Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option?
Abstract
:1. Introduction
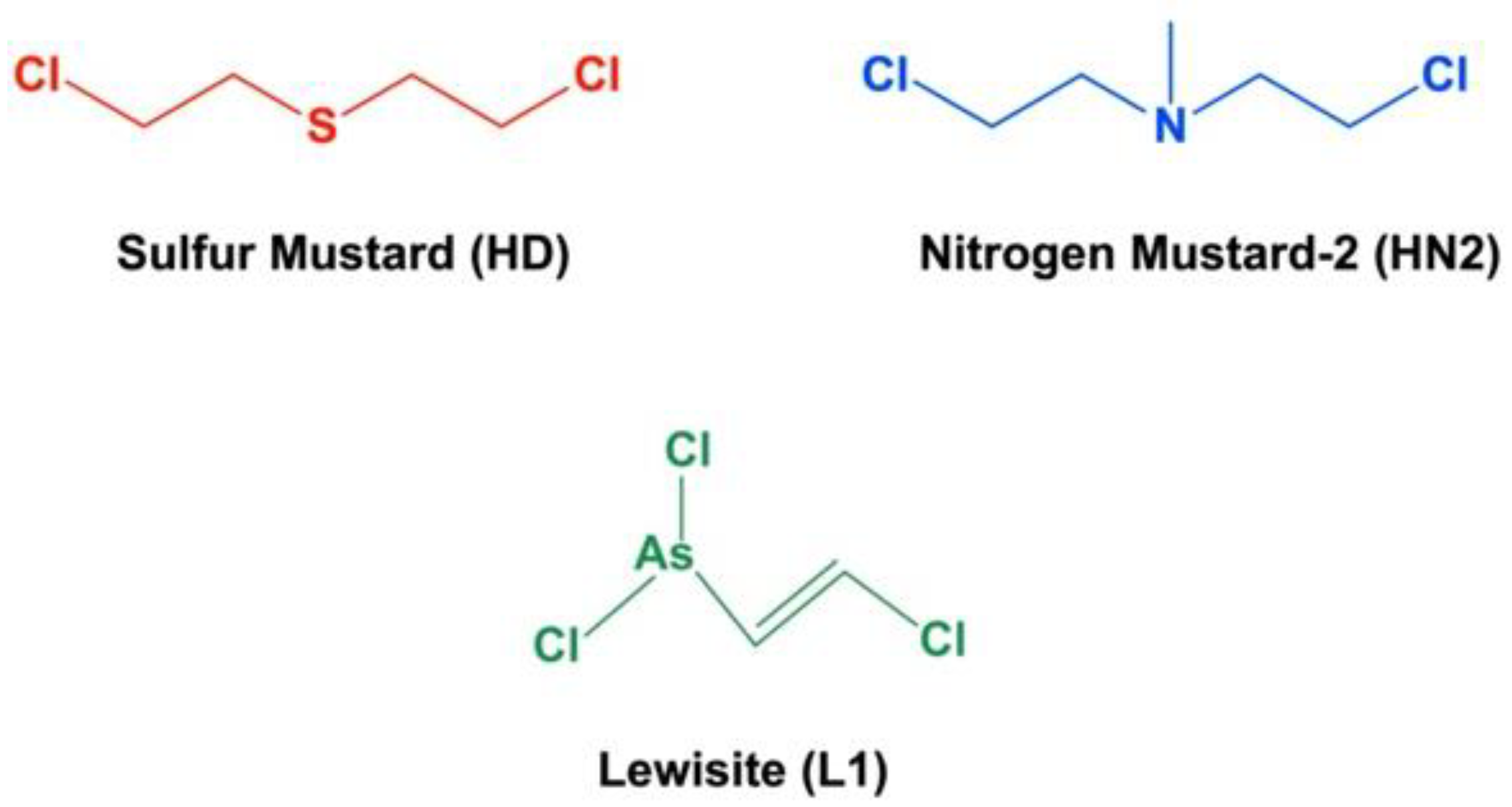
2. Target Organs and Acute Toxicity of Blister Agents: Focus on Melatonin Therapy and Safety Profile
3. Epigenetics and Long-Term Toxicity of Vesicants: A New Niche for Melatonin That Deserves Attention
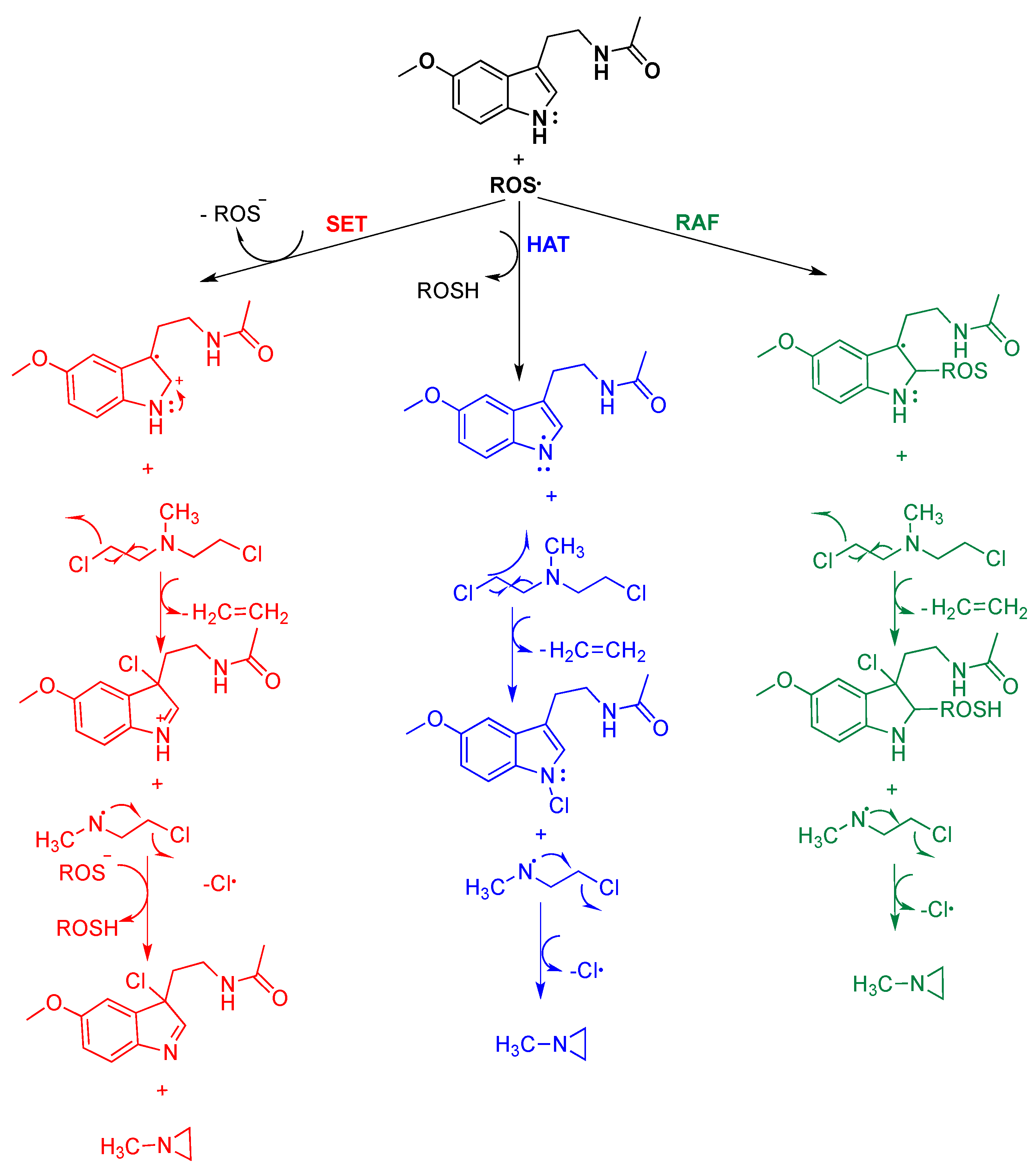
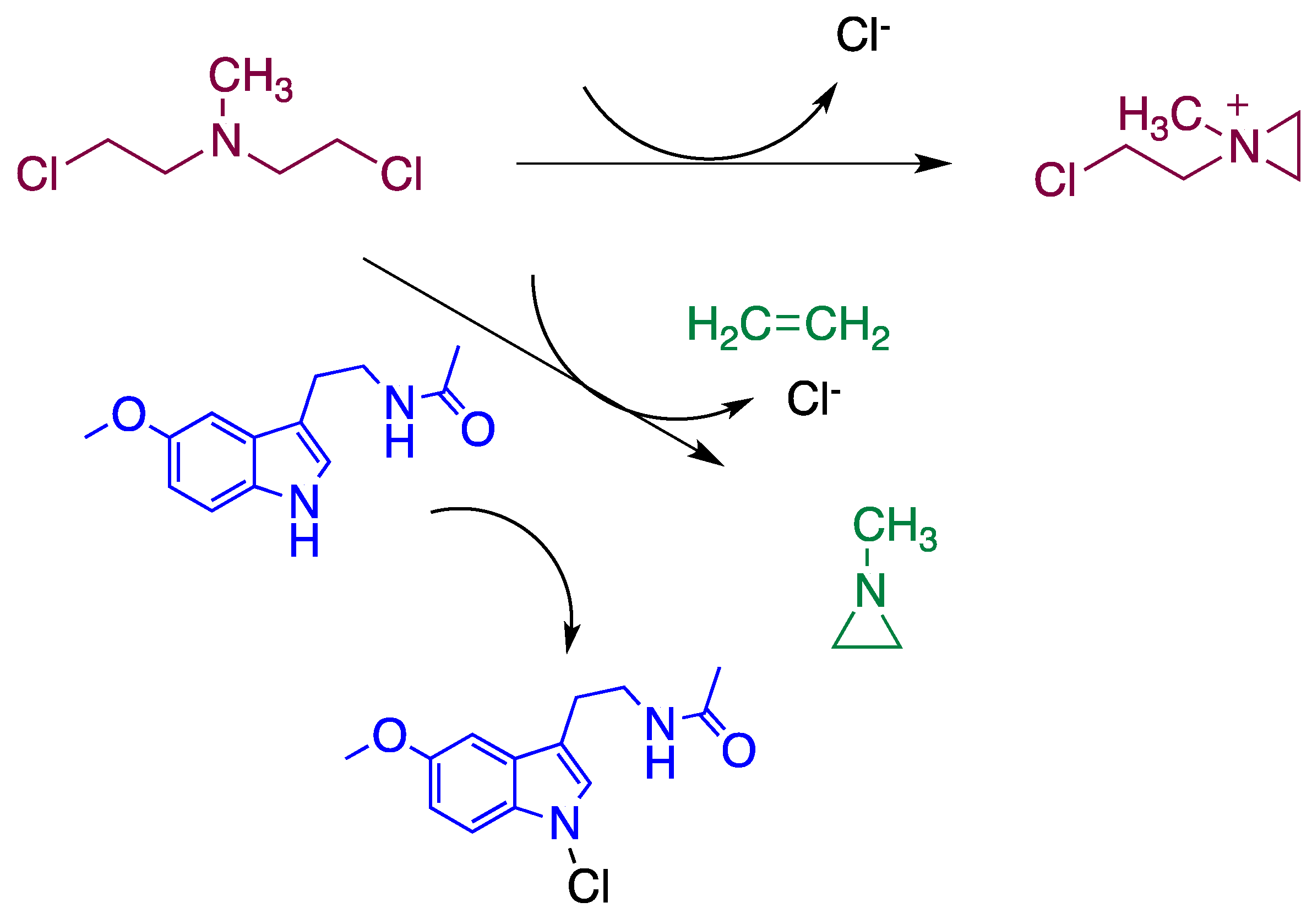
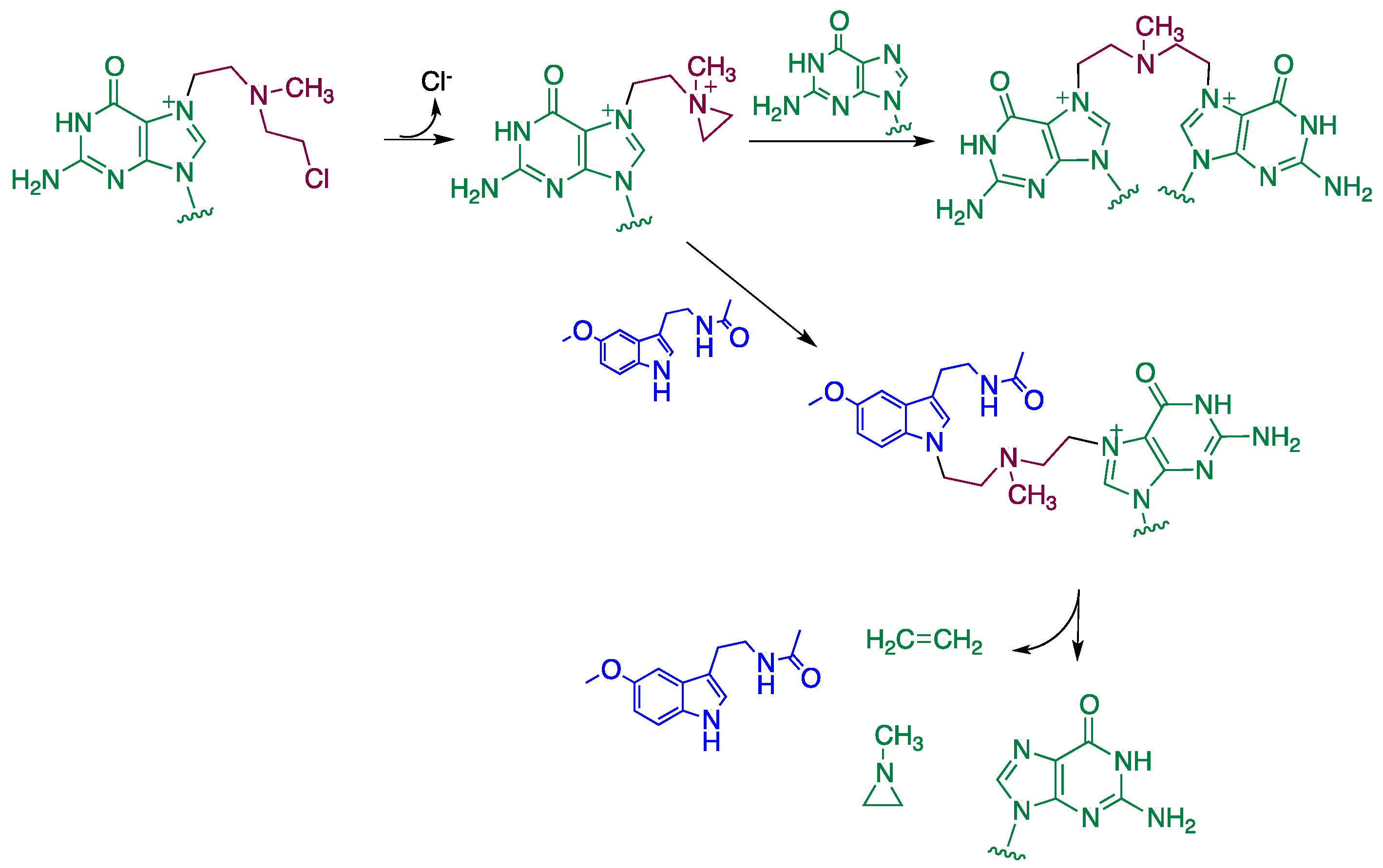
4. Reaction Mechanisms of Melatonin with Blistering Agents (HAT Mechanism and Counteract DNA Damage)
5. New Melatonin Galenic Developments Addressed to Reduce the Toxicity of Blister Agents
5.1. Oral Formulations
5.2. Topical Formulations
5.3. Parenteral Formulations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pita, R.; Anadón, A.; Romero, A.; Kuca, K. Chapter 7—Chemical Weapons of Mass Destruction and Terrorism: A Threat Analysis. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2020; pp. 79–94. [Google Scholar]
- Somani, S. Toxicokinetics and Toxicodynamics of Mustard. In Chemical Warfare Agents; Somani, S., Ed.; Academic Press: San Diego, CA, USA, 1992; pp. 13–50. [Google Scholar]
- Gilman, A.; Philips, F.S. The biological actions and therapeutic applications of the b-chloroethyl amines and sulfides. Science 1946, 103, 409–436. [Google Scholar] [CrossRef]
- Somani, S.M.; Babu, S.R. Toxicodynamics of sulfur mustard. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 419–435. [Google Scholar]
- Menendez-Pelaez, A.; Reiter, R.J. Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal Res. 1993, 15, 59–69. [Google Scholar] [CrossRef]
- Costa, E.J.; Shida, C.S.; Biaggi, M.H.; Ito, A.S.; Lamy-Freund, M.T. How melatonin interacts with lipid bilayers: A study by fluorescence and ESR spectroscopies. FEBS Lett. 1997, 416, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Luo, C.; Yang, Q.; Liu, Y.; Zhou, S.; Jiang, J.; Reiter, R.J.; Bhattacharya, P.; Cui, Y.; Yang, H.; Ma, H.; et al. The multiple protective roles and molecular mechanisms of melatonin and its precursor N-acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radic. Biol. Med. 2019, 130, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Radovic, M.; Ristic, L.; Krtinic, D.; Rancic, M.; Nickovic, V.; Vujnovic Zivkovic, Z.N.; Zivkovic, J.B.; Mirkovic, M.V.; Toskic, D.R.; Sokolovic, D. Melatonin treatment prevents carbon tetrachloride-induced acute lung injury in rats by mitigating tissue antioxidant capacity and inflammatory response. Bratisl. Lek Listy 2019, 120, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: Melatonin as a potential treatment. Expert Opin. Ther. Targets 2020, 24, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; de Los Rios, C.; Egea, J.; Del Pino, J.; Reiter, R.J. A review of metal-catalyzed molecular damage: Protection by melatonin. J. Pineal Res. 2014, 56, 343–370. [Google Scholar] [CrossRef]
- Almeida, L.L.; Pitombeira, G.; Teixeira, A.A.C.; Teixeira, V.W.; Silva Junior, V.A.; Vieira Filho, L.D.; Evencio Neto, J. Protective effect of melatonin against herbicides-induced hepatotoxicity in rats. Toxicol. Res. Camb. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Ali, B.H.; Abdelrahman, A.; Al Suleimani, Y.; Manoj, P.; Ali, H.; Nemmar, A.; Al Za’abi, M. Effect of concomitant treatment of curcumin and melatonin on cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2020, 131, 110761. [Google Scholar] [CrossRef]
- Ullah, U.; Badshah, H.; Malik, Z.; Uddin, Z.; Alam, M.; Sarwar, S.; Aman, A.; Khan, A.U.; Shah, F.A. Hepatoprotective effects of melatonin and celecoxib against ethanol-induced hepatotoxicity in rats. Immunopharmacol. Immunotoxicol. 2020, 42, 255–263. [Google Scholar] [CrossRef]
- Newman-Taylor, A.J.; Morris, A.J. Experience with mustard gas casualties. Lancet 1991, 337, 242. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Hefazi, M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam. Clin. Pharmacol. 2005, 19, 297–315. [Google Scholar] [CrossRef]
- Pita, R.; Vidal-Asensi, S. Cutaneous and systemic toxicology of vesicants used in warfare. Actas Dermosifiliogr. 2010, 101, 7–18. [Google Scholar] [CrossRef]
- Gu, T.Y. Mechanism and treatment of sulfur mustard-induced cutaneous injury. Chin. J. Traumatol. 2014, 17, 345–350. [Google Scholar] [PubMed]
- Kehe, K.; Szinicz, L. Medical aspects of sulphur mustard poisoning. Toxicology 2005, 214, 198–209. [Google Scholar] [CrossRef]
- Rice, P. Sulphur mustard injuries of the skin. Pathophysiology and management. Toxicol. Rev. 2003, 22, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Koçtürk, S.; Yüksel Egrilmez, M.; Aktan, Ş.; Oktay, G.; Resmi, H.; Şimşek Keskin, H.; Sert Serdar, B.; Erkmen, T.; Güner Akdogan, G.; Özkan, Ş. Melatonin attenuates the detrimental effects of UVA irradiation in human dermal fibroblasts by suppressing oxidative damage and MAPK/AP-1 signal pathway in vitro. Photodermatol. Photoimmunol. Photomed. 2019, 35, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Day, D.; Burgess, C.M.; Kircik, L.H. Assessing the Potential Role for Topical Melatonin in an Antiaging Skin Regimen. J. Drugs Dermatol. 2018, 17, 966–969. [Google Scholar] [PubMed]
- Martins Longaretti, L.; Luciano, J.A.; Strapazzon, G.; Pereira, M.; Damiani, A.P.; Rohr, P.; Rigo, F.K.; de Oliveira, C.A.; Steiner, B.T.; Vilela, T.C.; et al. Anti-genotoxic and anti-mutagenic effects of melatonin supplementation in a mouse model of melanoma. Drug Chem. Toxicol. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiol. Bethesda 2014, 29, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar] [CrossRef]
- Bora, N.S.; Mazumder, B.; Mandal, S.; Bhutia, Y.D.; Das, S.; Karmakar, S.; Chattopadhyay, P.; Dwivedi, S.K. Protective effect of a topical sunscreen formulation fortified with melatonin against UV-induced photodermatitis: An immunomodulatory effect via NF-κB suppression. Immunopharmacol. Immunotoxicol. 2019, 41, 130–139. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-inflammatory activity of melatonin: A focus on the role of NLRP3 inflammasome. Inflammation 2021. [Google Scholar] [CrossRef]
- Farré-Alins, V.; Narros-Fernández, P.; Palomino-Antolín, A.; Decouty-Pérez, C.; Lopez-Rodriguez, A.B.; Parada, E.; Muñoz-Montero, A.; Gómez-Rangel, V.; López-Muñoz, F.; Ramos, E.; et al. Melatonin reduces NLRP3 inflammasome activation by increasing α7 nAChR-mediated autophagic flux. Antioxidants 2020, 9, 1299. [Google Scholar] [CrossRef]
- Tekbas, O.F.; Ogur, R.; Korkmaz, A.; Kilic, A.; Reiter, R.J. Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J. Pineal Res. 2008, 44, 222–226. [Google Scholar] [CrossRef]
- Romero, A.; Gil Martín, E.; De los Rios, C.; Egea, J.; Ramos, E.; López-Muñoz, F.; Pita, R.; Juberías, A. Impact of melatonin effects on toxicology of vesicant chemical warfare agents: When science meets reality. Melatonin Res. 2020, 3, 101–119. [Google Scholar] [CrossRef]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Mohebbi, I.; Rastegar, M.; Kaviani, M.; Darband, S.G.; Jahanban-Esfahlan, R.; Nabavi, S.M.; Yousefi, B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018, 45, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Ghanei, M.; Harandi, A.A. Long term consequences from exposure to sulfur mustard: A review. Inhal. Toxicol. 2007, 19, 451–456. [Google Scholar] [CrossRef]
- Sciuto, A.M.; Kodavanti, U.P. Chapter 34—The Respiratory Toxicity of Chemical Warfare Agents. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2020; pp. 515–544. [Google Scholar] [CrossRef]
- Korkmaz, A.; Kunak, Z.I.; Paredes, S.D.; Yaren, H.; Tan, D.X.; Reiter, R.J. The use of melatonin to combat mustard toxicity. REVIEW. Neuroendocrinol. Lett. 2008, 29, 614–619. [Google Scholar]
- Ucar, M.; Korkmaz, A.; Reiter, R.J.; Yaren, H.; Oter, S.; Kurt, B.; Topal, T. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol. Lett. 2007, 173, 124–131. [Google Scholar] [CrossRef]
- Macit, E.; Yaren, H.; Aydin, I.; Kunak, Z.I.; Yaman, H.; Onguru, O.; Uysal, B.; Korkmaz, A.; Turel, S.; Kenar, L. The protective effect of melatonin and S-methylisothiourea treatments in nitrogen mustard induced lung toxicity in rats. Environ. Toxicol. Pharmacol. 2013, 36, 1283–1290. [Google Scholar] [CrossRef]
- Goldman, M.; Dacre, J.C. Lewisite: Its chemistry, toxicology, and biological effects. Rev. Environ. Contam. Toxicol. 1989, 110, 75–115. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Hefazi, M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin. Pharmacol. Toxicol. 2006, 99, 273–282. [Google Scholar] [CrossRef]
- Wang, B.; Zuo, X.; Peng, L.; Wang, X.; Zeng, H.; Zhong, J.; Li, S.; Xiao, Y.; Wang, L.; Ouyang, H.; et al. Melatonin ameliorates oxidative stress-mediated injuries through induction of HO-1 and restores autophagic flux in dry eye. Exp. Eye Res. 2021, 205, 108491. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Reiter, R.J.; Orhii, P.B.; Hara, M.; Poeggeler, B. Inhibitory effect of melatonin on cataract formation in newborn rats: Evidence for an antioxidative role for melatonin. J. Pineal Res. 1994, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog. Retin. Eye Res. 2020, 75, 100798. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, H.; Gu, Y.; Li, X.; Tao, L.; Lu, P. Melatonin exerts protective effects on diabetic retinopathy via inhibition of Wnt/β-catenin pathway as revealed by quantitative proteomics. Exp. Eye Res. 2021, 205, 108521. [Google Scholar] [CrossRef]
- Telek, H.H. Effects of Selenium and Melatonin on Ocular Ischemic Syndrome. Biomed. Res. Int. 2019, 2019, 8080564. [Google Scholar] [CrossRef]
- Gulati, K.; Thokchom, S.K.; Ray, A. Chapter 42—Impact of Chemical Warfare Agents on the Immune System. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2020; pp. 685–703. [Google Scholar] [CrossRef]
- Liu, J.; Wright, L.K.; Pope, C.N. Chapter 32—Chemical warfare agents and the nervous system. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2020; pp. 481–498. [Google Scholar] [CrossRef]
- Pacini, N.; Borziani, F. Action of melatonin on bone marrow depression induced by cyclophosphamide in acute toxicity phase. Neuroendocrinol. Lett. 2009, 30, 582–591. [Google Scholar]
- Kisby, G.E.; Springer, N.; Spencer, P.S. In vitro neurotoxic and DNA-damaging properties of nitrogen mustard. J. Appl. Toxicol. 2000, 20 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995, 9, 526–533. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S. Anti-warburg effect of melatonin: A Proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 2021, 22, 764. [Google Scholar] [CrossRef]
- Fernandez-Palanca, P.; Mendez-Blanco, C.; Fondevila, F.; Tunon, M.J.; Reiter, R.J.; Mauriz, J.L.; Gonzalez-Gallego, J. Melatonin as an antitumor agent against liver cancer: An updated systematic review. Antioxidants 2021, 10, 103. [Google Scholar] [CrossRef]
- Fu, Z.; Jiao, Y.; Wang, J.; Zhang, Y.; Shen, M.; Reiter, R.J.; Xi, Q.; Chen, Y. Cardioprotective role of melatonin in acute myocardial infarction. Front. Physiol. 2020, 11, 366. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Kamrava, S.K.; Moore, B.C.J.; Reiter, R.J.; Ghaznavi, H.; Kamali, M.; Mehrzadi, S. Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr. Drug Targets 2019, 20, 1112–1128. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Curro, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of human trials. Curr. Med. Chem. 2010, 17, 2070–2095. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, S.; Han, M.; Yang, Z.; Lv, J.; Deng, C.; Reiter, R.J.; Yang, Y. Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 52, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Ostadmohammadi, V.; Tabrizi, R.; Lankarani, K.B.; Heydari, S.T.; Amirani, E.; Reiter, R.J.; Asemi, Z. The effects of melatonin supplementation on inflammatory markers among patients with metabolic syndrome or related disorders: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2018, 26, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Gogenur, I. Pharmacokinetics of high-dose intravenous melatonin in humans. J. Clin. Pharmacol. 2016, 56, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Ramos, E.; Farre-Alins, V.; Egea, J.; Lopez-Munoz, F.; Reiter, R.J.; Romero, A. Melatonin’s efficacy in stroke patients; a matter of dose? A systematic review. Toxicol. Appl. Pharmacol. 2020, 392, 114933. [Google Scholar] [CrossRef]
- Cardinali, D.P. Are melatonin doses employed clinically adequate for melatonin-induced cytoprotection? Melatonin Res. 2019, 2, 106–132. [Google Scholar] [CrossRef]
- Khateri, S.; Ghanei, M.; Keshavarz, S.; Soroush, M.; Haines, D. Incidence of lung, eye, and skin lesions as late complications in 34,000 Iranians with wartime exposure to mustard agent. J. Occup. Environ. Med. 2003, 45, 1136–1143. [Google Scholar] [CrossRef]
- Shohrati, M.; Peyman, M.; Peyman, A.; Davoudi, M.; Ghanei, M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutan. Ocul. Toxicol. 2007, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Rowell, M.; Kehe, K.; Balszuweit, F.; Thiermann, H. The chronic effects of sulfur mustard exposure. Toxicology 2009, 263, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Hefazi, M.; Mahmoudi, M.; Jalali, E.; Attaran, D.; Maleki, M.; Razavi, M.E.; Zare, G.; Tabatabaee, A.; Jaafari, M.R. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam. Clin. Pharmacol. 2005, 19, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Tan, D.X.; Reiter, R.J. Acute and delayed sulfur mustard toxicity; novel mechanisms and future studies. Interdiscip. Toxicol. 2008, 1, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Yaren, H.; Kunak, Z.I.; Uysal, B.; Kurt, B.; Topal, T.; Kenar, L.; Ucar, E.; Oter, S. Epigenetic perturbations in the pathogenesis of mustard toxicity; hypothesis and preliminary results. Interdiscip. Toxicol. 2008, 1, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Beaudet, A.L. Epigenetics and Human Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a019497. [Google Scholar] [CrossRef]
- Imani, S.; Panahi, Y.; Salimian, J.; Fu, J.; Ghanei, M. Epigenetic: A missing paradigm in cellular and molecular pathways of sulfur mustard lung: A prospective and comparative study. Iran. J. Basic. Med. Sci. 2015, 18, 723–736. [Google Scholar]
- Rahmani, S.; Abdollahi, M. Novel treatment opportunities for sulfur mustard-related cancers: Genetic and epigenetic perspectives. Arch. Toxicol. 2017, 91, 3717–3735. [Google Scholar] [CrossRef]
- Panahi, Y.; Fattahi, A.; Zarei, F.; Ghasemzadeh, N.; Mohammadpoor, A.; Abroon, S.; Nojadeh, J.N.; Khojastefard, M.; Akbarzadeh, A.; Ghasemnejad, T. Next-generation sequencing approaches for the study of genome and epigenome toxicity induced by sulfur mustard. Arch. Toxicol. 2018, 92, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Yego, E.C.; Dillman, J.F., 3rd. Cytokine regulation by MAPK activated kinase 2 in keratinocytes exposed to sulfur mustard. Toxicol. In Vitro 2013, 27, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbpour, E.; Ghanei, M.; Qazvini, A.; Vahedi, E.; Panahi, Y. Gene expression profile of oxidative stress and antioxidant defense in lung tissue of patients exposed to sulfur mustard. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2016, 800–801, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Steinritz, D.; Schmidt, A.; Balszuweit, F.; Thiermann, H.; Simons, T.; Striepling, E.; Bolck, B.; Bloch, W. Epigenetic modulations in early endothelial cells and DNA hypermethylation in human skin after sulfur mustard exposure. Toxicol. Lett. 2016, 244, 95–102. [Google Scholar] [CrossRef]
- Venkatesh, S.; Workman, J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Deppe, J.; Steinritz, D.; Santovito, D.; Egea, V.; Schmidt, A.; Weber, C.; Ries, C. Upregulation of miR-203 and miR-210 affect growth and differentiation of keratinocytes after exposure to sulfur mustard in normoxia and hypoxia. Toxicol. Lett. 2016, 244, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rothmiller, S.; Wolf, M.; Worek, F.; Steinritz, D.; Thiermann, H.; Schmidt, A. Alteration of miRNA expression in a sulfur mustard resistant cell line. Toxicol. Lett. 2018, 293, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Wolf, M.; Rothmiller, S.; Worek, F.; Steinritz, D.; Thiermann, H. Cytostatic resistance profile of the sulfur mustard resistant keratinocyte cell line HaCaT/SM. Toxicol. Lett. 2018, 293, 16–20. [Google Scholar] [CrossRef]
- Schmidt, A.; Steinritz, D.; Thiermann, H.; Meineke, V.; Abend, M. Alteration of miRNA expression in early endothelial cells after exposure with sub-lethal sulfur mustard concentrations. Toxicol. Lett. 2016, 244, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Khafaei, M.; Samie, S.; Mowla, S.J.; Alvanegh, A.G.; Mirzaei, B.; Chavoshei, S.; Dorraj, G.S.; Esmailnejad, M.; Tavallaie, M.; Nourani, M. Evaluation of miR-9 and miR-143 expression in urine specimens of sulfur mustard exposed patients. Interdiscip. Toxicol. 2015, 8, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, A.; Reiter, R.J. Epigenetic regulation: A new research area for melatonin? J. Pineal Res. 2008, 44, 41–44. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics—Evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 2014, 15, 18221–18252. [Google Scholar] [CrossRef]
- Bahna, S.G.; Niles, L.P. Epigenetic induction of melatonin MT1 receptors by valproate: Neurotherapeutic implications. Eur. Neuropsychopharmacol. 2017, 27, 828–832. [Google Scholar] [CrossRef]
- Irmak, M.K.; Topal, T.; Oter, S. Melatonin seems to be a mediator that transfers the environmental stimuli to oocytes for inheritance of adaptive changes through epigenetic inheritance system. Med. Hypotheses 2005, 64, 1138–1143. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Vahedi, E.; Shohrati, M.; Panahi, Y.; Parvin, S. Nocturnal serum melatonin levels in sulfur mustard exposed patients with sleep disorders. J. R. Army Med. Corps 2017, 163, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Shohrati, M.; Vahedi, E.; Abdollahpour-Alitappeh, M.; Panahi, Y. Effect of melatonin administration on sleep quality in sulfur mustard exposed patients with sleep disorders. Iran. J. Pharm. Res. 2018, 17, 136–144. [Google Scholar]
- Sulkava, S.; Ollila, H.M.; Alasaari, J.; Puttonen, S.; Harma, M.; Viitasalo, K.; Lahtinen, A.; Lindstrom, J.; Toivola, A.; Sulkava, R.; et al. Common genetic variation near melatonin receptor 1A gene linked to job-related exhaustion in shift workers. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahtinen, A.; Puttonen, S.; Vanttola, P.; Viitasalo, K.; Sulkava, S.; Pervjakova, N.; Joensuu, A.; Salo, P.; Toivola, A.; Harma, M.; et al. A distinctive DNA methylation pattern in insufficient sleep. Sci. Rep. 2019, 9, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahriary, A.; Ghanei, M.; Rahmani, H. The systemic nature of mustard lung: Comparison with COPD patients. Interdiscip. Toxicol. 2017, 10, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Haim, A.; Zubidat, A.E. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [Green Version]
- Schwimmer, H.; Metzer, A.; Pilosof, Y.; Szyf, M.; Machnes, Z.M.; Fares, F.; Harel, O.; Haim, A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 2014, 31, 144–150. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, S.J.; Yoon, H.J.; Yu, S.Y.; Yang, H.; Jeong, S.I.; Hwang, S.Y.; Park, C.S.; Park, Y.S. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. Pineal Res. 2013, 54, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Mansour Razavi, S.; Salamati, P.; Saghafinia, M.; Abdollahi, M. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru 2012, 20, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pita, R.; Marco-Contelles, J.; Ramos, E.; Del Pino, J.; Romero, A. Toxicity induced by chemical warfare agents: Insights on the protective role of melatonin. Chem. Biol. Interact. 2013, 206, 134–142. [Google Scholar] [CrossRef] [Green Version]
- The United States Pharmacopeial Convention. The United States Pharmacopoeia: The National Formulary; The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019. [Google Scholar]
- Aykutoglu, G.; Tartik, M.; Darendelioglu, E.; Ayna, A.; Baydas, G. Melatonin and vitamin E alleviate homocysteine-induced oxidative injury and apoptosis in endothelial cells. Mol. Biol. Rep. 2020, 47, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Paredes, S.D.; Korkmaz, A.; Jou, M.J.; Tan, D.X. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 2008, 1, 137–149. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Zu, Y.; Wang, L.; Wu, W.; Deng, Y.; Zu, C.; Liu, Y. Melatonin-loaded silica coated with hydroxypropyl methylcellulose phthalate for enhanced oral bioavailability: Preparation, and in vitro-in vivo evaluation. Eur. J. Pharm. Biopharm. 2017, 112, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Wang, L.; Liu, Y.; Wu, W.; Zhong, C.; Zhang, Q.; Yang, J. Preparation, characterization and in vitro evaluation of melatonin-loaded porous starch for enhanced bioavailability. Carbohydr. Polym. 2018, 202, 125–133. [Google Scholar] [CrossRef]
- Munoz, H.; Castan, H.; Clares, B.; Ruiz, M.A. Obtaining fast dissolving disintegrating tablets with different doses of melatonin. Int. J. Pharm. 2014, 467, 84–89. [Google Scholar] [CrossRef]
- Lee, B.J.; Ryu, S.G.; Cui, J.H. Controlled release of dual drug-loaded hydroxypropyl methylcellulose matrix tablet using drug-containing polymeric coatings. Int. J. Pharm. 1999, 188, 71–80. [Google Scholar] [CrossRef]
- Albertini, B.; Di Sabatino, M.; Melegari, C.; Passerini, N. Formulating SLMs as oral pulsatile system for potential delivery of melatonin to pediatric population. Int. J. Pharm. 2014, 469, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Acuña-Castroviejo, D. Use of Melatonin for Treating and/or Preventing Mucositis. International Patent Application No. PCT/ES2012/070728, 2013. [Google Scholar]
- Escames Rosa, G.; Acuña Castroviejo, D.; López García, L.C. Composition Comprising Melatonin or Its Derivatives with Coenzyme Q10 and Use Thereof against Ageing of the Skin. International Patent Application No. PCT/ES2013/070817, 2014. [Google Scholar]
- Kikwai, L.; Kanikkannan, N.; Babu, R.J.; Singh, M. Effect of vehicles on the transdermal delivery of melatonin across porcine skin in vitro. J. Control. Release 2002, 83, 307–311. [Google Scholar] [CrossRef]
- Oh, H.J.; Oh, Y.K.; Kim, C.K. Effects of vehicles and enhancers on transdermal delivery of melatonin. Int. J. Pharm. 2001, 212, 63–71. [Google Scholar] [CrossRef]
- Gwak, H.S.; Kim, S.U.; Chun, I.K. Effect of vehicles and enhancers on the in vitro permeation of melatonin through hairless mouse skin. Arch. Pharm. Res. 2002, 25, 392–396. [Google Scholar] [CrossRef]
- Hoffmeister, C.R.; Durli, T.L.; Schaffazick, S.R.; Raffin, R.P.; Bender, E.A.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Hydrogels containing redispersible spray-dried melatonin-loaded nanocapsules: A formulation for transdermal-controlled delivery. Nanoscale Res. Lett. 2012, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- Kanikkannan, N.; Andega, S.; Burton, S.; Babu, R.J.; Singh, M. Formulation and in vitro evaluation of transdermal patches of melatonin. Drug Dev. Ind. Pharm. 2004, 30, 205–212. [Google Scholar] [CrossRef]
- Thakral, S.; Wolf, A.; Beilman, G.J.; Suryanarayanan, R. Development and in vivo evaluation of a novel lyophilized formulation for the treatment of hemorrhagic shock. Int. J. Pharm. 2018, 537, 162–171. [Google Scholar] [CrossRef]
- Daya, S.; Walker, R.B.; Glass, B.D.; Anoopkumar-Dukie, S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J. Pineal Res. 2001, 31, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.S.; Anoopkumar-Dukie, S.; Glass, B.D.; Antunes, E.M.; Lack, B.; Walker, R.B.; Daya, S. The identification of the UV degradants of melatonin and their ability to scavenge free radicals. J. Pineal Res. 2002, 32, 257–261. [Google Scholar] [CrossRef]
- Vlachou, M.; Eikosipentaki, A.; Xenogiorgis, V. Pineal hormone melatonin: Solubilization studies in model aqueous gastrointestinal environments. Curr. Drug Deliv. 2006, 3, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Molska, A.; Nyman, A.K.G.; Sofias, A.M.; Kristiansen, K.A.; Hak, S.; Wideroe, M. In vitro and in vivo evaluation of organic solvent-free injectable melatonin nanoformulations. Eur. J. Pharm. Biopharm. 2020, 152, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Agarwal, S.P.; Khanna, R. Modified release bi-layered tablet of melatonin using beta-cyclodextrin. Pharmazie 2003, 58, 642–644. [Google Scholar] [PubMed]
- Babu, R.J.; Dayal, P.; Singh, M. Effect of cyclodextrins on the complexation and nasal permeation of melatonin. Drug Deliv. 2008, 15, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.J.; Cui, J.H.; Parrott, K.A.; Ayres, J.W.; Sack, R.L. Percutaneous absorption and model membrane variations of melatonin in aqueous-based propylene glycol and 2-hydroxypropyl-beta-cyclodextrin vehicles. Arch. Pharm. Res. 1998, 21, 503–507. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, A.; Ramos, E.; López-Muñoz, F.; De Los Ríos, C.; Egea, J.; Gil-Martín, E.; Pita, R.; Torrado, J.J.; Serrano, D.R.; Juberias, A. Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option? Diseases 2021, 9, 27. https://doi.org/10.3390/diseases9020027
Romero A, Ramos E, López-Muñoz F, De Los Ríos C, Egea J, Gil-Martín E, Pita R, Torrado JJ, Serrano DR, Juberias A. Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option? Diseases. 2021; 9(2):27. https://doi.org/10.3390/diseases9020027
Chicago/Turabian StyleRomero, Alejandro, Eva Ramos, Francisco López-Muñoz, Cristóbal De Los Ríos, Javier Egea, Emilio Gil-Martín, René Pita, Juan J. Torrado, Dolores R. Serrano, and Antonio Juberias. 2021. "Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option?" Diseases 9, no. 2: 27. https://doi.org/10.3390/diseases9020027









