Developing a System Dynamic Model for Product Life Cycle Management of Generic Pharmaceutical Products: Its Relation with Open Innovation
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Dynamic (SD) Modeling Steps
2.1.1. Step 1: Problem Definition and Reference Mode
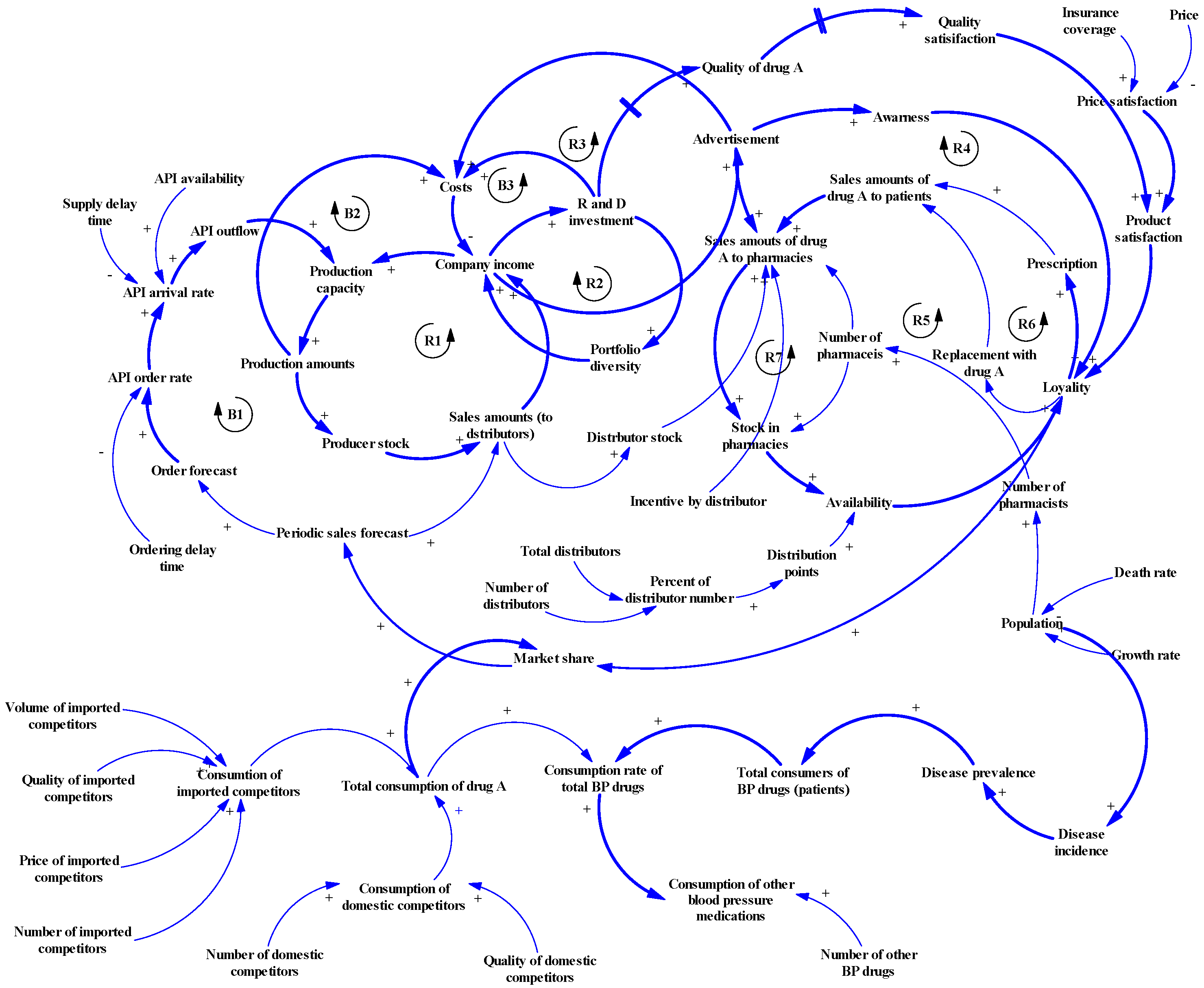
2.1.2. Step 2: Developing a Causal Loop Diagram
2.1.3. Step 3: Developing a Stock and Flow Diagram
2.1.4. Step 4: Testing the Model
2.2. Data Collection and Analysis
- First, we conceptualized the dynamic hypotheses based on an in-depth literature review and experts’ opinions.
- Second, we determined the causal loop of the pharmaceuticals PLC and confirmed their related relationships through a questionnaire to identify the causes of reference mode formation.
- Third, we ran a quantitative dynamic modeling based on real-world data and experts’ opinions regarding the Iranian pharmaceutical industry.
3. Results
3.1. PLC Subsystems of Generic Pharmaceutical Products
3.2. Determination of Reference Mode
3.3. System Dynamic Casual Loops for PLC
3.3.1. Subsystem of the Supply-Side
3.3.2. Subsystem of Demand-Side
- Patients and diseases: the number of patients depends on the disease incidence, which is affected by the population.
- Physicians and pharmacies: physicians play a role as gatekeepers between patients and pharmacies, and pharmacists as intermediate consumers have an essential role in drug selection. We found that loyalty to a manufacturer was an important factor to increase market share. The manufacturer’s marketing activities can affect loyalty by increasing physicians’ awareness and as a result increase consumption of related products (Feedback loop R4). Two other factors, including “product availability” and “product satisfaction”, along with “advertisement” lead to loyalty and ultimately increase patients’ consumption as a positive feedback loop R5. The intermediate consumers of pharmaceutical products are pharmacists; they usually choose the manufacturer of prescription drugs based on pharmacy inventory (availability), and quality and manufacturer advertisements (Feedback loop R6). Also, the number of pharmacies can enhance availability by increasing the pharmacies’ stock across the country (Positive feedback loop R7).
3.3.3. Competition Subsystem
3.4. Model Simulation: PLC System Behaviors
3.4.1. Subsystem of the Supply-Side
3.4.2. Subsystem of Demand-Side
3.4.3. Competition Subsystem
3.5. Model Validation
4. Discussion
4.1. Elements That Determine the PLC of a Generic Medicine
4.2. System Dynamic Model of PLM and Open Innovation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Variables | Unit | |
|---|---|---|
| Supply subsystem | Factors related to supply of raw materials | |
| 1. Inventory of raw materials | Percent | |
| 2. Delay in supply of raw materials | Number | |
| Factors related to manufacturers | ||
| 3. The amount of advertising activities | Percent | |
| 4. Production rate | Number/year | |
| 5. Warehouse stock | Number | |
| 6. Sales to distributor | Number/year | |
| 7. Company income | Rials | |
| 8. Market share | Percent | |
| 9. Costs | Rials | |
| 10. Research and development activities | Percent | |
| 11. Production capacity | Number | |
| 12. Sales forecast | Number | |
| 13. Raw material order rate | Number | |
| 14. Delay in raw material order | Number | |
| 15. Total number of product portfolio | Number | |
| 16. Product quality | - | |
| 17. Product availability | - | |
| Factors related to distributors | ||
| 18. Number of distribution points | Number | |
| 19. Sales to pharmacies | Number/year | |
| 20. Discount rate (incentive) | Number | |
| 21. Percentage of distribution centers | Number | |
| 22. Warehouse stocks of distributors | Percent | |
| Demand subsystem | Factors related to the disease and patients | |
| 23. Population | Number | |
| 24. Mortality rate | Number/year | |
| 25. Birth rate | Number/year | |
| 26. Disease prevalence | Number | |
| 27. Total number of people sought for treatment | Number | |
| 28. Total consumption of antihypertensive drugs | Number/year | |
| 29. Number of people treated | Number | |
| 30. Price satisfaction | - | |
| 31. Quality satisfaction | - | |
| 32. Product satisfaction | - | |
| 33. Existence of insurance coverage | 0/1 | |
| Factors related to the pharmacies | ||
| 34. Number of pharmacies | Number | |
| 35. Warehouse stocks of pharmacies | Number | |
| 36. Sales to patients | Number/year | |
| 37. Loyalty to the manufacturer | - | |
| Factors related to the physicians | ||
| 38. Product satisfaction | - | |
| 39. Loyalty to the manufacturer | - | |
| Competition subsystem | Factors related to the competition | |
| 40. Number of competitors from other families | Number | |
| 41. Consumption of competitors from other therapeutic families | Number/year | |
| 42. Consumption of domestic competitors of drug A | Number/year | |
| 43. Number of domestic competitors | Number | |
| 44. Number of foreign competitors | Number | |
| 45. Price of foreign competitors | Rials | |
| 46. Volume of competitors imports | Number/year | |
| 47. Import volume of competitors of the same family A | Number |

References
- Stark, J. Product Lifecycle Management Lifecycle Management, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 2. [Google Scholar]
- Cespi, D.; Beach, E.S.; Swarr, T.E.; Passarini, F.; Vassura, I.; Dunn, P.J.; Anastas, P.T. Life cycle inventory improvement in the pharmaceutical sector: Assessment of the sustainability combining PMI and LCA tools. Green Chem. 2015, 17, 3390–3400. [Google Scholar] [CrossRef]
- López, A.S.; Del Valle, C.; Escalona, M.J.; Lee, V.; Goto, M. Patient lifecycle management: An approach for clinical processes. Lect. Notes Comput. Sci. 2017, 9044, 694–700. [Google Scholar] [CrossRef]
- Carlos, F. A simulation model to evaluate pharmaceutical supply chain costs in hospitals: The case of a Colombian hospital. DARU J. Pharm. Sci. 2020, 28, 1–12. [Google Scholar] [CrossRef]
- Emara, Y.; Lehmann, A.; Siegert, M.W.; Finkbeiner, M. Modeling pharmaceutical emissions and their toxicity-related effects in life cycle assessment (LCA): A review. Integr. Environ. Assess. Manag. 2019, 15, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajapati, V.; Dureja, H. Product lifecycle management in pharmaceuticals. J. Med. Mark. 2012, 12, 150–158. [Google Scholar] [CrossRef]
- Hein, T. Product Lifecycle Management for the Pharmaceutical Industry; Oracle Life Sciences: Austin, TX, USA, 2012; pp. 1–9. [Google Scholar]
- Montserrat Peñarroya-Farell, F.M. Business Model Dynamics from Interaction with Open Innovation. J. Open Innov. Technol. Mark. Complex. Artic. 2021, 7, 81. [Google Scholar] [CrossRef]
- Henry Chesbrough, M.B. Explicating Open Innovation: Clarifying an Emerging Paradigm for Understanding Innovation; Oxford Scholarship Online: Oxford, UK, 2013. [Google Scholar]
- Feldhusen, J.; Gebhardt, B.; Macke, N.; Nurcahya, E.; Bungert, F. Development of Methods to Support the Implementation of a PDMS; Springer: Dordrecht, The Netherland, 2006. [Google Scholar]
- Martin Eigner, R.S. Product Lifecycle Management-Ein Leitfaden für Product Development und Life Cycle Management, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gürtler, M.R. Bridging the Gap: From Open Innovation to an Open Product-Life-Cycle by Using Open-X Methodologies; Springer: Delhi, India, 2013. [Google Scholar]
- Githens, G. Product Lifecycle Management: Driving the Next Generation of Lean Thinking by Michael Grieves; McGraw Hill: New York, NY, USA, 2007; Volume 24. [Google Scholar]
- de Oliveira, P.S.G.; da Silva, D.; da Silva, L.F.; dos Santos Lopes, M.; Helleno, A. Factors that influence product life cycle management to develop greener products in the mechanical industry. Int. J. Prod. Res. 2016, 54, 4547–4567. [Google Scholar] [CrossRef]
- Dunne, S.S.; Dunne, C.P. What do people really think of generic medicines? A systematic review and critical appraisal of literature on stakeholder perceptions of generic drugs. BMC Med. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Colgan, S.L.E.; Faasse, K.; Pereira, J.A.; Grey, A.; Petrie, K.J. Changing perceptions and efficacy of generic medicines: An intervention study. Health Psychol. 2016, 35, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Dixit, A.; Kumar, N.; Kumar, S. Use of Generic Medicines: Challenges and Benefits. J. Health Manag. 2018, 20, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Kebriaeezadeh, A.; Koopaei, N.N.; Abdollahiasl, A.; Nikfar, S.; Mohamadi, N. Trend analysis of the pharmaceutical market in Iran; 1997–2010; policy implications for developing countries. DARU J. Pharm. Sci. 2013, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, N.; Mehralian, G.; Rasekh, H.R.; Tayeba, H. Pharmaceutical innovation and market share: Evidence from a generic market. Int. J. Pharm. Healthc. Mark. 2016, 10, 376–389. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Agheli, L. Determining the structure of pharmaceutical industry in Iran. Int. J. Pharm. Healthc. Mark. 2019, 13, 101–115. [Google Scholar] [CrossRef]
- Carnahan, S.; Agarwal, R.; Campbell, B. The Effect of Firm Compensation Structures on the Mobility and Entrepreneurship of Extreme Performers. Strateg. Manag. J. 2010, 2345, 1–43. [Google Scholar] [CrossRef]
- Komninos, I.D. Product Life Cycle Management; Urban and Regional Innovation Research Unit: Thessaloniki, Greece, 2002. [Google Scholar]
- Jernigan, J.M.; Smith, M.C.; Banahan, B.F.; Juergens, J.P. Descriptive Analysis of the 15-Year Product Life Cycles of a Sample of Pharmaceutical Products. J. Pharm. Mark. Manage. 1991, 6, 3–36. [Google Scholar] [CrossRef]
- Grabowski, H.; Vernon, J. A new look at the returns and risks to pharmaceutical R&D. Manag. Sci. 1990, 36, 804–821. [Google Scholar]
- Bergström, R.; Höög, S. The Impact of Over-the-Counter Switches on the Product Life Cycles of 15 Pharmaceutical Products in Sweden. J. Pharm. Mark. Manage. 1994, 9, 25–68. [Google Scholar] [CrossRef]
- Bauer, H.H.; Fischer, M. Product life cycle patterns for pharmaceuticals and their impact on R&D profitability of late mover products. Int. Bus. Rev. 2000, 9, 703–725. [Google Scholar] [CrossRef]
- Fischer, M.; Leeflang, P.S.H.; Verhoef, P.C. Drivers of peak sales for pharmaceutical brands. Quant. Mark. Econ. 2010, 8, 429–460. [Google Scholar] [CrossRef] [Green Version]
- Tahmasebi, N.; Zadeh, A.K.; Imani, A.; Golestani, M. Evaluation of factors affecting sales of prescription medicines by econometric methods in Iran. Pharm. Sci. 2013, 19, 101–107. [Google Scholar]
- Dhaval Dave, H.S. the Impact of Direct-To-Consumer Advertising on Pharmaceutical. Natl. Bur. Econ. Res. 2010, 79, 1–2. [Google Scholar]
- Kapedanovska, A.; Naumovska, Z.; Sterjev, Z. The advertising influence on pharmacist recommendations and consumer selection of over-the-counter drugs. Proc. Maced. Pharm. Bull. 2016, 62, 107–108. [Google Scholar]
- Mehralian, G.; Sharif, Z.; Yousefi, N.; Akhgari, M. Physicians’ loyalty to branded medicines in low-middle-income countries: A structural equation modeling. J. Generic Med. 2017, 13, 9–18. [Google Scholar] [CrossRef]
- Afshar Kazemi, M.A.; Eshlaghy, A.T.; Tavasoli, S. Developing the product strategy via product life cycle simulation according to the system dynamics approach. Appl. Math. Sci. 2011, 5, 845–862. [Google Scholar]
- Pimpão, P.; Correia, A.; Duque, J.; Zorrinho, C. Diffusion patterns in loyalty programs. Adv. Cult. Tour. Hosp. Res. 2016, 12, 115–126. [Google Scholar] [CrossRef]
- Abdollahiasl, A.; Kebriaeezadeh, A.; Dinarvand, R.; Abdollahi, M.; Cheraghali, A.M.; Jaberidoost, M.; Nikfar, S. A system dynamics model for national drug policy. DARU J. Pharm. Sci. 2014, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yan, H.; Qi, J. What do chinese entrepreneurs think about entrepreneurship: A case study of popular essays on Zhisland. J. Open Innov. Technol. Mark. Complex. 2020, 6, 86. [Google Scholar] [CrossRef]
- Davahli, M.R.; Karwowski, W.; Taiar, R. A system dynamics simulation applied to healthcare: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 5741. [Google Scholar] [CrossRef] [PubMed]
- Binti Safri, S.; Binti Bazin, N.E.N. Conceptualization of factors influencing new product introduction within shorter product life cycle. Proc. Conf. Data Min. Optim. 2012, 143–148. [Google Scholar] [CrossRef]
- Moosivand, A.; Ghatari, A.R.; Rasekh, H.R. Supply chain challenges in pharmaceutical manufacturing companies: Using qualitative system dynamics methodology. Iran. J. Pharm. Res. 2019, 18, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.W.H. Research on Optimization of Pooling System and Its Application in Drug Supply Chain Based on Big Data Analysis. Int. J. Telemed. Appl. 2017. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Hayati, Z. A System Dynamic Model for Analyzing Bullwhip Effect in Drug Supply Chain Considering Targeted Subsidy Plan. Appl. Econ. Stud. Iran 2018, 7, 37–42. [Google Scholar]
- Akhlaghinia, N.; Ghatari, A.R.; Moghbel, A.; Yazdian, A. Developing a System Dynamic Model for Pharmacy Industry. Ind. Eng. Manag. Syst. 2018, 17, 662–668. [Google Scholar] [CrossRef]
- Zali, M.R.; Najafian, M.; Colabi, A.M. System Dynamics Modeling in Entrepreneurship Research: A Review of the Literature. Int. J. Supply Oper. Manag. 2014, 1, 347–370. [Google Scholar]
- Ford, D.N. A system dynamics glossary. Syst. Dyn. Rev. 2019, 35, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Salim, H.K.; Stewart, R.A.; Sahin, O.; Dudley, M. Systems approach to end-of-life management of residential photovoltaic panels and battery energy storage system in Australia. Renew. Sustain. Energy Rev. 2020, 134, 110176. [Google Scholar] [CrossRef]
- Pejić-Bach, M.; Čerić, V. Developing system dynamics models with “step-by-step” approach. J. Inf. Organ. Sci. 2007, 31, 171–185. [Google Scholar]
- IFDA Official Iranian Drug Statistics; Food and Drug Administration of Iran: Tehran, Iran, 2018.
- Seifert, E. OriginPro 9.1: Scientific data analysis and graphing software-Software review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Official Reports of Companies Listed on Tehran Stock Exchange; Codal Publishers’ Information System: Tehran, Iran, 2019.
- Ventana Systems. Ventana Simulation Environment (Users Guide: Version 5); Ventana Systems: Harvard, MA, USA, 2007. [Google Scholar]
- Isee Systems. Getting Started with iThink and STELLA Copyright, Trademarks, and Conditions of Use; Mac2Win Porting Technology: Lebanon, PA, USA, 2012; Available online: https://static1.squarespace.com/static/5829e14b414fb518a2bf6124/t/5af4aaee2b6a289bcc1640c9/1525983988315/GettingStartedwithiThinkandSTELLA.pdf (accessed on 11 November 2021).
- Food and Drug Administration of The Islamic Republic of Iran, Tehran, Iran. Available online: https://www.fda.gov.ir/ (accessed on 11 November 2021).
- Merkuryeva, G.; Valberga, A.; Smirnov, A. Demand forecasting in pharmaceutical supply chains: A case study. Procedia Comput. Sci. 2019, 149, 3–10. [Google Scholar] [CrossRef]
- Ahmad, N. Sale Forecasting of Merck Pharma Company using ARMA Model. Res. J. Financ. Account. 2015, 6, 30–36. [Google Scholar]
- Mccarthy, T.M.; Davis, D.F.; Golicic, S.L.; Mentzer, J.T. The evolution of sales forecasting management: A 20-year longitudinal study of forecasting practices. J. Forecast. 2006, 25, 303–324. [Google Scholar] [CrossRef]
- Zhang, G.; Qiu, H. Competitive Product Identification and Sales Forecast Based on Consumer Reviews. Math. Probl. Eng. 2021. [Google Scholar] [CrossRef]
- Fortsch, S.M.; Choi, J.H.; Khapalova, E.A. Competition can help predict sales. J. Forecast. 2021, 1–14. [Google Scholar] [CrossRef]
- Barat, S. Global Marketing Management. J. Glob. Mark. 2009, 22, 329–331. [Google Scholar] [CrossRef]
- Cheraghali, A.M. Trends in Iran Pharmaceutical Market. Iran. J. Pharm. Res. IJPR 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Fardazar, F.E.; Asiabar, A.S.; Safari, H.; Asgari, M.; Saber, A.; Azar, A.A.E.F. Policy analysis of Iranian pharmaceutical sector; A qualitative study. Risk Manag. Healthc. Policy 2019, 12, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheraghali, A.M. Current status of biopharmaceuticals in Iran’s pharmaceutical market. Generics Biosimilars Initiat. J. 2013, 2, 26–29. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Bakhtiari, N.; Safarey, R.; Ghari, T. Pharmaceutical industry in export marketing: A closer look at competitiveness. Int. J. Pharm. Healthc. Mark. 2019, 13, 331–345. [Google Scholar] [CrossRef]
- Seyedifar, M.; Nikfar, S.; Asl, A.A.; Rasekh, H.R.; Ehsani, A.; Kebriaeezadeh, A. An Evaluation of the Policy and the Procedures of Successful Pharmaceutical Exporters and the Comparison Iranian Counterpart Policy. J. Pharm. Pharm. Manag. 2015, 12, A246–A247. [Google Scholar] [CrossRef] [Green Version]
- Schumock, G.T.; Walton, S.M.; Park, H.Y.; Nutescu, E.A.; Blackburn, J.C.; Finley, J.M.; Lewis, R.K. Factors that Influence Prescribing Decisions. Ann. Pharmacother. 2004, 38, 557–562. [Google Scholar] [CrossRef]
- Delpasand, K.; Tavakkoli, S.N.; Kiani, M.; Abbasi, M.; Afshar, L. Ethical challenges in the relationship between the pharmacist and patient in Iran. Int. J. Hum. RIGHTS Healthc. 2020, 13. [Google Scholar] [CrossRef]
- Bambra, C.; Riordan, R.; Ford, J.; Matthews, F. The COVID-19 pandemic and health inequalities. J. Epidemiol. Community Health 2020, 74, 964–968. [Google Scholar] [CrossRef]
- Civaner, M. Sale strategies of pharmaceutical companies in a “pharmerging” country: The problems will not improve if the gaps remain. Health Policy 2012, 106, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, A.S.; Soltani, S.; Mehdizadeh, M. Competitive advantage and its impact on new product development strategy (Case study: Toos Nirro technical firm). J. Open Innov. Technol. Mark. Complex. 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Xu, M.; Song, H.; Jiang, W.; Zhang, Y. R&D-Marketing Integration and Performance—Evidence Provided by Agricultural Science and Technology Enterprises. J. Serv. Sci. Manag. 2014, 07, 18–29. [Google Scholar] [CrossRef] [Green Version]
- MAAM Leenders, B.W. The effectiveness of different mechanisms for integrating marketing and R&D. J. Prod. Innov. 2002, 19, 305–317. [Google Scholar]
- Becker, M.C.; Lillemark, M. Marketing/R&D integration in the pharmaceutical industry. Res. Policy 2006, 35, 105–120. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Torabi, S.A.; Zahiri, B. A robust possibilistic programming approach for pharmaceutical supply chain network design. Comput. Chem. Eng. 2015, 82, 115–128. [Google Scholar] [CrossRef]
- Izadi, A.; Kimiagari, A. mohammad Distribution network design under demand uncertainty using genetic algorithm and Monte Carlo simulation approach: A case study in pharmaceutical industry. J. Ind. Eng. Int. 2014, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bastani, P.; Dehghan, Z.; Kashfi, S.M.; Dorosti, H.; Mohammadpour, M.; Mehralian, G.; Ravangard, R. Strategies to improve pharmaceutical supply chain resilience under politico-economic sanctions: The case of Iran. J. Pharm. Policy Pract. 2021, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.Y.H.; Zheng, P.; Chen, C.-H. A state-of-the-art survey of Digital Twin: Techniques, engineering product lifecycle management and business innovation perspectives. J. Intell. Manuf. 2020, 31, 1313–1337. [Google Scholar] [CrossRef]
- Ameri, F.; Dutta, D. Product Lifecycle Management: Closing the Knowledge Loops. Comput. Aided. Des. Appl. 2013, 2, 577–590. [Google Scholar] [CrossRef]
- Gečevska, V.; Štefanić, N.; Veža, I.; Čuš, F. Sustainable business solutions trough lean product lifecycle management. Acta Tech. Corviniensis Bull. Eng. 2012, 5, 135–142. [Google Scholar]
- Matsokis, A. An Ontology-Based Approach for Closed-Loop Product Lifecycle Management Aristeidis; EPFL: Lausanne, Switzerland, 2010; Volume 4823. [Google Scholar]
- Urbinati, A.; Chiaroni, D.; Chiesa, V.; Frattini, F. The role of digital technologies in open innovation processes: An exploratory multiple case study analysis. R&D Manag. 2020, 50, 136–160. [Google Scholar] [CrossRef]
- Marilungoa, E.; Coscia, E.; Quaglia, A.; Peruzzini, M.; Germani, M. Open Innovation for ideating and designing new Product Service Systems. Procedia CIRP 2016, 47, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Letizia, M.; Tim, M. How do large multinational companies implement open innovation? Technovation 2011, 31, 586–597. [Google Scholar]
- Su, J.; Yang, Y.; Zhang, X. Knowledge transfer efficiency measurement with application for open innovation networks. Int. J. Technol. Manag. 2019, 81, 118–138. [Google Scholar]
- Díaz, M.M.; Duque, C.M. Open innovation through customer satisfaction: A logit model to explain customer recommendations in the hotel sector. J. Open Innov. Technol. Mark. Complex. 2021, 7, 180. [Google Scholar] [CrossRef]
- Mention, A.L. Co-operation and co-opetition as open innovation practices in the service sector: Which influence on innovation novelty? Technovation 2011, 31, 44–53. [Google Scholar] [CrossRef]
- Bullinger, A.C.; Rass, M.; Adamczyk, S.; Moeslein, K.M.; Sohn, S. Open innovation in health care: Analysis of an open health platform. Health Policy 2012, 105, 165–175. [Google Scholar] [CrossRef]
- Giustina Secundo, A.T. Knowledge transfer in open innovation A classification framework for healthcare ecosystems. Bus. Process Manag. J. 2017, 25, 144–163. [Google Scholar] [CrossRef]
- Department, Z.K. Implementing Open Innovation Using Quality Management Systems: The Role of Organizational Commitment and Customer Loyalty. J. Open Innov. Technol. Mark. Complex. 2019, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Hughes, B.; Wareham, J. Knowledge arbitrage in global pharma: A synthetic view of absorptive capacity and open innovation. R&D Manag. 2010, 40, 324–343. [Google Scholar]
- Amrina, U.; Hidayatno, A.; Zagloel, T.Y.M. A Model-Based Strategy for Developing Sustainable Cosmetics Small and Medium Industries with System Dynamics. J. Open Innov. Technol. Mark. Complex. Artic. 2021, 7, 225. [Google Scholar] [CrossRef]





| PLC Type | Linear (Upward and Downward Trends) | Binominal (Upward and Downward Trends) | Overshoot and Collapse | Oscillating | No Line Fitted |
|---|---|---|---|---|---|
| Number | 60 | 54 | 110 | 267 | 36 |
| Percent | 11.38 | 10.25 | 20.87 | 50.66 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, A.; Mohammadzadeh, M.; Zare, H. Developing a System Dynamic Model for Product Life Cycle Management of Generic Pharmaceutical Products: Its Relation with Open Innovation. J. Open Innov. Technol. Mark. Complex. 2022, 8, 14. https://doi.org/10.3390/joitmc8010014
Mousavi A, Mohammadzadeh M, Zare H. Developing a System Dynamic Model for Product Life Cycle Management of Generic Pharmaceutical Products: Its Relation with Open Innovation. Journal of Open Innovation: Technology, Market, and Complexity. 2022; 8(1):14. https://doi.org/10.3390/joitmc8010014
Chicago/Turabian StyleMousavi, Atefeh, Mehdi Mohammadzadeh, and Hossein Zare. 2022. "Developing a System Dynamic Model for Product Life Cycle Management of Generic Pharmaceutical Products: Its Relation with Open Innovation" Journal of Open Innovation: Technology, Market, and Complexity 8, no. 1: 14. https://doi.org/10.3390/joitmc8010014
APA StyleMousavi, A., Mohammadzadeh, M., & Zare, H. (2022). Developing a System Dynamic Model for Product Life Cycle Management of Generic Pharmaceutical Products: Its Relation with Open Innovation. Journal of Open Innovation: Technology, Market, and Complexity, 8(1), 14. https://doi.org/10.3390/joitmc8010014






