Analysis | 93 | 75 | 101 | 68 |
| Objective Response Rate | 50% | 83% | 82% | 93% |
| Complete Response | 40% | 60% | 54% | 67% |
| 12 month RFS/PFS | 65% | 59% | 44% | 61% |
| 12 month OS | 49% (estimated) | 76% | 59% | 83% |
| Patients Studied in Safety Analysis | 111 | 75 | 101 | 68 |
| Percent with any Grade AE | 100% | 100% | 100% | 100% |
| CRS | 64 (58%) | 58 (77%) | 94 (93%) | 61 (91%) |
| CRS Grading System | Penn Criteria [12] | Penn Criteria [12] | Lee Criteria [13] | Lee Criteria [13] |
| Tocilizumab Use | 16 (14%) | 36 (48%) | 49 (48.5%) | 42 (61.8%) |
| Hypotension | 29 (26%) | 22(29%) | 60 (59%) | 35 (51%) |
| Hypotension requiring inotropic support or shock | 8 (9%) | 13 (17%) | 14 (14%) | 15 (22%) |
| Pulmonary Edema | NR | 5 (6.7%) | NR | NR |
| Left Ventricular Dysfunction | NR | 3 (4.0%) | NR | NR |
| Cardiac Arrest | NR | 3(4.0%) | NR | NR |
| Cardiac Failure | NR | 2 (2.7%) | NR | NR |
| Tachycardia | 12 (11%) | 3 (4.0%) | 39 (39%) | 21 (31%) |
| CD19-CAR-T Cardiovascular Events | Shalabi et al. (2020) [28] | Burstein et al. (2018) [29] | Fitzgerald et al. (2017) [30] |
|---|---|---|---|
| Patient Population | Pediatric (n = 52) | Pediatric (n = 98) | Pediatric (n = 39) |
| Treatment Indication | |||
| B-ALL | 50 (96.1%) | 90 (97%) | 39 (100%) |
| NHL | 2 (3.9%) | 1 (1%) | 0 |
| Multiple Myeloma | 0 | 0 | 0 |
| T-ALL | 0 | 1 (1%) | 0 |
| PML | 0 | 1 (1%) | 0 |
| CRS Grading System | Penn Criteria [12] ASTCT Consensus Criteria [14] | Penn Criteria [12] | Penn Criteria [12] |
| Cardiotoxic Events | |||
| Pre-existing Cardiomyopathy/Structural Disease/Arrhythmia | 6 (11.5%) | 10 (11%)/1(5%) | NR |
| Hypotension Requiring Inotropic Support | 9 (24.3%) | 24 (24%) | 13 (33%) |
| Troponemia | NR | NR | NR |
| Ventricular Systolic Dysfunction | 6 (11.5%) | 10 (10%) | 1 (2%) |
| Tachycardia | 36 (69.2%) | NR | NR * |
| Arrhythmia | NR | NR | NR |
| ST segment changes | NR | 6 (6%) | NR |
| Cardiac Arrest/ Cardiac Death | 1 (2.7%) | 0 | NR |
| Required Tocilizumab | 14 (37.8%) | 21 (21%) | 13 (33%) |
| CD19-CAR-T Cardiovascular Events | Ganatra et al. (2020) [18] | Alvi et al. (2019) [31] | Lefebvre et al. (2020) [32] |
|---|---|---|---|
| Patient Population | Adults (n = 187) | Adult (n = 137) | Adult (n = 145) |
| Treatment Indication | |||
| B-ALL | 1 (0.5%) | 0 | 36 (25%) |
| NHL | 185 (98.7%) | 119 (88%) | 43 (30%) |
| Multiple Myeloma | 0 | 11 (8%) | 0 |
| T-ALL | 0 | 0 | 0 |
| PML | 1 (0.5%) | 0 | 0 |
| CLL | 0 | 0 | 66 (46%) |
| CRS Grading System | Lee Criteria [13] | Lee Criteria [13] | ASTCT consensus Criteria [14] |
| Number with Cardiotoxic event | 12 (6.4%) | 17 (12%) | 31 (21.3%) |
| Pre-existing Cardiomyopathy/Structural Disease/Arrhythmia | 1 (0.5%)/4 (2.1%)/3 (1.6%) | 5 (3.6%)/10 (7.3%)/18 (13%) | 1 (0.7%)/5 (3.4%)/5 (3.4%) |
| Hypotension/shock Requiring Inotropic Support | 5 (2.6%) | 6 (4%) | 33 (22.7%) |
| Troponemia | NR | 29 (21%) | NR |
| CHF/Ventricular Systolic Dysfunction | 12 (6.4%) | 8 (6%) | 21 (14.5%) |
| Sinus Tachycardia | NR | 6 (4.4%) | NR |
| Arrhythmia | 5 (3.6%) | 13 (8.9%) | |
| ST segment changes | NR | NR | NR |
| Cardiac Arrest/ Cardiac Death | 3 (1.6%) | 6 (4.4%) | 2 (1.4%) |
| Required Tocilizumab | 12 (6.4%) | 56 (40.9%) | 15 (10.3%) |
| Predictive Risk Factors for CRS [25,27,34,35] | Risk Factors for Cardiotoxicity in Pediatric Patients [28,29] | Risk Factors for Cardiotoxicity in Adult Patients [18,31,32,35] |
|---|---|---|
| High disease burden | Pre-Treatment Blasts >25% on bone marrow biopsy | Concomitant CRS (grade 3 or 4 CRS) |
| High CAR-T dose | Lower Pre-CAR-T Treatment baseline EF | Troponin elevation |
| High intensity lymphodepleting regimen | Pre-existing diastolic dysfunction | Older Age |
| Pre-existing endothelial activation | Higher Baseline Creatinine | |
| Severe thrombocytopenia | Aspirin, statin, insulin, beta blocker, RAA medication use | |
| Addition of fludarabine to cyclophosphamide during lymphodepletion | Hyperlipidemia | |
| Higher peak of C reactive protein | CAD | |
| Older patient age | Aortic Stenosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, E.A.; Gentille, C.; Trachtenberg, B.; Pingali, S.R.; Anand, K. Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases 2021, 9, 20. https://doi.org/10.3390/diseases9010020
Burns EA, Gentille C, Trachtenberg B, Pingali SR, Anand K. Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases. 2021; 9(1):20. https://doi.org/10.3390/diseases9010020
Chicago/Turabian StyleBurns, Ethan A., Cesar Gentille, Barry Trachtenberg, Sai Ravi Pingali, and Kartik Anand. 2021. "Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management" Diseases 9, no. 1: 20. https://doi.org/10.3390/diseases9010020
APA StyleBurns, E. A., Gentille, C., Trachtenberg, B., Pingali, S. R., & Anand, K. (2021). Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases, 9(1), 20. https://doi.org/10.3390/diseases9010020







