Colorimetric Gas Detection Using Molecular Devices and an RGB Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. PCN-224 Synthesis
2.3. Films Preparation
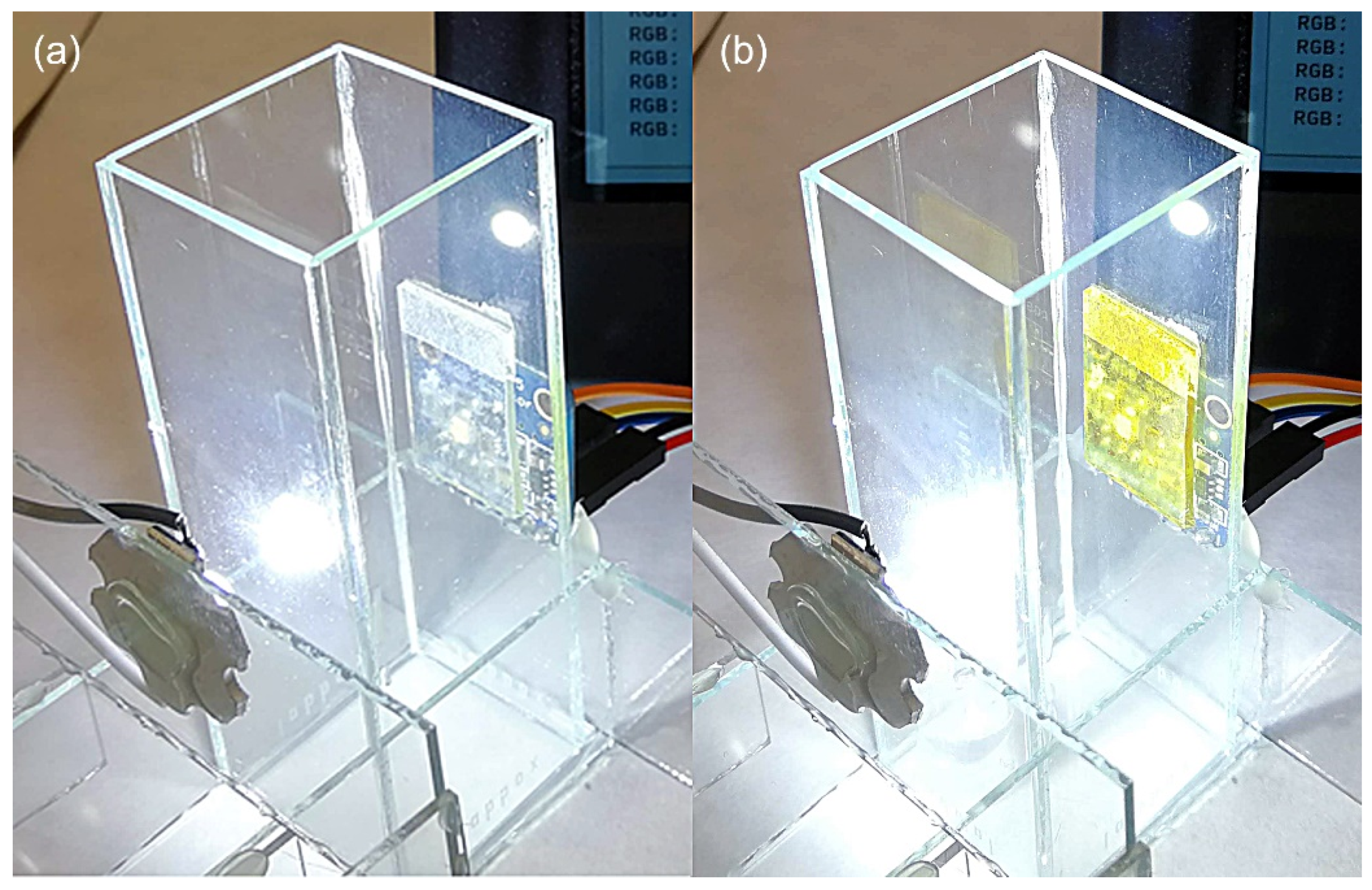
2.3.1. Phosgene Detection Films
2.3.2. Biogenic Amines Detection Films
2.4. Gas Sensing Experiments
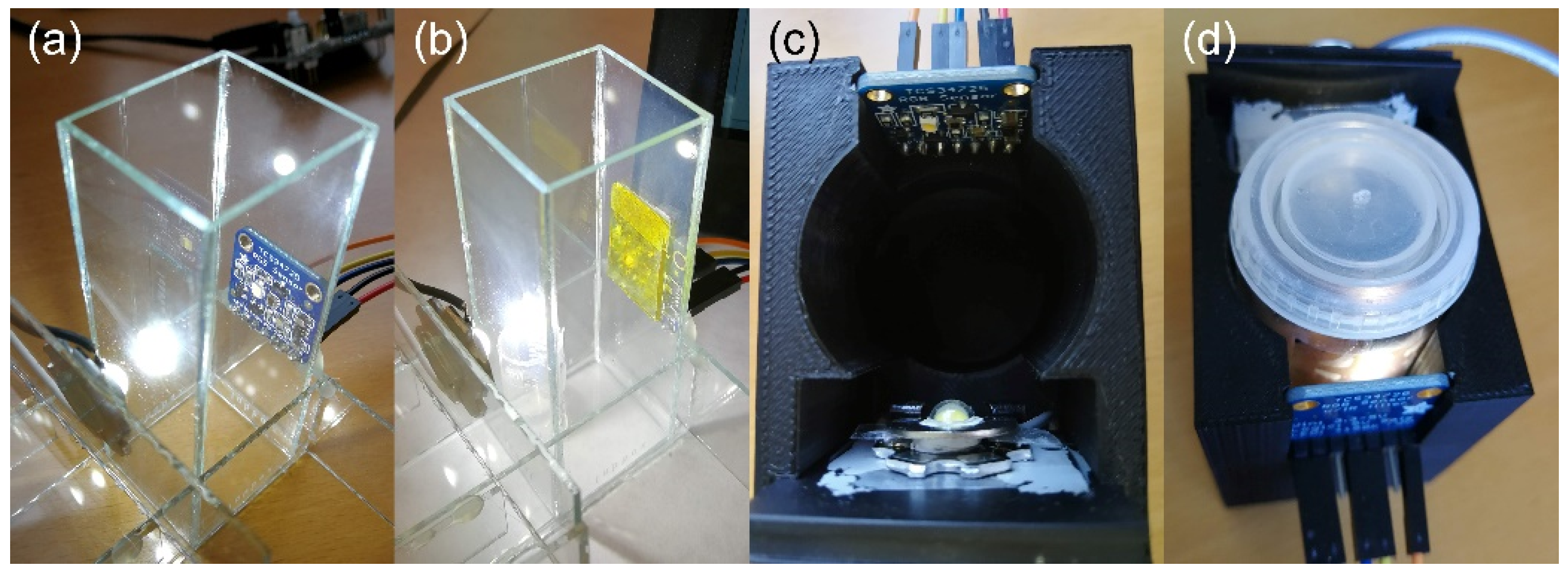
2.5. Prototype
2.5.1. RGB Sensor and Interfacing
2.5.2. Light Sources
2.5.3. Sample Holders
2.5.4. Data Acquisition
3. Results and Discussion
3.1. Light Source
3.2. Sensing
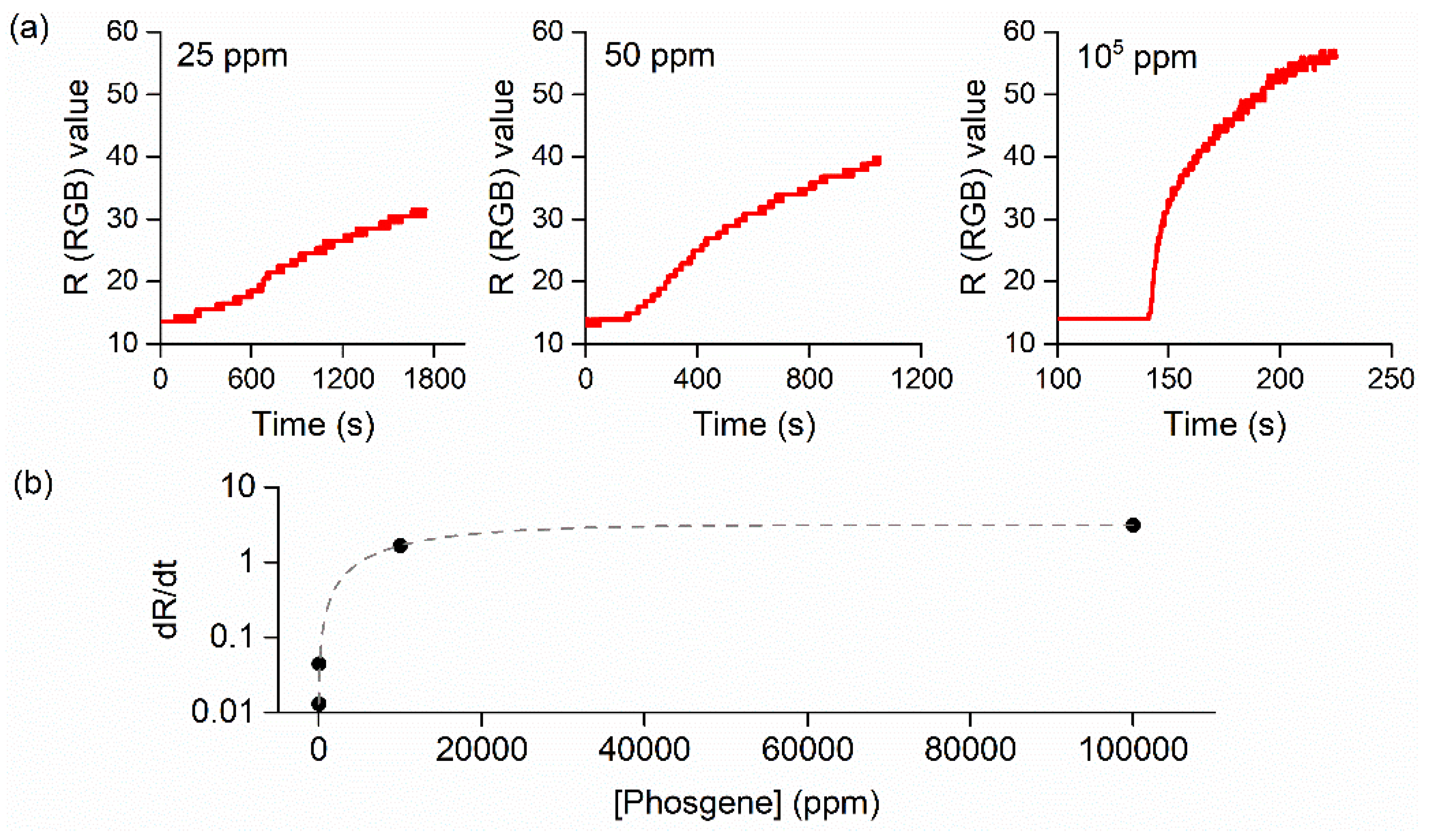
3.2.1. Phosgene Detection
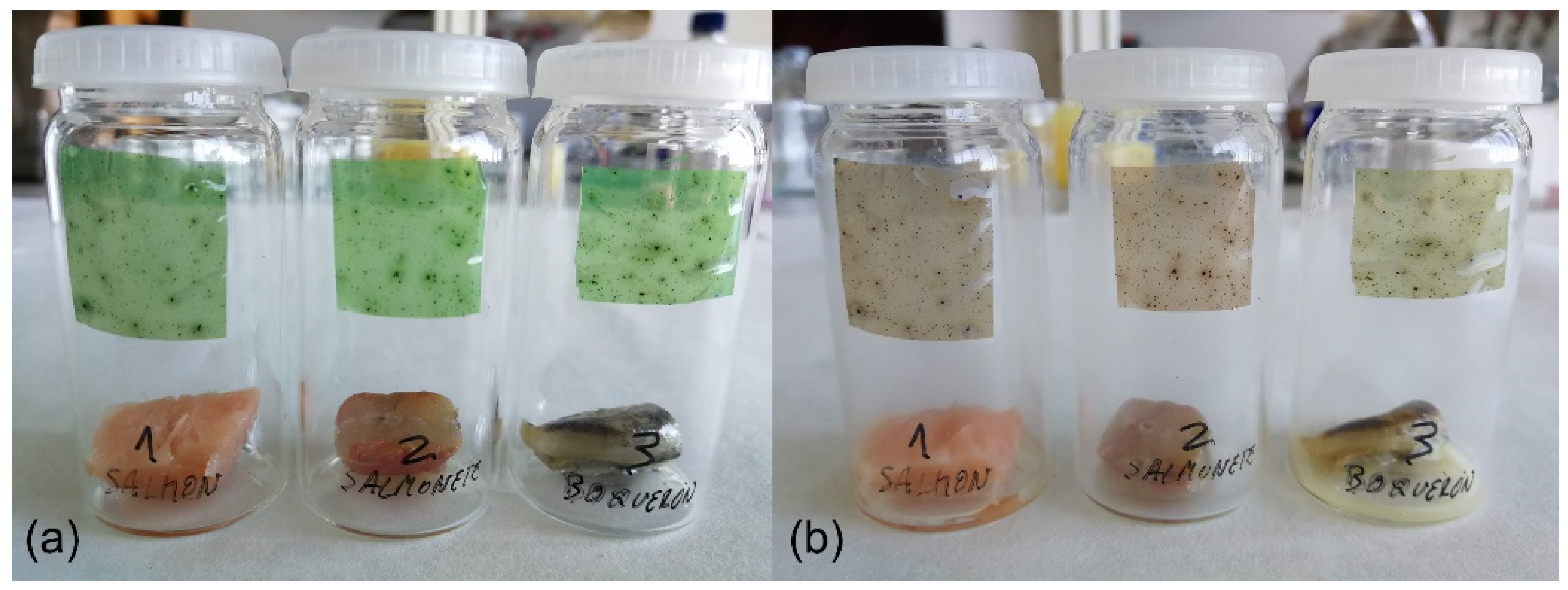
3.2.2. Biogenic Amines Detection
3.3. Comparison with Other Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellerbee, A.K.; Phillips, S.T.; Siegel, A.C.; Mirica, K.A.; Martinez, A.W.; Striehl, P.; Jain, N.; Prentiss, M.; Whitesides, G.M. Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper. Anal. Chem. 2009, 81, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Linder, V.; Parviz, B.A.; Siegel, A.; Whitesides, G.M. An Integrated Approach to a Portable and Low-Cost Immunoassay for Resource-Poor Settings. Angew. Chem.-Int. Ed. 2004, 43, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.D.; Chun, H.J.; Yoon, H.C. The transformation of common office supplies into a low-cost optical biosensing platform. Biosens. Bioelectron. 2014, 59, 259–268. [Google Scholar] [CrossRef]
- Albert, D.R.; Todt, M.A.; Davis, H.F. A low-cost quantitative absorption spectrophotometer. J. Chem. Educ. 2012, 89, 1432–1435. [Google Scholar] [CrossRef]
- Quagliano, J.M.; Marks, C.A. Demystifying spectroscopy with secondary students: Designing and using a custom-built spectrometer. J. Chem. Educ. 2013, 90, 1409–1410. [Google Scholar] [CrossRef]
- Anzalone, G.C.; Glover, A.G.; Pearce, J.M. Open-source colorimeter. Sensors 2013, 13, 5338–5346. [Google Scholar] [CrossRef] [Green Version]
- Asheim, J.; Kvittingen, E.V.; Kvittingen, L.; Verley, R. A simple, small-scale lego colorimeter with a light-emitting diode (LED) used as detector. J. Chem. Educ. 2014, 91, 1037–1039. [Google Scholar] [CrossRef]
- Rohit; Kanwar, L.; Rao, K.K. Development of a low-cost portable colorimeter for the estimation of fluoride in drinking water. Sens. Actuators B Chem. 2010, 149, 245–251. [Google Scholar] [CrossRef]
- Sumriddetchkajorn, S.; Chaitavon, K.; Intaravanne, Y. Mobile-platform based colorimeter for monitoring chlorine concentration in water. Sens. Actuators B Chem. 2014, 191, 561–566. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef]
- Cohen, A.R.; Seidl-Friedman, J. HemoCue® system for hemoglobin measurement. Evaluation in anemic and nonanemic children. Am. J. Clin. Pathol. 1988, 90, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mieczkowska, E.; Koncki, R.; Tymecki, L. Hemoglobin determination with paired emitter detector diode. Anal. Bioanal. Chem. 2011, 399, 3293–3297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, K.; Kimoto, H.; Noguchi, T.; Hatta, M.; Kawakami, H.; Suzue, T. Colorimetric pH measurement for seawater samples using a three light-emitting diodes detector and a calibration method for temperature dependence. Anal. Sci. 2014, 30, 1135–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christodouleas, D.C.; Nemiroski, A.; Kumar, A.A.; Whitesides, G.M. Broadly Available Imaging Devices Enable High-Quality Low-Cost Photometry. Anal. Chem. 2015, 87, 9170–9178. [Google Scholar] [CrossRef]
- Knagge, K.; Raftery, D. Construction and Evaluation of a LEGO Spectrophotometer for Student Use. Chem. Educ. 2002, 7, 371–375. [Google Scholar] [CrossRef]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. A simple and cost-effective method, as an appropriate alternative for visible spectrophotometry: Development of a dopamine biosensor. Analyst 2009, 134, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; van Oordt, T.; Schneider, E.M.; Zengerle, R.; von Stetten, F.; Luong, J.H.T. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015, 67, 248–255. [Google Scholar] [CrossRef]
- Filippini, D.; Svensson, S.P.S.; Lundström, I. Computer screen as a programmable light source for visible absorption characterization of (bio)chemical assays. Chem. Commun. 2003, 240–241. [Google Scholar] [CrossRef]
- de Oliveira, H.J.S.; de Almeida, P.L.; Sampaio, B.A.; Fernandes, J.P.A.; Pessoa-Neto, O.D.; de Lima, E.A.; de Almeida, L.F. A handheld smartphone-controlled spectrophotometer based on hue to wavelength conversion for molecular absorption and emission measurements. Sens. Actuators B Chem. 2017, 238, 1084–1091. [Google Scholar] [CrossRef]
- Benavides, M.; Mailier, J.; Hantson, A.L.; Muñoz, G.; Vargas, A.; Van Impe, J.; Wouwer, A. Vande Design and test of a low-cost RGB sensor for online measurement of microalgae concentration within a photo-bioreactor. Sensors 2015, 15, 4766–4780. [Google Scholar] [CrossRef]
- Seelye, M.; Sen Gupta, G.; Bailey, D.; Seelye, J. Low cost colour sensors for monitoring plant growth in a laboratory. In Proceedings of the 2011 IEEE International Instrumentation and Measurement Technology, Hangzhou, China, 10–12 May 2011; pp. 972–977. [Google Scholar] [CrossRef]
- Salmerón, J.F.; Gómez-Robledo, L.; Carvajal, M.Á.; Huertas, R.; Moyano, M.J.; Gordillo, B.; Palma, A.J.; Heredia, F.J.; Melgosa, M. Measuring the colour of virgin olive oils in a new colour scale using a low-cost portable electronic device. J. Food Eng. 2012, 111, 247–254. [Google Scholar] [CrossRef]
- De La Torre, C.; Muñiz, R.; Pérez, M.A. A new, low-cost, on-line RGB colorimeter for wine industry based on optical fibers. In Proceedings of the 19th IMEKO World Congress, Lisbon, Portugal, 6–11 September 2009; Volume 4, pp. 2353–2357. [Google Scholar]
- Ariono, M.R.E.; Budiman, F.; Silalahi, D.K. Design of Banana Ripeness Classification Device Based on Alcohol Level and Color Using a Hybrid Adaptive Neuro-Fuzzy Inference System Method BT. In Proceedings of the 1st International Conference on Electronics, Biomedical Engineering, and Health Informatics, Surabaya, Indonesia, 8–9 October 2020; Lecture Notes in Electrical, Engineering. Triwiyanto, Nugroho, H.A., Rizal, A., Caesarendra, W., Eds.; Springer: Singapore, 2021; pp. 107–117. [Google Scholar]
- Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. An Optical Dosimeter for the Selective Detection of Gaseous Phosgene with Ultralow Detection Limit. ACS Sens. 2018, 3, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Sousaraei, A.; Queirós, C.; Moscoso, F.G.; Silva, A.M.G.; Lopes-Costa, T.; Pedrosa, J.M.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Reversible Protonation of Porphyrinic Metal-Organic Frameworks Embedded in Nanoporous Polydimethylsiloxane for Colorimetric Sensing. Adv. Mater. Interfaces 2021, 8, 2001759. [Google Scholar] [CrossRef]
- Feng, D.; Chung, W.C.; Wei, Z.; Gu, Z.Y.; Jiang, H.L.; Chen, Y.P.; Darensbourg, D.J.; Zhou, H.C. Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. Luminescent MOF crystals embedded in PMMA/PDMS transparent films as effective NO2gas sensors. Mol. Syst. Des. Eng. 2020, 5, 1048–1056. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.J.M.; Guillén, M.G.M.G.; Lopes-Costa, T.; Pinto, S.M.A.S.M.A.; Calvete, M.J.F.M.J.F.; Pereira, M.M.M.M. Optical detection of amine vapors using ZnTriad porphyrin thin films. Sens. Actuators B Chem. 2015, 210, 28–35. [Google Scholar] [CrossRef]
- Ryan, T.A.; Ryan, C.; Seddon, E.A.; Seddon, K.R. Phosgene and Related Carbonyl Halides; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 9780080538808. [Google Scholar]
- Cowles, J.C. Theory of Dual-Wavelength Spectrophotometry for Turbid Samples. J. Opt. Soc. Am. 1965, 55, 690–692. [Google Scholar] [CrossRef]
- Swanson, C.; Lee, S.; Aranyosi, A.J.; Tien, B.; Chan, C.; Wong, M.; Lowe, J.; Jain, S.; Ghaffari, R. Rapid light transmittance measurements in paper-based microfluidic devices. Sens. Bio-Sens. Res. 2015, 5, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Fu, L.; Zhang, W.; Feng, W.; Chen, L. Portable paper-based device for quantitative colorimetric assays relying on light reflectance principle. Electrophoresis 2014, 35, 1152–1159. [Google Scholar] [CrossRef]




| Device | Cost | Sensor | Light Source | Output | Post- Processing | Programming |
|---|---|---|---|---|---|---|
| This study | ∼25 € | RGB | White LED | RGB values | No | Yes (Python) |
| Ellerbee et al. [1] | ∼$50 | Photodetector | Tricolor LED | Transmittance | No | No |
| Christodouleas et al. [14] | $90/ unspecified (a) | CCD/CMOS image sensors | White LED | RGB-resolved absorbance | Yes | No |
| Swanson et al. [32] | ∼$20 (b) | Photodiode | Monochromatic LED | Transmittance | No | Yes (C) |
| Li et al. [33] | ∼$20 | Color sensor (unspecified) | LED (unspecified) | Reflected light intensity | No | Yes (C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roales, J.; Moscoso, F.G.; Vargas, A.P.; Lopes-Costa, T.; Pedrosa, J.M. Colorimetric Gas Detection Using Molecular Devices and an RGB Sensor. Chemosensors 2023, 11, 92. https://doi.org/10.3390/chemosensors11020092
Roales J, Moscoso FG, Vargas AP, Lopes-Costa T, Pedrosa JM. Colorimetric Gas Detection Using Molecular Devices and an RGB Sensor. Chemosensors. 2023; 11(2):92. https://doi.org/10.3390/chemosensors11020092
Chicago/Turabian StyleRoales, Javier, Francisco G. Moscoso, Alejandro P. Vargas, Tânia Lopes-Costa, and José M. Pedrosa. 2023. "Colorimetric Gas Detection Using Molecular Devices and an RGB Sensor" Chemosensors 11, no. 2: 92. https://doi.org/10.3390/chemosensors11020092





