Childhood Hearing Impairment in Senegal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval Statement
2.2. Operational Definitions
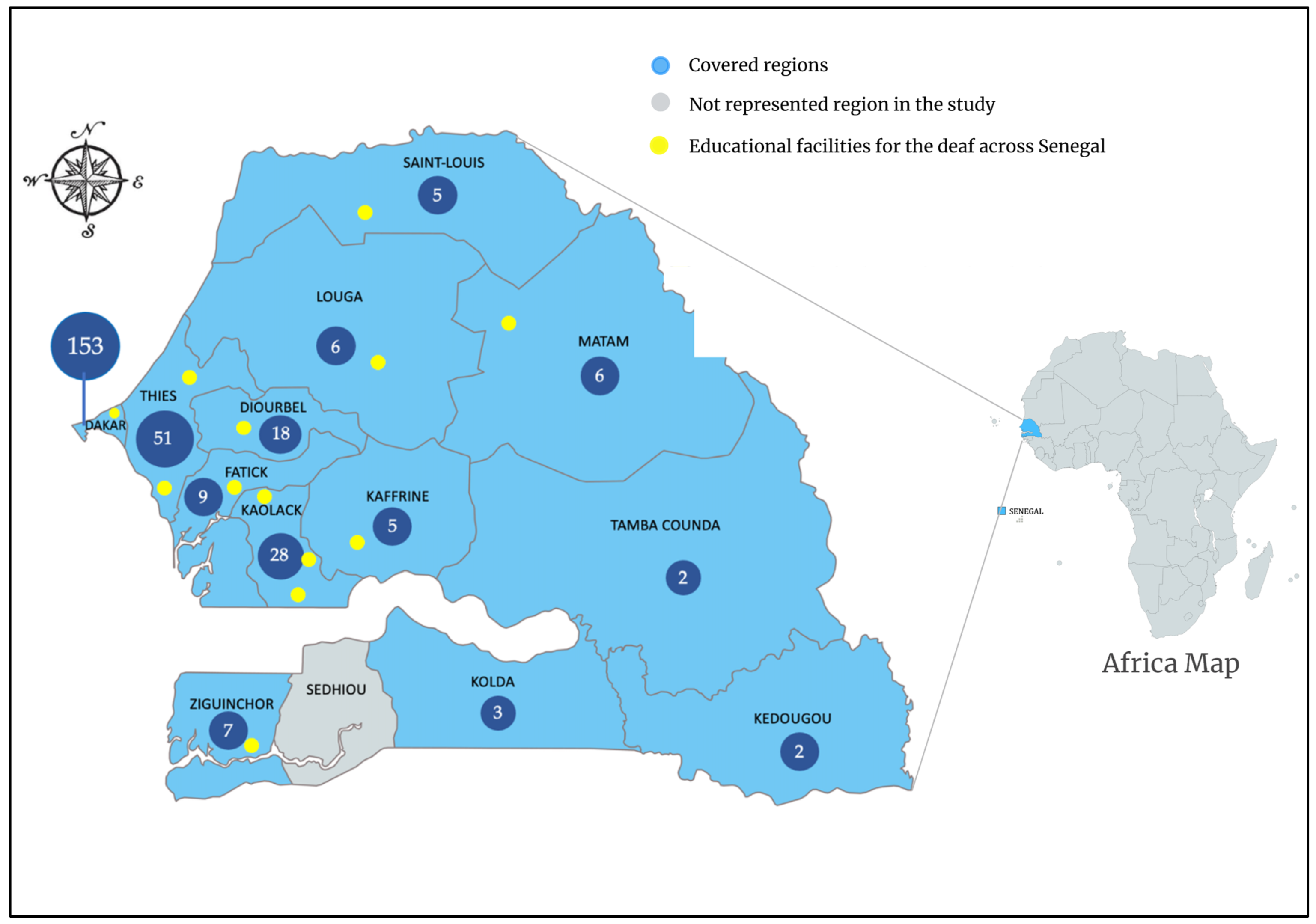
2.3. Study Settings, Populations, and Procedures
2.4. Audiological Evaluation
2.5. Data Analysis
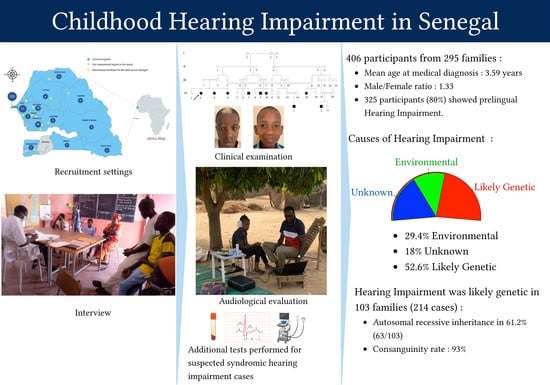
3. Results
3.1. Participant Demographics
3.2. Audiometric Characteristics and Management of Permanent HI
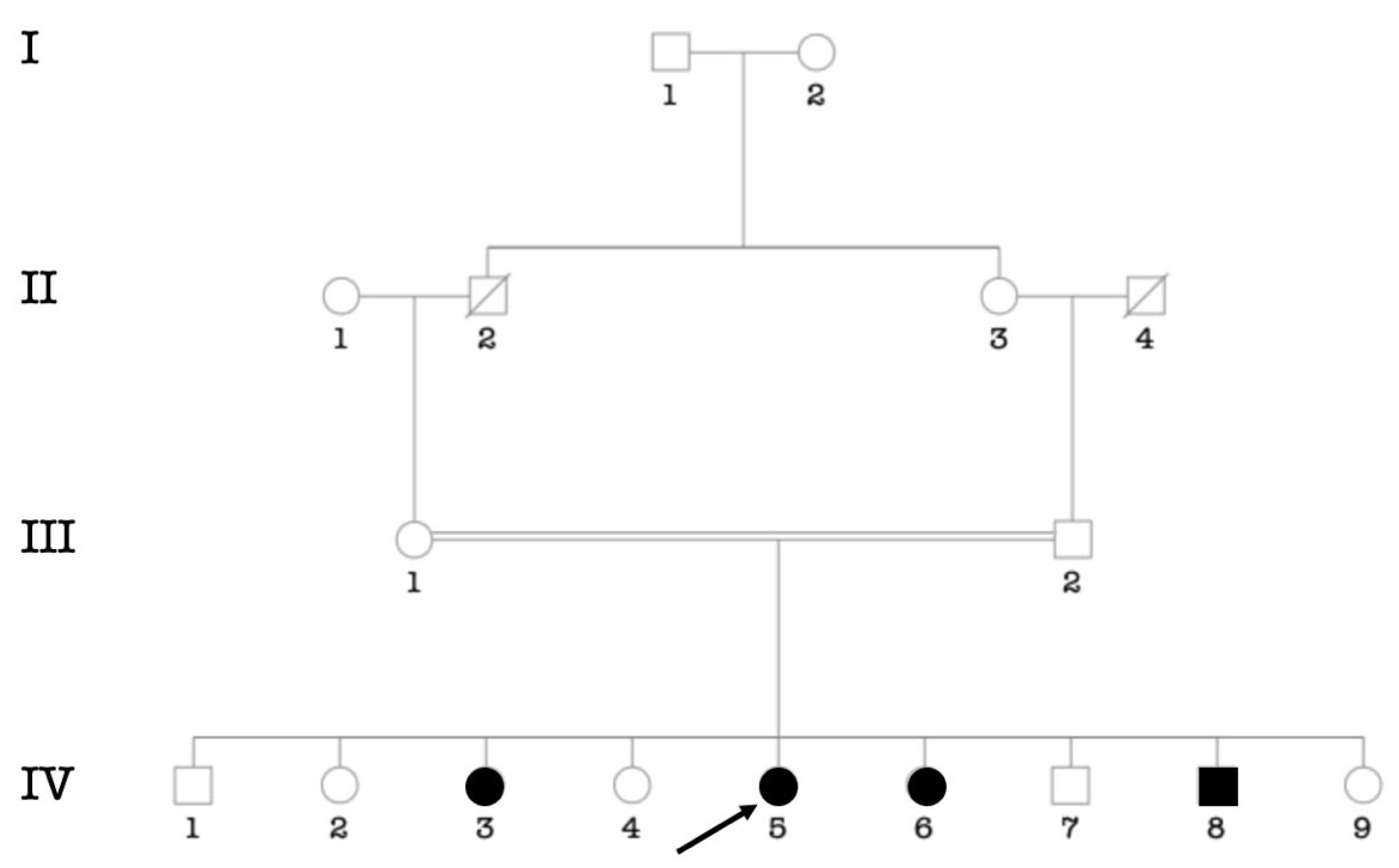
3.3. Etiologies of Childhood Hearing Impairment in Senegal
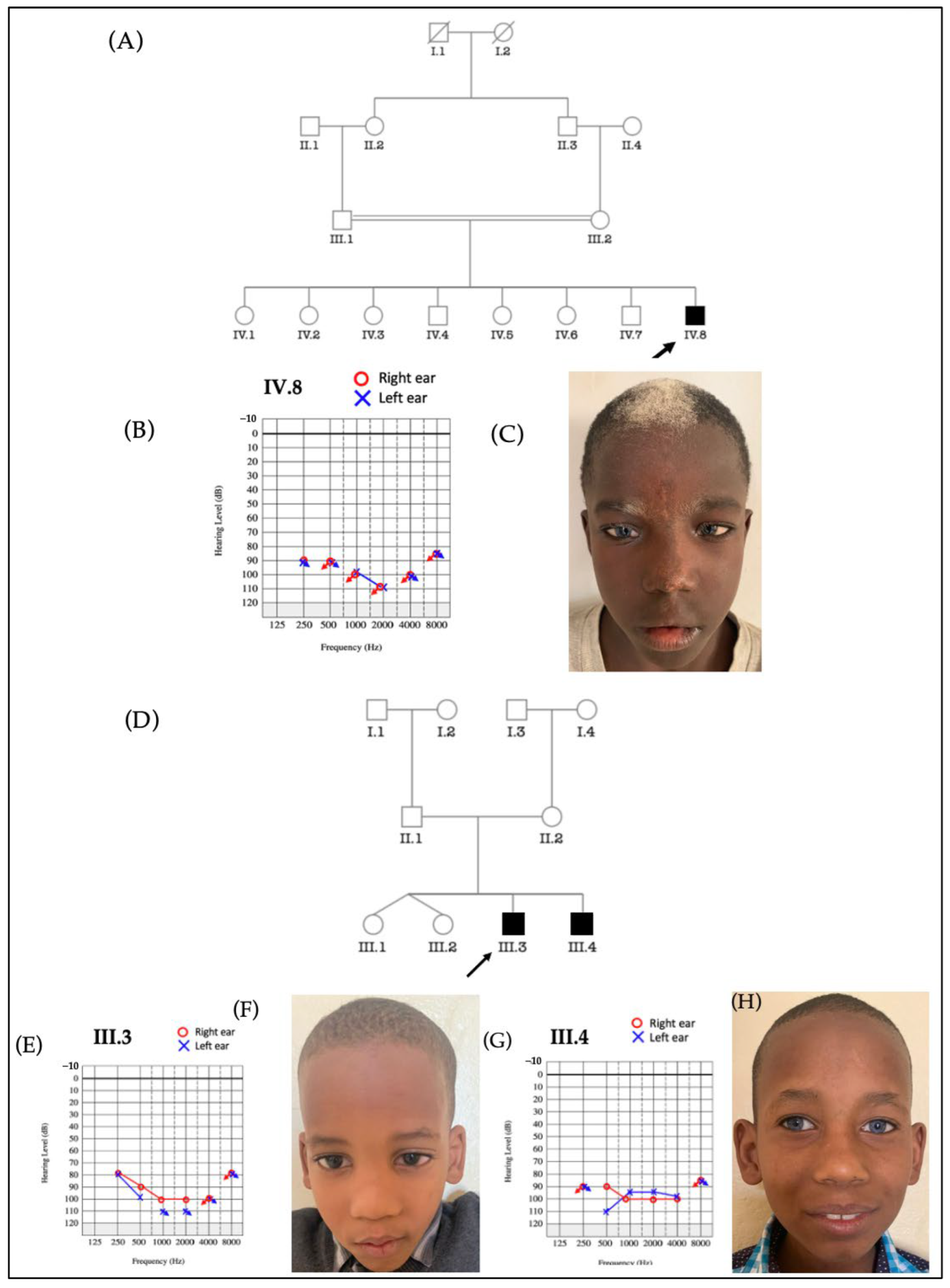
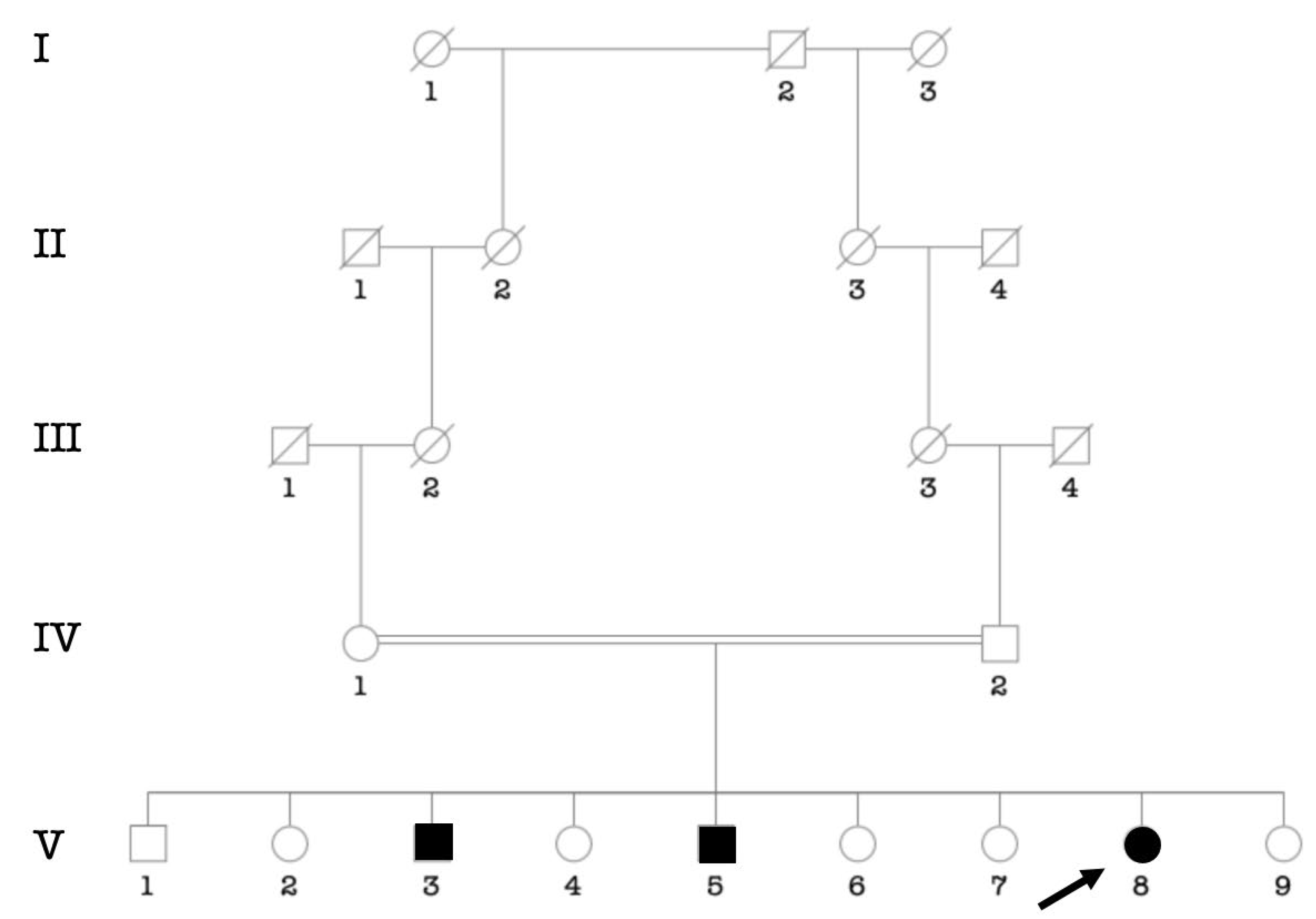
3.3.1. Waardenburg Syndrome (WS)
3.3.2. Labyrinthine Aplasia, Microtia, and Microdontia (LAMM) Syndrome
3.3.3. Pendred Syndrome
3.3.4. Diabetes-Deafness Syndrome
3.3.5. Usher Syndrome
3.3.6. Down’s Syndrome
3.3.7. Alport Syndrome
3.3.8. Uncharacterized Syndrome
3.4. Consanguinity Rate among the Main Ethnic Groups
3.5. Comparison of Our Results to Other Studies in the Neighboring Countries of Senegal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2013 DALYs and HALE Collaborators; Murray, C.J.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Addressing the Rising Prevalence of Hearing Loss. Available online: https://apps.who.int/iris/handle/10665/260336 (accessed on 17 August 2022).
- Stephenson, J. WHO Report Predicts Hearing Loss for 1 in 4 People Worldwide by 2050. JAMA Health Forum 2021, 2, e210357. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Fischer, C.; Tran, K.; Rachakonda, T.; Kallogjeri, D.; Lieu, J.E.C. Quality of Life in Children with Hearing Impairment: Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2016, 155, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, K.; Erixon, E.; Löfkvist, U.; Mäki-Torkko, E. The impact of permanent early-onset unilateral hearing impairment in children-A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2019, 120, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Grosse, S.D.; Riehle-Colarusso, T.; Gaffney, M.; Mason, C.A.; Shapira, S.K.; Sontag, M.K.; Braun, K.V.N.; Iskander, J. CDC Grand Rounds: Newborn Screening for Hearing Loss and Critical Congenital Heart Disease. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 888–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, I.; Mackey, A.; Rosenhall, U. Prevalence of childhood hearing impairment in the County of Stockholm-a 40-year perspective from Sweden and other high-income countries. Int. J. Audiol. 2020, 59, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Desalew, A.; Feto Gelano, T.; Semahegn, A.; Geda, B.; Ali, T. Childhood hearing impairment and its associated factors in sub-Saharan Africa in the 21st century: A systematic review and meta-analysis. SAGE Open Med. 2020, 8, 2050312120919240. [Google Scholar] [CrossRef]
- Stevens, G.; Flaxman, S.; Brunskill, E.; Mascarenhas, M.; Mathers, C.D.; Finucane, M.; Global Burden of Disease Hearing Loss Expert Group. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur. J. Public Health 2013, 23, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Mehra, S.; Eavey, R.D.; Keamy, D.G. The epidemiology of hearing impairment in the United States: Newborns, children, and adolescents. Otolaryngol. Head Neck Surg. 2009, 140, 461–472. [Google Scholar] [CrossRef]
- Ching, T.Y.C.; Oong, R.; van Wanrooy, E. The ages of intervention in regions with and without universal newborn hearing screening and prevalence of childhood hearing impairment in Australia. Aust. N. Z. J. Audiol. 2006, 28, 137–150. [Google Scholar] [CrossRef]
- Fortnum, H.M.; Summerfield, A.Q.; Marshall, D.H.; Davis, A.C.; Bamford, J.M. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: Questionnaire based ascertainment study. BMJ 2001, 323, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Wonkam, A.; Noubiap, J.J.N.; Djomou, F.; Fieggen, K.; Njock, R.; Toure, G.B. Aetiology of childhood hearing loss in Cameroon (sub-Saharan Africa). Eur. J. Med. Genet. 2013, 56, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yalcouyé, A.; Traoré, O.; Taméga, A.; Maïga, A.B.; Kané, F.; Oluwole, O.G.; Guinto, C.O.; Kéita, M.; Timbo, S.K.; DeKock, C.; et al. Etiologies of Childhood Hearing Impairment in Schools for the Deaf in Mali. Front. Pediatr. 2021, 9, 726776. [Google Scholar] [CrossRef] [PubMed]
- Sako, A.B. Particularities of hearing disorders in inhabitants of Mali. Vestn. Otorinolaringol. 1990, 1, 18–20. [Google Scholar]
- Lebeko, K.; Bosch, J.; Noubiap, J.J.N.; Dandara, C.; Wonkam, A. Genetics of hearing loss in Africans: Use of next generation sequencing is the best way forward. Pan. Afr. Med. J. 2015, 20, 383. [Google Scholar] [CrossRef]
- Smith, R.J.H.; Bale, J.F.; White, K.R. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Ayub, M. Genetics Of Human Hereditary Hearing Impairment. J. Ayub. Med. Coll. Abbottabad. 2017, 29, 671–676. [Google Scholar] [PubMed]
- Lefranc, M.P.; Lefranc, G. Consanguinity. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 158–162. [Google Scholar]
- Ndiaye, S.; Sarr, I.; Ayad, M. Enquête Démographique et de Santé au Sénégal 1986. 1988. Available online: https://dhsprogram.com/publications/publication-FR34-DHS-Final-Reports.cfm (accessed on 18 August 2022).
- Ndadi, T.K.J.; Ndiaye, M.; Randriamalala, N.A.; Witti, A.A.; Coly, I.J.; Watt, H.M.; Diom, S.E.; Diallo, B.K.; Raji, A. Child Deafness in Sub-Saharan Africa: Experience of Two ENT Services in Casamance, South of Senegal. Int. J. Otolaryngol. Head Neck Surg. 2021, 10, 92–101. [Google Scholar] [CrossRef]
- Dia, Y.; Adadey, S.M.; Diop, J.P.D.; Aboagye, E.T.; Ba, S.A.; De Kock, C.; Ly, C.A.T.; Oluwale, O.G.; Sène, A.R.G.; Sarr, P.D.; et al. GJB2 is a Major Cause of Non-Syndromic Hearing Impairment in Senegal. Biology 2022, 11, 795. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Davis, A.C.; Hoffman, H.J. Hearing loss grades and the International classification of functioning, disability and health. Bull. World Health Organ. 2019, 97, 725–728. [Google Scholar] [CrossRef]
- McPherson, B.; Holborow, C.A. A study of deafness in West Africa: The Gambian Hearing Health Project. Int. J. Pediatr. Otorhinolaryngol. 1985, 10, 115–135. [Google Scholar] [CrossRef]
- Moctar, E.C.M.; Riahi, Z.; El Hachmi, H.; Veten, F.; Meiloud, G.; Bonnet, C.; Abdelhak, S.; Errami, M.; Houmeida, A. Etiology and associated GJB2 mutations in Mauritanian children with non-syndromic hearing loss. Eur. Arch. Otorhinolaryngol. 2016, 273, 3693–3698. [Google Scholar] [CrossRef]
- Diallo, A.O.; Keita, A.; Odzili, F.A.; Balde, Y.; Diallo, M.T.; Conde, B.; Sylla, A.V. Surdité de l’Enfant à Conakry (Guinée): Analyse d’un Groupe de 124 Élèves d’une École de Sourds-Muets. Health Sci. Dis. 2017, 18. Available online: https://www.hsd-fmsb.org/index.php/hsd/article/view/807 (accessed on 12 August 2022).
- CDC. Data and Statistics About Hearing Loss in Children|CDC. 2021. Available online: https://www.cdc.gov/ncbddd/hearingloss/data.html (accessed on 30 August 2022).
- Vartiainen, E.; Kemppinen, P.; Karjalainen, S. Prevalence and etiology of bilateral sensorineural hearing impairment in a Finnish childhood population. Int. J. Pediatr. Otorhinolaryngol. 1997, 41, 175–185. [Google Scholar] [CrossRef]
- Gaye, B.; Diop, M.; Narayanan, K.; Offredo, L.; Reese, P.; Antignac, M.; Diop, V.; Mbacké, A.B.; Chatenet, L.B.; Marijon, E.; et al. Epidemiological transition in morbidity: 10-year data from emergency consultations in Dakar, Senegal. BMJ Glob. Health 2019, 4, e001396. [Google Scholar] [CrossRef] [Green Version]
- OMS|Bureau Régional Pour l’Afrique. Le Programme élargi de Vaccination du Sénégal Compte Désormais 11 Antigènes. Available online: https://www.afro.who.int/fr/news/le-programme-elargi-de-vaccination-du-senegal-compte-desormais-11-antigenes (accessed on 30 August 2022).
- Nkoumou Ngoa, G. Gratuité des soins et utilisation des services de santé maternelle Une analyse d’impact au Sénégal. L’Actualité Économique 2020, 96, 159–193. [Google Scholar] [CrossRef]
- Adadey, S.M.; Manyisa, N.; Mnika, K.; De Kock, C.; Nembaware, V.; Quaye, O.; Amedofu, G.K.; Awandare, G.A.; Wonkam, A. GJB2 and GJB6 Mutations in Non-Syndromic Childhood Hearing Impairment in Ghana. Front. Genet. 2019, 10, 841. [Google Scholar] [CrossRef]
- Bowman, D.M.; Brown, D.K.; Kimberley, B.P. An examination of gender differences in DPOAE phase delay measurements in normal-hearing human adults. Hear Res. 2000, 142, 1–11. [Google Scholar] [CrossRef]
- Wonkam, E.T.; Chimusa, E.; Noubiap, J.J.; Adadey, S.M.; Fokouo, J.V.; Wonkam, A. GJB2 and GJB6 Mutations in Hereditary Recessive Non-Syndromic Hearing Impairment in Cameroon. Genes 2019, 10, 844. [Google Scholar] [CrossRef] [Green Version]
- MCHB. Early Hearing Detection and Intervention (EHDI). Available online: https://mchb.hrsa.gov/programs-impact/early-hearing-detection-intervention-ehdi (accessed on 28 August 2022).
- Degue-Hear-Entendre. Available online: https://www.degue.org/ (accessed on 28 August 2022).
- le Roux, T.; Swanepoel, D.W.; Louw, A.; Vinck, B.; Tshifularo, M. Profound childhood hearing loss in a South Africa cohort: Risk profile, diagnosis and age of intervention. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 8–14. [Google Scholar] [CrossRef]
- Mazamay, S.; Guégan, J.-F.; Diallo, N.; Bompangue, D.; Bokabo, E.; Muyembe, J.-J.; Taty, N.; Vita, T.P.; Broutin, H. An overview of bacterial meningitis epidemics in Africa from 1928 to 2018 with a focus on epidemics “outside-the-belt”. BMC Infect. Dis. 2021, 21, 1027. [Google Scholar] [CrossRef]
- Wonkam, A.; Adadey, S.M.; Schrauwen, I.; Aboagye, E.T.; Wonkam-Tingang, E.; Esoh, K.; Popel, K.; Manyisa, N.; Jonas, M.; Dekock, C.; et al. Exome sequencing of families from Ghana reveals known and candidate hearing impairment genes. Commun. Biol. 2022, 5, 369. [Google Scholar] [CrossRef] [PubMed]
- Cantrelle, P. L’endogamie des populations du Fouta Sénégalais. Population 1960, 15, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Bayazit, Y.A.; Yilmaz, M. An overview of hereditary hearing loss. ORL J. Otorhinolaryngol. Relat. Spec. 2006, 68, 57–63. [Google Scholar] [CrossRef]
- Nance, W.E. The genetics of deafness. Ment. Retard. Dev. Disabil. Res. Rev. 2003, 9, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Vona, B.; Nanda, I.; Hofrichter, M.A.H.; Shehata-Dieler, W.; Haaf, T. Non-syndromic hearing loss gene identification: A brief history and glimpse into the future. Mol. Cell. Probes 2015, 29, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Hertzano, R.; Elkon, R. High throughput gene expression analysis of the inner ear. Hear Res. 2012, 288, 77–88. [Google Scholar] [CrossRef]






| Characteristics | N | % | ||
|---|---|---|---|---|
| Onset | Prelingual | Before 2 years old | 325 | 80.05 |
| Perilingual | Between 2 and 4 years old | 29 | 7.14 | |
| Post lingual | After 4 years old | 52 | 12.81 | |
| Age at medical diagnosis | [0–2 years] | 159 | 39.16 | |
| [2–4 years] | 112 | 27.58 | ||
| After 4 years | 134 | 33.00 | ||
| Sociodemographic | Sex | Male | 232 | 57.14 |
| Female | 174 | 42.86 | ||
| Level of education | Preschool | 94 | 23.15 | |
| Primary | 180 | 44.33 | ||
| Secondary | 13 | 3.20 | ||
| Tertiary | 1 | 0.25 | ||
| None | 69 | 17 | ||
| Too young to attend a school | 49 | 12.07 |
| Characteristics | n | % | |
|---|---|---|---|
| Type of HI | Sensorineural | 362 | 89.16 |
| Conductive | 37 | 9.11 | |
| Mixed | 7 | 1.72 | |
| Degree of HI | Moderate (40–60 dB) | 65 | 16.01 |
| Severe (61–80 dB) | 66 | 16.26 | |
| Profound (≥81 dB) | 275 | 67.73 | |
| Symmetry of HI | Symmetric | 381 | 93.84 |
| Asymmetric | 25 | 6.16 | |
| Laterality of HI | Bilateral | 401 | 98.77 |
| Unilateral | 5 | 1.23 | |
| Curve pattern | Flat | 301 | 81.79 |
| Sloping | 45 | 12.23 | |
| Ascendant | 9 | 2.45 | |
| No Curve (Total hearing loss) | 13 | 3.53 | |
| Hearing rehabilitation | Conventional hearing aids | 15 | 3.70 |
| Cochlear implantation | 3 | 0.74 | |
| Absence of hearing rehabilitation | 388 | 95.56 |
| Etiologies | n | % | ||
|---|---|---|---|---|
| Environmental | Chronic otitis media | 38 (38 families) | 9.36 | |
| Meningitis | 25 (25 families) | 6.16 | ||
| Prematurity | 11 (11 families) | 2.71 | ||
| Head Trauma | 9 (9 families) | 2.22 | ||
| Neonatal asphyxia | 9 (9 families) | 2.22 | ||
| Cerebral malaria | 6 (6 families) | 1.48 | ||
| Neonatal infection | 7 (7 families) | 1.72 | ||
| Neonatal jaundice | 2 (2 families) | 0.49 | ||
| Ototoxicity | 2 (2 families) | 0.49 | ||
| Low birth weight | 2 (2 families) | 0.49 | ||
| Eustachian Tube Dysfunction | 2 (2 families) | 0.49 | ||
| Others * | 7 (7 families) | 1.72 | ||
| Likely genetic | Non-Syndromic HI | Autosomal recessive | 153 (59 families) | 37.69 |
| Autosomal dominant | 7 (3 families) | 1.72 | ||
| Recessive X-linked | 7 (3 families) | 1.72 | ||
| Sporadic | 27 (27 families) | 6.65 | ||
| Syndromic HI | Waardenburg type 2 | 5 (4 families) | 1.23 | |
| Pendred Syndrome | 4 (1 family) | 0.99 | ||
| Usher type 2 Syndrome | 3 (1 family) | 0.74 | ||
| Diabetes-deafness Syndrome | 3 (1 family) | 0.74 | ||
| LAMM Syndrome | 1 (1 family) | 0.25 | ||
| Down’s Syndrome | 1 (1 family) | 0.25 | ||
| Alport Syndrome | 1 (1 family) | 0.24 | ||
| Non-Specific Syndrome | 2 (1 family) | 0.49 | ||
| Unknown | 72 (72 families) | 17.74 | ||
| Total | 406 (295 families) | 100 | ||
| Ethnic Groups | Number of Families | Consanguinity Rate (%) |
|---|---|---|
| Wolof | 53 | 90.6 |
| Fulani | 10 | 100 |
| Serer | 21 | 100 |
| Manding | 2 | 50 |
| Other * | 4 | 75 |
| Total | 90 | 93 |
| Gambia | Mali | Mauritania | Guinea | Present Study | |
|---|---|---|---|---|---|
| Year of publication | 1985 | 2021 | 2016 | 2017 | 2022 |
| Reference | [24] | [14] | [25] | [26] | -- |
| Number of patients | 257 | 117 (100 families) | 139 (113 families) | 124 | 406 (295 families) |
| Hereditary | 8% | 29.3% | 44.4% | 18.5% | 52.6% |
| Consanguinity rate | -- | 55.5% | 61.3% | -- | 93% |
| Contribution of GJB2 | -- | 0% | 9.4% | -- | 34.1% |
| Chronic otitis | -- | 7.7% | -- | -- | 9.36% |
| Cerebrospinal meningitis | 31% | 40% | 13% | 42% | 6.16% |
| Ototoxic medication | -- | 14.3% | 3.6% | 16.1% | 0.49% |
| Cerebral malaria | 16% | -- | -- | -- | 1.48% |
| Prematurity | -- | 15.7% | -- | -- | 2.17% |
| Head trauma | -- | -- | -- | -- | 2.22% |
| Neonatal asphyxia | -- | 2.8% | -- | 8.1% | 2.22% |
| Neonatal jaundice | -- | -- | -- | -- | 0.49% |
| Measles | 2% | -- | 2.9% | -- | -- |
| Rubella | 1.5% | -- | -- | -- | -- |
| Unknown | 54.4% | 11.3% | 5.7% | 22.6% | 17.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dia, Y.; Loum, B.; Dieng, Y.J.K.B.; Diop, J.P.D.; Adadey, S.M.; Aboagye, E.T.; Ba, S.A.; Touré, A.A.; Niang, F.; Diaga Sarr, P.; et al. Childhood Hearing Impairment in Senegal. Genes 2023, 14, 562. https://doi.org/10.3390/genes14030562
Dia Y, Loum B, Dieng YJKB, Diop JPD, Adadey SM, Aboagye ET, Ba SA, Touré AA, Niang F, Diaga Sarr P, et al. Childhood Hearing Impairment in Senegal. Genes. 2023; 14(3):562. https://doi.org/10.3390/genes14030562
Chicago/Turabian StyleDia, Yacouba, Birame Loum, Yaay Joor Koddu Biigé Dieng, Jean Pascal Demba Diop, Samuel Mawuli Adadey, Elvis Twumasi Aboagye, Seydi Abdoul Ba, Abdoul Aziz Touré, Fallou Niang, Pierre Diaga Sarr, and et al. 2023. "Childhood Hearing Impairment in Senegal" Genes 14, no. 3: 562. https://doi.org/10.3390/genes14030562