Internal Flesh Browning in Apple and Its Predisposing Factors—A Review
Abstract
:1. Introduction
2. Physiological Disorders
3. Internal Flesh Browning
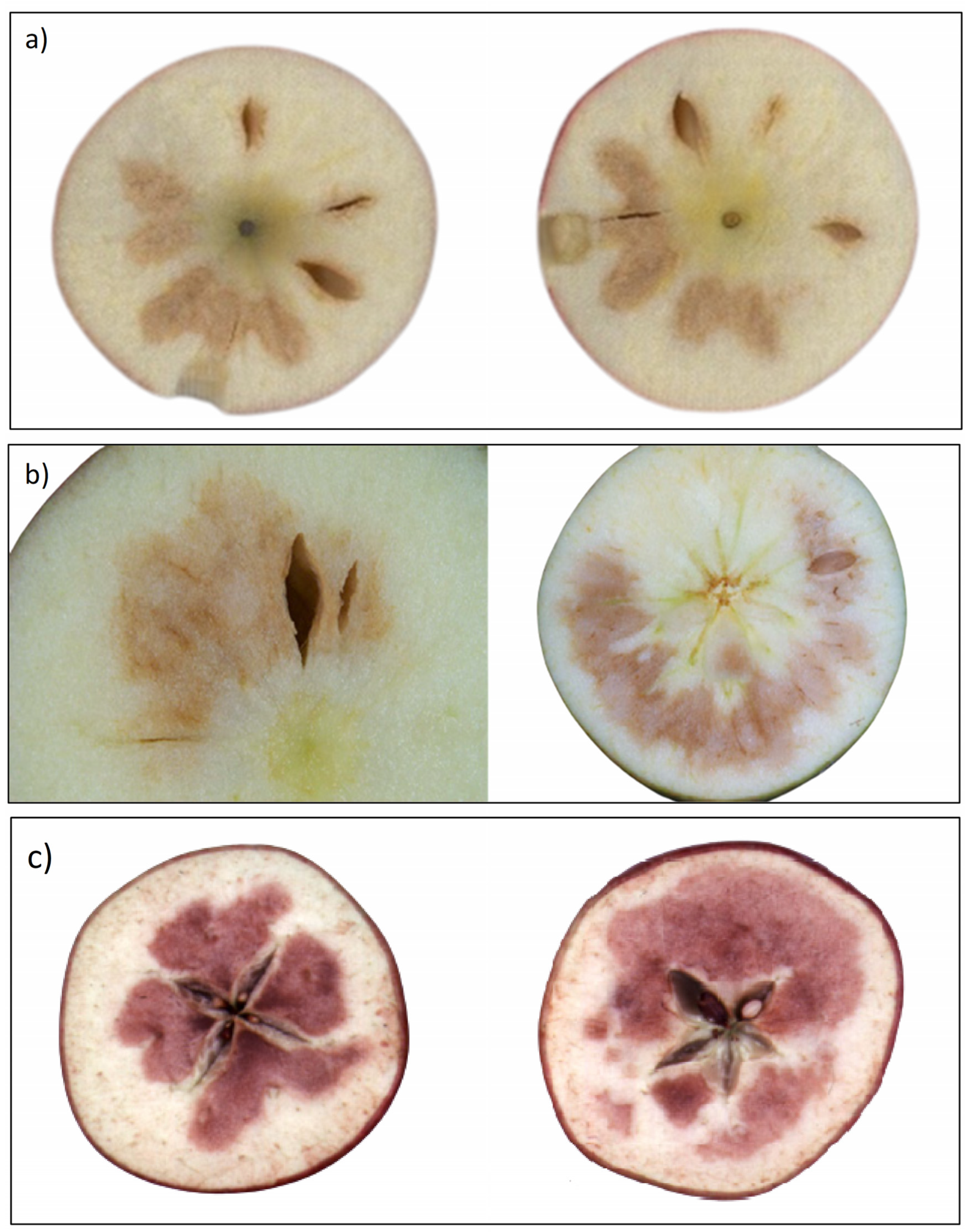
3.1. Types of Internal Flesh Browning
3.1.1. Chilling Injury
| Type of IFB Disorder | Sub-Category | Characteristic Feature/Symptoms | Cultivar | Reference |
|---|---|---|---|---|
| Chilling injury | Low temperature breakdown | Browning of vascular bundles and some portion of fruit flesh while other portions of flesh remain unaffected | ‘Cox’s Orange Pippin’ ‘McIntosh’ | Johnson and Yogaratnam [76] Webster and Lidster [77] Bramlage [39] Meheriuk et al. [78] Watkins et al. [79] |
| Core browning/Core flush | Browning of core region | ‘Empire’ | Meheriuk et al. [78] Little and Holmes [65] Watkins and Liu [63] | |
| Internal browning | Discolouration of fruit flesh | _ | Meheriuk et al. [78] Little and Holmes [65] Watkins et al. [79] | |
| Diffuse flesh browning | Browning of entire cortex tissue browning, while vascular bundles remain unaffected | ‘Cripps Pink’ ‘Empire’ | James and Jobling [28] Watkins and Liu [63] Bergman et al. [36] Butler [35] Crouch et al. [44] Moggia et al. [42] | |
| Firm flesh browning | Browning of flesh tissue, while the affected tissue remains firm and juicy, hence differentiating it from senescent breakdown | ‘Empire’ | Watkins and Liu [63] Lee et al. [66] | |
| Soggy breakdown | Typically develops irregular dark brownish portions in flesh, with sharp demarcation from healthy tissue, which distinguish it from DFB | ‘Honeycrisp’ | Watkins et al. [74] Leisso et al. [75] Tong et al. [64] | |
| CO2 injury | Brown heart | Browning of vascular tissue extending to mid-cortex along with irregular, sunken dry patches on fruit skin | ‘Fuji’ | Smock [80] Moon and Koo [81] Meheriuk et al. [78] Johnson et al. [82] Volz et al. [83] Argenta et al. [84] James et al. [85] |
| Braeburn browning disorder | Brown portions in fruit flesh followed by formation of lens-shaped pits or cavities | ‘Cripps Pink’ ‘Fuji’ ‘Bramley′s Seedling’ ‘Braeburn’ ‘Elstar’ | Blanpied et al. [86] Elgar et al. [31] Lau [32] Streif and Saquet [30] James et al. [48] | |
| Senescent related IFB disorders | Radial flesh browning | Browning of vascular tissue, while cortex tissue remains unaffected | ‘Cripps Pink’ | James and Jobling [28] Bergman et al. [36] Butler [35] Crouch et al. [44] Moggia et al. [42] |
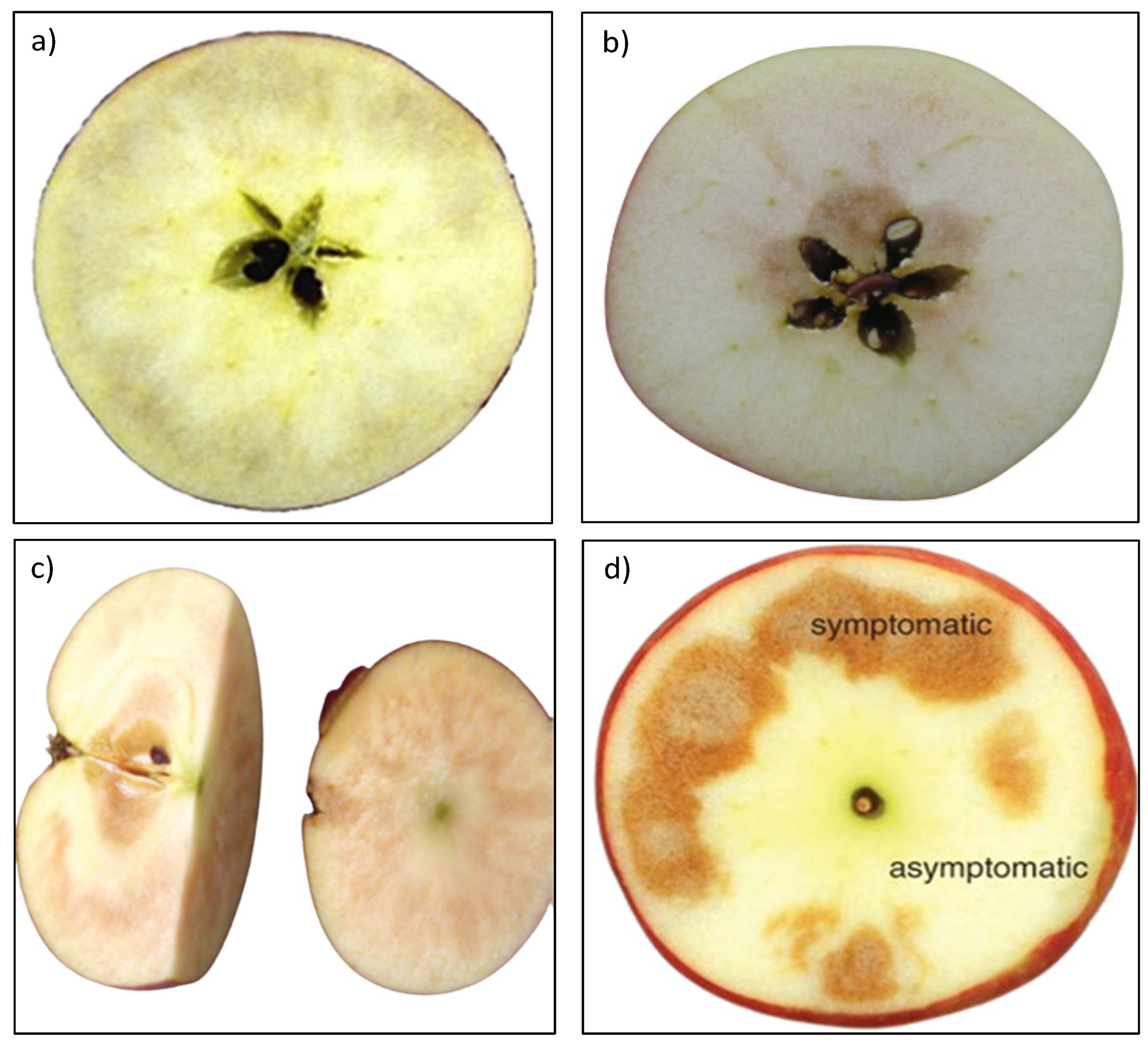
3.1.2. CO2 Injury
3.1.3. Senescent Related IFB Disorders
3.2. Factors Contributing to Internal Flesh Browning and Fruit Quality Deterioration
3.2.1. Pre-Harvest Orchard Factors
Fruit Size
Crop Load
Fruit Maturity at Harvest
Cultivar
3.2.2. Climatic Factors
Seasonal Temperatures
Growing Degree Days
3.2.3. Mineral Nutrition
Nitrogen
Phosphorus
Potassium
Calcium
4. Commercially Available Pre-Harvest Technologies and Their Effectiveness against IFB-Related Disorders
4.1. 1-Methylcyclopropene (1-MCP)
4.2. Aminoethoxyvinylglycine (AVG)
4.3. Diphenylamine (DPA)
4.4. Other Technologies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuri, J.A.; Moggia, C.; Sepulveda, A.; Poblete-Echeverría, C.; Valdés-Gómez, H.; Torres, C.A. Effect of cultivar, rootstock, and growing conditions on fruit maturity and postharvest quality as part of a six-year apple trial in Chile. Sci. Hortic. 2019, 253, 70–79. [Google Scholar] [CrossRef]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Ma, Y.; Li, M.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.; et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef]
- Cornille, A.; Gladieux, P.; Smulders, M.J.M.; Roldán-Ruiz, I.; Laurens, F.; Le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New insight into the history of domesticated Apple: Secondary contribution of the European Wild Apple to the genome of cultivated varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [Green Version]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Palmer, J.; Prive, J.; Tustin, D. Temperature. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CABI Publishing: Oxon, UK, 2003; pp. 217–236. [Google Scholar]
- Forsline, P.L.; Aldwinckle, H.S.; Dickson, E.E.; Luby, J.J.; Hokanson, S.C. Collection, maintenance, characterization, and utilization of wild apples of Central Asia. Hortic. Rev. 2003, 29, 1–62. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations-FAOSTAT Statistical Database. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 August 2022).
- Hort Innovation. Australian-Horticulture-Statistics-Handbook-Fruit. 2022. Available online: https://www.horticulture.com.au/contentassets/a68c8934a8bf40b4becdc487bacdb60f/hort-innovation-ahsh-20-21-fruit.pdf (accessed on 1 August 2022).
- APAL. Apples Next Priority for China Access, Joyce Confirms. 2017. Available online: https://apal.org.au/apples-next-priority-market-access-china-joyce-confirms/ (accessed on 6 July 2022).
- Di Guardo, M.; Tadiello, A.; Farneti, B.; Lorenz, G.; Masuero, D.; Vrhovsek, U.; Costa, G.; Velasco, R.; Costa, F. A multidisciplinary approach providing new insight into fruit flesh browning physiology in apple (Malus x domestica Borkh.). PLoS ONE 2013, 8, e78004. [Google Scholar] [CrossRef]
- Watkins, C.B.; Mattheis, J.P. Apple. In Postharvest Physiological Disorders in Fruits and Vegetables, 1st ed.; Tonetto de Freitas, S., Pareek, S., Eds.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2019; pp. 165–207. [Google Scholar]
- Kumar, R.; Kumar, V. Physiological disorders in perennial woody tropical and subtropical fruit crops-a review. Indian J. Agric. Sci. 2016, 86, 703–717. [Google Scholar]
- Sandhu, S.; Gill, B.S. Physiological Disorders of Fruit Crops; New India Publishing Agency: New Delhi, India, 2013. [Google Scholar]
- Bramlage, W.J.; Weis, S.A. Effects of temperature, light, and rainfall on superficial scald susceptibility in apples. HortScience 1997, 32, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Emongor, V.; Murr, D.; Lougheed, E. Preharvest factors that predispose apples to superficial scald. Postharvest Biol. Technol. 1994, 4, 289–300. [Google Scholar] [CrossRef]
- Ferguson, I.; Volz, R.; Woolf, A. Preharvest factors affecting physiological disorders of fruit. Postharvest Biol. Technol. 1999, 15, 255–262. [Google Scholar] [CrossRef]
- Curry, E.A.; Torres, C.; Neubauer, L. Preharvest lipophilic coatings reduce lenticel breakdown disorder in ‘Gala’ apples. HortTechnology 2008, 18, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Nissen, R.; Bound, S.; Adhikari, R.; Cover, I. Factors Affecting Postharvest Management of Apples: A Guide to Optimising Quality; Tasmanian Institute of Agriculture, Fruit Growers Tasmania, and Department of Agriculture and Water Resources: Hobart, TAS, Australia, 2018. [Google Scholar]
- Donahue, D.J.; Reig Córdoba, G.; Elone, S.E.; Wallis, A.E.; Basedow, M.R. ‘Honeycrisp’ bitter pit response to rootstock and region under Eastern New York climatic conditions. Plants 2021, 10, 983. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Kalcsits, L. Rootstock affects scion nutrition and fruit quality during establishment and early production of ‘Honeycrisp’ apple. HortScience 2021, 56, 261–269. [Google Scholar] [CrossRef]
- Mathew, A.; Parpia, H. Food browning as a polyphenol reaction. Adv. Food. Res. 1971, 19, 75–145. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants-recent progress. Phytochemistry 1986, 26, 11–20. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Hatoum, D.; Buts, K.; Hertog, M.L.A.T.M.; Geeraerd, A.H.; Schenk, A.; Vercammen, J.; Nicolai, B.M. Effects of pre- and postharvest factors on browning in Braeburn. Hortic. Sci. 2014, 41, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.; Schimanski, L.; Jennings, D. Investigating internal browning of Tasmanian ‘Pink Lady’ apples. Acta Hortic. 2003, 628, 161–166. [Google Scholar] [CrossRef]
- Brown, G.; Schimanski, L.; Jennings, D. The effect of seasonality, maturity and colour treatments on internal browning in ‘Pink Lady’™ apples. Acta Hortic. 2005, 682, 1013–1020. [Google Scholar] [CrossRef]
- De Castro, E.; Barrett, D.M.; Jobling, J.; Mitcham, E.J. Biochemical factors associated with a CO2-induced flesh browning disorder of ‘Pink Lady’ apples. Postharvest Biol. Technol. 2008, 48, 182–191. [Google Scholar] [CrossRef]
- James, H.; Jobling, J. The flesh browning disorder of ‘Pink Lady’ apples. N. Y. Fruit Q. 2008, 16, 23–28. [Google Scholar]
- Jobling, J.; Brown, G.; Mitcham, E.; Tanner, D.; Tustin, S.; Wilkinson, I.; Zanella, A. Flesh browning of ‘Pink Lady’TM Apples: Why do symptoms occur? Results from an international collaborative study. Acta Hortic. 2005, 682, 851–858. [Google Scholar] [CrossRef]
- Streif, J.; Saquet, A.A. Internal flesh browning of ‘Elstar’ Apples as influenced by pre- and postharvest factors. Acta Hortic. 2003, 599, 523–527. [Google Scholar] [CrossRef]
- Elgar, H.J.; Burmeister, D.M.; Watkins, C.B. Storage and handling effects on a CO2-related internal browning disorder of ‘Braeburn’ apples. HortScience 1998, 33, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Lau, O.L. Effect of growing season, harvest maturity, waxing, low O2 and elevated CO2 on flesh browning disorders in ‘Braeburn’ apples. Postharvest Biol. Technol. 1998, 14, 131–141. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Sánchez-Contreras, J.; Santos, L.S.; Misle, E.; Yuri, J.A. Assessing apple coming from agroclimatic differing locations: A tool for evaluating impact of environmental variation on postharvest. N. Z. J. Crop Hortic. Sci. 2022, 1–20. [Google Scholar] [CrossRef]
- McCormick, R.J.; Biegert, K.; Streif, J. Occurrence of physiological browning disorders in stored ‘Braeburn’ apples as influenced by orchard and weather conditions. Postharvest Biol. Technol. 2021, 177, 111534. [Google Scholar] [CrossRef]
- Butler, L. Internal Flesh Browning of ‘Cripps’ Pink’apple (Malus domestica Borkh.) as Influenced by Pre-Harvest Factors and the Evaluation of near Infrared Reflectance Spectroscopy as a Non-Destructive Method for Detecting Browning. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Bergman, H.; Crouch, E.; Crouch, I.; Jooste, M.; Majoni, J. Update on the possible causes and management strategies of flesh browning disorders in ‘Cripps Pink’ apples. S. Afr. Fruit J. 2012, 11, 56–59. [Google Scholar]
- James, H.J. Understanding the Flesh Browning Disorder of ‘Cripps Pink’ Apples. Ph.D. Thesis, University of Sydney, Sydney, NSW, Australia, 2007. [Google Scholar]
- Elgar, H.; Watkins, C.; Lallu, N. Harvest date and crop load effects on a carbon dioxide–related storage injury of ‘Braeburn’ apple. HortScience 1999, 34, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Bramlage, W.J. Interactions of orchard factors and mineral nutrition on quality of pome fruit. Acta Hortic. 1993, 326, 15–28. [Google Scholar] [CrossRef]
- De Castro, E.; Biasi, B.; Mitcham, E.; Tustin, S.; Tanner, D.; Jobling, J. Carbon dioxide-induced flesh browning in Pink Lady Apples. J. Am. Soc. Hortic. Sci. 2007, 132, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Neilson, G.H.; Neilson, D. Nutritional requirements of apple. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 267–302. [Google Scholar]
- Moggia, C.; Pereira, M.; Yuri, J.A.; Torres, C.A.; Hernández, O.; Icaza, M.G.; Lobos, G.A. Preharvest factors that affect the development of internal browning in apples cv. Cripp’s Pink: Six-years compiled data. Postharvest Biol. Technol. 2015, 101, 49–57. [Google Scholar] [CrossRef]
- Doe, J.W.; Schoeman, L.; Crouch, E.M. Effect of harvest maturity, storage condition and duration, on internal flesh browning and quality of ‘Rosy Glow’ apples grown in South Africa. Acta Hortic. 2018, 1201, 41–48. [Google Scholar] [CrossRef]
- Crouch, E.M.; Jooste, M.; Majoni, T.J.; Crouch, I.J.; Bergman, H. Harvest maturity and storage duration influencing flesh browning in South African ‘Cripps Pink’ apples. Acta Hortic. 2015, 1079, 121–127. [Google Scholar] [CrossRef]
- Streif, J. Internal browning disorders in stored Pip fruit. Acta Hortic. 2008, 804, 315–320. [Google Scholar] [CrossRef]
- Volz, R.K.; Biasi, W.V.; Mitcham, E.J. Fermentative volatile production in relation to carbon dioxide-induced flesh browning in ‘Fuji’ Apple. HortScience 1998, 33, 1231–1234. [Google Scholar] [CrossRef] [Green Version]
- James, H.; Nock, J.; Watkins, C. Internal browning in ‘Empire’ apples in relation to harvest date. N. Y. Fruit Q. 2010, 18, 11–14. [Google Scholar]
- James, H.; Jobling, J.; Tanner, D. Investigating structural and physiological differences between radial and diffuse types of flesh browning in ‘Cripps Pink’ apples. Acta Hortic. 2008, 768, 77–84. [Google Scholar] [CrossRef]
- Jones, K.; Bound, S.; Oakford, M.; Koen, T. Control of pudding spot in Crofton apple. Aust. J. Exp. Agric. 1992, 32, 503–506. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Mitcham, E.J. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar] [CrossRef]
- Bonomelli, C.; Mogollón, R.; Tonetto de Freitas, S.; Zoffoli, J.P.; Contreras, C. Nutritional relationships in bitter pit-affected fruit and the feasibility of Vis-NIR models to determine calcium concentration in ‘Fuji’ apples. Agronomy 2020, 10, 1476. [Google Scholar] [CrossRef]
- Martins, C.R.; Hoffmann, A.; Rombaldi, C.V.; Farias, R.d.M.; Teodoro, A.V. Apple biological and physiological disorders in the orchard and in postharvest according to production system. Rev. Bras. Frutic. 2013, 35, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.; Mattheis, J.; Giordani, L.; Reid, M.; Mullin, K. Fruit canopy positioning affects fruit calcium and potassium concentrations, disorder incidence, and fruit quality for ‘Honeycrisp’ apple. Can. J. Plant Sci. 2019, 99, 761–771. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Sydney, Australia, 1990. [Google Scholar]
- Kays, S.J.; Paull, R.E. Postharvest Physiology of Perishable Plant Products; Exon Press: Athens, GA, USA, 2004; 526p. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 7th ed.; WH Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- James, H.J.; Jobling, J.J. Contrasting the structure and morphology of the radial and diffuse flesh browning disorders and CO2 injury of ‘Cripps Pink’ apples. Postharvest Biol. Technol. 2009, 53, 36–42. [Google Scholar] [CrossRef]
- Macheix, J.; Fleuriet, A.; Billot, J. The main phenolics of fruits. In Fruit Phenolics; CRC Press, Taylor & Francis Group: Boca Raton FL, USA, 1990; pp. 1–98. [Google Scholar]
- Mellidou, I.; Buts, K.; Hatoum, D.; Ho, Q.T.; Johnston, J.W.; Watkins, C.B.; Schaffer, R.J.; Gapper, N.E.; Giovannoni, J.J.; Rudell, D.R.; et al. Transcriptomic events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 2014, 14, 328. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.E. The Biology of Apples and Pears; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar]
- Marangoni, A.; Palma, T.; Stanley, D. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–217. [Google Scholar] [CrossRef]
- Watkins, C.B.; Liu, F.W. Temperature and carbon dioxide interactions on quality of controlled atmosphere-stored ‘Empire’ apple. HortScience 2010, 45, 1708–1712. [Google Scholar] [CrossRef] [Green Version]
- Tong, C.B.S.; Chang, H.-Y.; Boldt, J.K.; Ma, Y.B.; Deell, J.R.; Moran, R.E.; Bourgeois, G.; Plouffe, D. Diffuse flesh browning in ‘Honeycrisp’ apple fruit is associated with low temperatures during fruit growth. HortScience 2016, 51, 1256–1264. [Google Scholar] [CrossRef]
- Little, C.R.; Holmes, R.J. Storage Technology for Apples and Pears: A Guide to Production, Postharvest Treatment and Storage of Pome Fruit in Australia; Department of Natural Resources and Environment, Agriculture Victoria: Knoxfield, Australia, 2000; 528p. [Google Scholar]
- Lee, J.; Cheng, L.; Rudell, D.R.; Watkins, C.B. Antioxidant metabolism of 1-methylcyclopropene (1-MCP) treated ‘Empire’ apples during controlled atmosphere storage. Postharvest Biol. Technol. 2012, 65, 79–91. [Google Scholar] [CrossRef]
- Saba, K.M.; Watkins, C.B. Flesh browning development of ‘Empire’ apple during a shelf life period after 1-methylcyclopropene (1-MCP) treatment and controlled atmosphere storage. Sci. Hortic. 2020, 261, 108938. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Rickard, B.J.; Nock, J.F.; Watkins, C.B. An economic analysis of harvest timing to manage the physiological storage disorder firm flesh browning in ‘Empire’ apples. Postharvest Biol. Technol. 2015, 107, 1–8. [Google Scholar] [CrossRef]
- Jung, S.-K.; Watkins, C.B. Involvement of ethylene in browning development of controlled atmosphere-stored ‘Empire’ apple fruit. Postharvest Biol. Technol. 2011, 59, 219–226. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Nock, J.F.; Watkins, C.B. Peroxidase and polyphenoloxidase activities in relation to flesh browning of stem-end and calyx-end tissues of ‘Empire’ apples during controlled atmosphere storage. Postharvest Biol. Technol. 2015, 108, 1–7. [Google Scholar] [CrossRef]
- Lee, J.; Leisso, R.; Rudell, D.R.; Watkins, C.B. 1-Methylcyclopropene differentially regulates metabolic responses in the stem-end and calyx-end flesh tissues of ‘Empire’ apple during long-term controlled atmosphere storage. Postharvest Biol. Technol. 2022, 192, 112018. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, L.; Rudell, D.R.; Nock, J.F.; Watkins, C.B. Antioxidant metabolism in stem and calyx end tissues in relation to flesh browning development during storage of 1-methylcyclopropene treated ‘Empire’ apples. Postharvest Biol. Technol. 2019, 149, 66–73. [Google Scholar] [CrossRef]
- Lee, J.; Rudell, D.R.; Davies, P.J.; Watkins, C.B. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 2012, 8, 742–753. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F.; Weis, S.A.; Jayanty, S.; Beaudry, R.M. Storage temperature, diphenylamine, and pre-storage delay effects on soft scald, soggy breakdown and bitter pit of ‘Honeycrisp’ apples. Postharvest Biol. Technol. 2004, 32, 213–221. [Google Scholar] [CrossRef]
- Leisso, R.S.; Buchanan, D.A.; Lee, J.; Mattheis, J.P.; Sater, C.; Hanrahan, I.; Watkins, C.B.; Gapper, N.; Johnston, J.W.; Schaffer, R.J.; et al. Chilling-related cell damage of apple (Malus × domestica Borkh.) fruit cortical tissue impacts antioxidant, lipid and phenolic metabolism. Physiol. Plant. 2015, 153, 204–220. [Google Scholar] [CrossRef]
- Johnson, D.; Yogaratnam, N. The effects of phosphorus sprays on the mineral composition and storage quality of ‘Cox’s Orange Pippin’ apples. J. Hortic. Sci. 1978, 53, 171–178. [Google Scholar] [CrossRef]
- Webster, D.; Lidster, P. Effects of phosphate sprays on McIntosh apple fruit and leaf composition, flesh firmness and susceptibility to low-temperature disorders. Can. J. Plant Sci. 1986, 66, 617–626. [Google Scholar] [CrossRef]
- Meheriuk, P.; Prange, M.; Lidster, R.; Porritt, S. Postharvest Disorders of Apples and Pears; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1994; Volume 1737. [Google Scholar]
- Watkins, C.; Kupferman, E.; Rosenberger, D. Apple. Postharvest quality management guidelines. In The Commercial Storage of Fruits, Vegetables and Florist and Nursery Crops; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; USDA: Washington, DC, USA, 2002. [Google Scholar]
- Smock, R. Nomenclature for internal storage disorders of apples. HortScience 1977, 12, 306–308. [Google Scholar]
- Moon, P.Y.; Koo, L.S. Susceptibility of ‘Fuji’ apples to low-oxygen injury and high-carbon dioxide injury during CA storage. Hortic. Environ. Biotechnol. 1992, 33, 38–43. [Google Scholar]
- Johnson, D.S.; Dover, C.J.; Colgan, R.J. Effect of rate of establishment of ca conditions on the development of CO2-injury in ‘Bramley’s Seedling’ apples. Acta Hortic. 1998, 464, 351–356. [Google Scholar] [CrossRef]
- Volz, R.K.; Biasi, W.V.; Grant, J.A.; Mitcham, E.J. Prediction of controlled atmosphere-induced flesh browning in ‘Fuji’ apple. Postharvest Biol. Technol. 1998, 13, 97–107. [Google Scholar] [CrossRef]
- Argenta, L.; Fan, X.; Mattheis, J. Delaying establishment of controlled atmosphere or CO2 exposure reduces ‘Fuji’ apple CO2 injury without excessive fruit quality loss. Postharvest Biol. Technol. 2000, 20, 221–229. [Google Scholar] [CrossRef]
- James, H.; Brown, G.; Mitchan, E.; Tanner, D.; Tustin, S.; Wilkinson, I.; Zanella, A.; Jobling, J. Flesh browning in ‘Pink Lady’ Apples: Research results have helped to change market specifications for blush colour which is an added bonus for growers. Acta Hortic. 2005, 687, 175–180. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Johnson, D.S.; Lau, O.L.; Lidster, P.D.; Lougheed, E.C.; Porritt, S.W. Apples. In Controlled-Atmosphere Disorders of Commercial Fruits and Vegetables; Communications Branch, Agriculture Canada: Ottawa, ON, Canada, 1990. [Google Scholar]
- Argenta, L.C.; Anese, R.D.O.; Thewes, F.R.; Wood, R.M.; Nesi, C.N.; Neuwald, D.A. Maintenance of ‘Luiza’ apple fruit quality as affected by postharvest practices. Rev. Bras. Frutic. 2022, 44, e-905. [Google Scholar] [CrossRef]
- Schotsmans, W.; Verlinden, B.E.; Lammertyn, J.; Nicolaï, B.M. The relationship between gas transport properties and the histology of apple. J. Sci. Food Agric. 2004, 84, 1131–1140. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Schenk, A.; Delele, M.A.; Rolletschek, H.; Vercammen, J.; Nicolaï, B.M. Genotype effects on internal gas gradients in apple fruit. J. Exp. Bot. 2010, 61, 2745–2755. [Google Scholar] [CrossRef] [Green Version]
- Dražeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Air volume measurement of ‘Braeburn’ apple fruit. J. Exp. Bot. 2004, 55, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Janssen, S.; Verboven, P.; Nugraha, B.; Wang, Z.; Boone, M.; Josipovic, I.; Nicolaï, B.M. 3D pore structure analysis of intact ‘Braeburn’ apples using X-ray micro-CT. Postharvest Biol. Technol. 2020, 159, 111014. [Google Scholar] [CrossRef]
- Chigwaya, K.; Plessis, A.D.; Viljoen, D.W.; Crouch, I.J.; Crouch, E.M. Use of X-ray computed tomography and 3D image analysis to characterize internal browning in ‘Fuji’ apples after exposure to CO2 stress. Sci. Hortic. 2021, 277, 109840. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Schenk, A.; Nicolaï, B.M. Controlled atmosphere storage may lead to local ATP deficiency in apple. Postharvest Biol. Technol. 2013, 78, 103–112. [Google Scholar] [CrossRef]
- Herremans, E.; Verboven, P.; Defraeye, T.; Rogge, S.; Ho, Q.T.; Hertog, M.L.A.T.M.; Verlinden, B.E.; Bongaers, E.; Wevers, M.; Nicolai, B.M. X-ray CT for quantitative food microstructure engineering: The apple case. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 324, 88–94. [Google Scholar] [CrossRef]
- Argenta, L.; Fan, X.; Mattheis, J. Development of internal browning in ‘Fuji’ apples during storage. In Proceedings of the Washington Tree Fruit Postharvest Conference, Wenatchee, WA, USA, 13–14 March 2001; pp. 1–4. [Google Scholar]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Farina, A.; Pifferi, A.; Spinelli, L.; Verlinden, B.E.; Torricelli, A. Non destructive detection of brown heart in ‘Braeburn’ apples by time-resolved reflectance spectroscopy. Procedia Food Sci. 2011, 1, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.J.; Burmeister, D.M. Magnetic resonance imaging of browning development in ‘Braeburn’ apple during controlled-atmosphere storage under high CO2. HortScience 1999, 34, 915–919. [Google Scholar] [CrossRef]
- Contreras, C.; Alsmairat, N.; Beaudry, R. Prestorage conditioning and diphenylamine improve resistance to controlled-atmosphere-related injury in ‘Honeycrisp’ apples. HortScience 2014, 49, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Cross, J.; Berrie, A.; Johnson, D.; Pennell, D.; Luton, M.; Ashdown, C. The Best Practice Guide for UK Apple Production; Agriculture and Horticulture Development Board: East Malling, UK, 2012; pp. 1–51. [Google Scholar]
- Prange, R.; Delong, J.; Nichols, D.; Harrison, P. Effect of fruit maturity on the incidence of bitter pit, senescent breakdown, and other post-harvest disorders in ‘Honeycrisp’TM apple. J. Hortic. Sci. Biotechnol. 2011, 86, 245–248. [Google Scholar] [CrossRef]
- Watkins, C. Principles and practices of postharvest handling and stress. In Apples: Botany, Production and Uses; Ferree, D., Warrington, I., Eds.; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Sánchez-Contreras, J.; Rudell, D.; Mattheis, J.; Torres, C.A. Sphingolipids associated with flesh browning onset and development in ‘Cripps Pink’ apples (Malus domestica Borkh.). Postharvest Biol. Technol. 2021, 180, 111623. [Google Scholar] [CrossRef]
- James, H.; Jobling, J.; Tanner, D.; Tustin, S.; Wilkinson, I. Climatic conditions during growth relate to risk of ‘Pink Lady’TM apples developing flesh browning during storage. Acta Hortic. 2010, 857, 197–204. [Google Scholar] [CrossRef]
- Deyman, K.L.; Chiu, G.; Liu, J.; Brikis, C.J.; Trobacher, C.P.; DeEll, J.R.; Shelp, B.J.; Bozzo, G.G. Effects of elevated CO2 and 1-methylcyclopropene on storage-related disorders of Ontario-grown Empire apples. Can. J. Plant Sci. 2014, 94, 857–865. [Google Scholar] [CrossRef]
- Reid, M.; Kalcsits, L. Water deficit timing affects physiological drought Response, fruit size, and bitter pit development for ‘Honeycrisp’ apple. Plants 2020, 9, 874. [Google Scholar] [CrossRef]
- Lidster, P.D.; Porritt, S.W.; Mason, J.; Eaton, G.W. Spartan apple breakdown as affected by orchard factors, nutrient content and fruit quality. Can. J. Plant Sci. 1975, 55, 443–446. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M.; Harker, R. Harvest date and fruit size affect postharvest softening of apple fruit. J. Hortic. Sci. Biotechnol. 2002, 77, 355–360. [Google Scholar] [CrossRef]
- Lee, J.; Mattheis, J.P.; Rudell, D.R. Fruit size affects physiological attributes and storage disorders in cold-stored ‘Royal Gala’ apples. HortScience 2013, 48, 1518–1524. [Google Scholar] [CrossRef] [Green Version]
- Harker, F.R.; Redgwell, R.J.; Hallet, I.C. Texture of fresh fruit. Hortic. Rev. 1997, 20, 121–224. [Google Scholar] [CrossRef]
- Watkins, C.B.; Erkan, M.; Nock, J.F.; Iungerman, K.A.; Beaudry, R.M.; Moran, R.E. Harvest date effects on maturity, quality, and storage disorders of ‘Honeycrisp’ apples. HortScience 2005, 40, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Malladi, A.; Hirst, P.M. Increase in fruit size of a spontaneous mutant of ‘Gala’ apple (Malus×domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 2010, 61, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- Mann, H.; Bedford, D.; Luby, J.; Vickers, Z.; Tong, C. Relationship of instrumental and sensory texture measurements of fresh and stored apples to cell number and size. HortScience 2005, 40, 1815–1820. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, R.S.; Bound, S.A.; Hunt, I. Crop load and thinning methods impact yield, nutrient content, fruit quality, and physiological disorders in ‘Scilate’ apples. Agronomy 2022, 12, 1989. [Google Scholar] [CrossRef]
- Karim, S.K.A.; Allan, A.C.; Schaffer, R.J.; David, K.M. Cell division controls final fruit size in three apple (Malus × domestica) cultivars. Horticulturae 2022, 8, 657. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Ferguson, I.B. Crop load interactions in apple. Hortic. Rev. 2005, 31, 231–290. [Google Scholar] [CrossRef]
- Jones, K.; Bound, S.; Koen, T.; Oakford, M. Effect of timing of hand thinning on the cropping potential of ‘Red Fuji’ apple trees. Aust. J. Exp. Agric. 1992, 32, 417–420. [Google Scholar] [CrossRef]
- Jones, K.M.; Koen, T.B. Manipulation of blossom density and the effects of ethephon thinning of Golden Delicious. Acta Hortic. 1986, 179, 653–658. [Google Scholar] [CrossRef]
- Jones, K.M.; Koen, T.B.; Oakford, M.J.; Bound, S. Thinning ‘Red Fuji’ apples with ethephon or NAA. J. Hortic. Sci. 1989, 64, 527–532. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ Apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Ferguson, I.; Watkins, C. Bitter pit in apple fruit. Hortic. Rev. 1989, 11, 289–355. [Google Scholar] [CrossRef]
- Kalcsits, L.; van der Heijden, G.; Reid, M.; Mullin, K. Calcium absorption during fruit development in ‘Honeycrisp’ apple measured using 44Ca as a stable isotope tracer. HortScience 2017, 52, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Sharples, R.O. Fruit-thinning effects on the development and storage quality of Cox’s Orange Pippin Apple fruits. J. Hortic. Sci. 1968, 43, 359–371. [Google Scholar] [CrossRef]
- Johnson, D.S. The effect of flower and fruit thinning on the firmness of ‘Cox’s Orange Pippin’ apples at harvest and after storage. J. Hortic. Sci. 1992, 67, 95–101. [Google Scholar] [CrossRef]
- Mpelasoka, B.; Behboudian, M.; Ganesh, S. Fruit quality attributes and their interrelationships of ‘Braeburn’ apple in response to deficit irrigation and to crop load. Gartenbauwissenschaft 2001, 66, 247–253. [Google Scholar]
- Opara, L.U.; Studman, C.J.; Banks, N.H. Physico-mechanical properties of “Gala” apples and stem-end splitting as influenced by orchard management practices and harvest date. J. Agric. Eng. Res. 1997, 68, 139–146. [Google Scholar] [CrossRef]
- Tough, H.J.; Park, D.G.; Crutchley, K.J.; Bartholomew, F.B.; Craig, G. Effect of crop load on mineral status, maturity and quality of ‘Braeburn’ (Malus domestica Borkh.) apple fruit. Acta Hortic. 1998, 464, 53–58. [Google Scholar] [CrossRef]
- Volz, R.; Oliver, M.; Legg, S.; Stanley, J.; Morgan, C.; Bradley, A.; Pidakala, P. Prediction of BBD (HR99P11.05); Technical Report No 2001/160. HortResearch report to ENZA (NZ) International; 2000; p. 21. [Google Scholar]
- Kondo, S. Effect of environmental conditions on the contents of sugars and ascorbic acid in ‘Senshu’ apple fruit. J. Jap. Soc. Food. Sci. Technol. 1992, 39, 1112–1118. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. The effect of harvest date on aroma compound production from ‘Golden Delicious’ apple fruit and relationship to respiration and ethylene production. Postharvest Biol. Technol. 1996, 8, 259–269. [Google Scholar] [CrossRef]
- DeEll, J.R.; Khanizadeh, S.; Saad, F.; Ferree, D.C. Factors affecting apple fruit firmness-a review. J. Am. Pomol. Soc. 2001, 55, 8–26. [Google Scholar]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Kingston, C. Maturity indices for apple and pear. Hortic. Rev. 1992, 13, 407–432. [Google Scholar] [CrossRef]
- Jung, S.-K.; Choi, H.-S. Browning of early and late-harvested ‘Empire’ apples affected by cold storage and 1-MCP. Agronomy 2020, 10, 1050. [Google Scholar] [CrossRef]
- Doerflinger, F.; Rickard, B.; Nock, J.; Watkins, C. Early harvest is a critical factor in decreasing flesh browning development of ‘Empire’ apples. N. Y. Fruit Q. 2015, 23, 30–34. [Google Scholar]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Purvis, A.C. Regulation of oxidative stress in horticultural crops. HortScience 2004, 39, 930–932. [Google Scholar] [CrossRef]
- Harada, T.; Kurahashi, W.; Yanai, M.; Wakasa, Y.; Satoh, T. Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 2005, 105, 447–456. [Google Scholar] [CrossRef]
- Ting, V.J.L.; Silcock, P.; Bremer, P.J.; Biasioli, F. X-ray micro-computer tomographic method to visualize the microstructure of different apple cultivars. J. Food Sci. 2013, 78, E1735–E1742. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Chanona-Pérez, J.J.; Méndez Méndez, J.V.; Perea-Flores, M.J.; Cervantes-Sodi, F.; Hernández-Hernández, H.M.; Marin-Bustamante, M.Q. Physicochemical, structural and nanomechanical study elucidating the differences in firmness among four apple cultivars. Postharvest Biol. Technol. 2021, 171, 111342. [Google Scholar] [CrossRef]
- DeEll, J.R.; Murr, D.P.; Mueller, R.; Wiley, L.; Porteous, M.D. Influence of 1-methylcyclopropene (1-MCP), diphenylamine (DPA), and CO2 concentration during storage on ‘Empire’ apple quality. Postharvest Biol. Technol. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Saquet, A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’ pears and ‘Jonagold’ apples during controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Köpcke, D. 1-Methylcyclopropene (1-MCP) and dynamic controlled atmosphere (DCA) applications under elevated storage temperatures: Effects on fruit quality of ‘Elstar’,‘Jonagold’and ‘Gloster’apple (Malus domestica Borkh.). European J. Hortic. Sci. 2015, 80, 25–32. [Google Scholar] [CrossRef]
- Williamson, V.G.; Frisina, C.; Tareen, M.N.; Stefanelli, D. Storage performance of two ‘Pink Lady®’ clones differs, but 1-MCP treatment is beneficial, regardless of maturity at harvest. Sci. Hortic. 2018, 235, 142–151. [Google Scholar] [CrossRef]
- Argenta, L.C.; do Amarante, C.V.T.; Betinelli, K.S.; Brancher, T.L.; Nesi, C.N.; Vieira, M.J. Comparison of fruit attributes of ‘Fuji’ apple strains at harvest and after storage. Sci. Hortic. 2020, 272, 109585. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, C.; Wang, C.; Golding, J.B.; Ru, L. Comparative transcriptome reveals molecular mechanism in apple genotypes differing in CO2 tolerance in CA storage. Postharvest Biol. Technol. 2022, 185, 111807. [Google Scholar] [CrossRef]
- Bergh, O. Effect of time of hand-thinning on apple fruit size. S. Afr. J. Plant Soil 1990, 7, 1–10. [Google Scholar] [CrossRef]
- Stanley, C.; Tustin, D.; Lupton, G.; McArtney, S.; Cashmore, W.; Silva, H.D. Towards understanding the role of temperature in apple fruit growth responses in three geographical regions within New Zealand. J. Hortic. Sci. Biotechnol. 2000, 75, 413–422. [Google Scholar] [CrossRef]
- Park, Y.M. Gas Exchange in Apples: Pathway for Gas Exchange, Changes in Resistance to Gas Diffusion during Fruit Development and Storage, and Factors Affecting the Changes; Cornell University: Ithaca, NY, USA, 1990. [Google Scholar]
- Argenta, L.C.; do Amarante, C.V.T.; de Freitas, S.T.; Brancher, T.L.; Nesi, C.N.; Mattheis, J.P. Fruit quality of ‘Gala’ and ‘Fuji’ apples cultivated under different environmental conditions. Sci. Hortic. 2022, 303, 111195. [Google Scholar] [CrossRef]
- Corrêa, T.R.; Steffens, C.A.; Amarante, C.V.T.D.; Brackmann, A.; Silveira, J.P.G.; Tanaka, H.; Both, V. Quality of ‘Fuji’ apples stored under controlled atmosphere and influenceof climate on the incidence of internal browning. Pesqui. Agropecu. Bras. 2010, 45, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, E.; Fallahi, B.; Neilsen, G.H.; Neilsen, D.; Peryea, F.J. Effects of mineral nutrition on fruit quality and nutritional disorders in Apples. Acta Hortic. 2010, 868, 49–60. [Google Scholar] [CrossRef]
- Perring, M. Mineral composition of apples. VII.—The relationship between fruit composition and some storage disorders. J. Sci. Food Agric. 1968, 19, 186–192. [Google Scholar] [CrossRef]
- Doryanizadeh, M.; Ghasemnezhad, M.; Sabouri, A. Estimation of postharvest quality of “Red Delicious” apple fruits based on fruit nutrient elements composition. J. Agric. Sci. 2017, 9, 164–173. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, L. Differential effects of nitrogen supply on skin pigmentation and flesh starch breakdown of ‘Gala’ apple. HortScience 2011, 46, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Marcelle, R. Mineral nutrition and fruit quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- Bramlage, W.J.; Drake, M.; Lord, W.J. The influence of mineral nutrition on the quality and storage performance of pome fruits grown in North America. Acta Hortic. 1980, 92, 29–40. [Google Scholar] [CrossRef]
- Sharples, R.O. The influence of orchard nutrition on the storage quality of apples and pears grown in the United Kingdom. Acta Hortic. 1980, 92, 17–28. [Google Scholar] [CrossRef]
- Shear, C.; Faust, M. Nutritional ranges in deciduous tree fruits and nuts. Hortic. Rev. 1980, 2, 142–163. [Google Scholar] [CrossRef]
- Dris, R.; Niskanen, R.; Fallahi, E. Nitrogen and calcium nutrition and fruit quality of commercial apple cultivars grown in Finland. J. Plant Nutr. 1998, 21, 2389–2402. [Google Scholar] [CrossRef]
- Perring, M.A.; Jackson, C.H. The mineral composition of apples. Calcium concentration and bitter pit in relation to mean mass per apple. J. Sci. Food Agric. 1975, 26, 1493–1502. [Google Scholar] [CrossRef]
- Taylor, B.; Goubran, F. The phosphorus nutrition of the apple tree. I. Influence of rate of application of superphosphate on the performance of young trees. Aust. J. Agric. Res. 1975, 26, 843–853. [Google Scholar] [CrossRef]
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley & Sons, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Bramlage, W.; Drake, M.; Weis, S. Comparisons of calcium chloride, calcium phosphate, and a calcium chelate as foliar sprays for ‘McIntosh’ apple trees. J. Am. Soc. Hortic. Sci. 1985, 110, 786–789. [Google Scholar] [CrossRef]
- Yogaratnam, N.; Sharples, R.O. Supplementing the nutrition of Bramley’s Seedling Apple with phosphorus sprays II. Effects on fruit composition and storage quality. J. Hortic. Sci. 1982, 57, 53–59. [Google Scholar] [CrossRef]
- Neuwald, D.A.; Kittemann, D.; Streif, J. Possible prediction of physiological storage disorders in ‘Braeburn’ apples comparing fruit of different orchards. Acta Hortic. 2008, 796, 211–216. [Google Scholar] [CrossRef]
- Veltman, R.H.; Lenthéric, I.; Van der Plas, L.H.W.; Peppelenbos, H.W. Internal browning in pear fruit (Pyrus communis L. cv Conference) may be a result of a limited availability of energy and antioxidants. Postharvest Biol. Technol. 2003, 28, 295–302. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Hakerlerler, H.; Oktay, M.; Eryüce, N.; Yagmur, B. Effect of potassium sources on the chilling tolerance of some vegetable seedlings grown in hotbeds. In Food Security in the WANA Region, The Essential Need for Balanced Fertilization; Johnston, A.E., Ed.; International Potash Institute: Zug, Switzerland, 1997; pp. 317–327. [Google Scholar]
- Singer, S.; El-Tohamy, W. Chilling and water stress injury in bean, Phaseolus vulgaris L. seedlings reduced by pretreatment with CaCl2, mefluidide, KCl and MgCl2. Egypt. J. Hortic. 1996, 23, 77–87. [Google Scholar]
- Neuwald, D.A.; Sestari, I.; Kittemann, D.; Streif, J.; Weber, A.; Brackmann, A. Can mineral analysis be used as a tool to predict ‘Braeburn’ browning disorders (BBD) in apple in commercial controlled atmosphere (CA) storage in Central Europe? Erwerbs-Obstbau 2014, 56, 35–41. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants: Development, Visual and Analytical Diagnosis; Gustav Fisher: Jena, Germany, 1992. [Google Scholar]
- Ghafir, S.; Gadalla, S.; Murajei, B.; El-Nady, M. Physiological and anatomical comparison between four different apple cultivars under cold-storage conditions. Afr. J. Plant Sci. 2009, 3, 133–138. [Google Scholar]
- Clark, C.J.; McGlone, V.A.; Jordan, R.B. Detection of Brownheart in ‘Braeburn’ apple by transmission NIR spectroscopy. Postharvest Biol. Technol. 2003, 28, 87–96. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.R.; Steffens, C.A.; Amarante, C.V.T.D.; Miqueloto, A.; Brackmann, A.; Ernani, P.R. Multivariate analysis of mineral content associated with flesh browning disorder in ‘Fuji’ apples produced in Southern Brazil. Bragantia 2017, 76, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.A. Non-destructive measurement of calcium and potassium in apple and pear using handheld X-ray fluorescence. Front. Plant Sci. 2016, 7, 442. [Google Scholar] [CrossRef] [Green Version]
- Marcelle, R.D. Relationships between mineral content, lipoxygenase activity, levels of 1-aminocyclopropane-1-carboxylic acid and ethylene emission in apple fruit flesh disks (cv. Jonagold) during storage. Postharvest Biol. Technol. 1991, 1, 101–109. [Google Scholar] [CrossRef]
- Wójcik, P.; Filipczak, J.; Wójcik, M. Effects of prebloom sprays of tryptophan and zinc on calcium nutrition, yielding and fruit quality of ‘Elstar’ apple trees. Sci. Hortic. 2019, 246, 212–216. [Google Scholar] [CrossRef]
- Marcelle, R.D. Ethylene formation, lipoxygenase activity and calcium content in Apple (cv. Jonagold). Acta Hortic. 1989, 258, 61–68. [Google Scholar] [CrossRef]
- Majoni, T.J.; Jooste, M.; Crouch, E.M. The effect of 1-MCP and storage duration on the storage potential and flesh browning development on ‘Cripps Pink’ apples stored under controlled atmosphere conditions. Acta Hortic. 2013, 1007, 49–56. [Google Scholar] [CrossRef]
- Wood, R.M.; Proske, M.; De Freitas, S.T.; Scheer, C.; Vögele, R.T.; Neuwald, D.A. Seasonal variation in calcium and ascorbic acid content at harvest related to internal browning in ‘Braeburn’ apple during controlled atmosphere storage. Sci. Hortic. 2022, 297, 110943. [Google Scholar] [CrossRef]
- Suzuki, A.; Basso, C. Soils and apple tree nutrition. In The Culture of the Apple Tree; EPAGRI: Florianopolis, Brazil, 2006; pp. 341–381. [Google Scholar]
- Watkins, C.B. The effect of 1-MCP on the development of physiological storage disorders in horticultural crops. Stewart Postharvest Rev. 2007, 2, 11. [Google Scholar] [CrossRef]
- Watkins, C.B. Overview of 1-Methylcyclopropene trials and uses for edible horticultural crops. HortScience 2008, 43, 86–94. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Watkins, C.B.; Gapper, N.E.; Nock, J.F.; Giovannoni, J.J.; Rudell, D.A.; Leisso, R.; Lee, J.; Buchanan, D.; Mattheis, J.; Hertog, M.L.A.T.M.; et al. Interactions between 1-MCP and controlled atmospheres on quality and storage disorders of fruits and vegetables. Acta Hortic. 2015, 1071, 45–58. [Google Scholar] [CrossRef]
- Bekele, E.A.; Beshir, W.F.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A.H. Metabolomics of the effect of 1-MCP and CA during ripening and storage in apples. Acta Hortic. 2015, 1079, 223–228. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.M.; Mattheis, J.P. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–32. [Google Scholar] [CrossRef]
- Mattheis, J.P. How 1-Methylcyclopropene has altered the Washington state apple industry. HortScience 2008, 43, 99–101. [Google Scholar] [CrossRef]
- Watkins, C.B. Managing physiological processes in fruits and vegetables with inhibitors of ethylene biosynthesis and perception. Acta Hortic. 2010, 880, 301–310. [Google Scholar] [CrossRef]
- Watkins, C.B. Pre- and postharvest inhibition of ethylene production and action by 1-MCP and the quality of apples and other horticultural products. Acta Hortic. 2016, 1120, 1–10. [Google Scholar] [CrossRef]
- Lee, J.; Kang, I.-K.; Nock, J.F.; Watkins, C.B. Effects of preharvest and postharvest applications of 1-Methylcyclopropene on fruit quality and physiological disorders of ‘Fuji’ apples during storage at warm and cold Temperatures. HortScience 2019, 54, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Moran, R.E.; McManus, P. Firmness retention, and prevention of coreline browning and senescence in ‘Macoun’ apples with 1-Methylcyclopropene. HortScience 2005, 40, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Mattheis, J.P.; Rudell, D.R. Storage temperature and 1-Methylcyclopropene treatment affect storage disorders and physiological attributes of ‘Royal Gala’ apples. HortScience 2016, 51, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Lafer, G. Storability and fruit quality of ‘Golden Delicious’ as affected by harvest date, AVG and 1-MCP treatments. J. Fruit Ornam. Plant Res. 2006, 14, 203–211. [Google Scholar]
- Lafer, G. Storability and fruit quality of ‘Braeburn’ apples as affected by harvest date, 1-MCP treatment and different storage conditions. Acta Hortic. 2008, 796, 179–184. [Google Scholar] [CrossRef]
- Argenta, L.C.; Mattheis, J.P.; Fan, X. Interactive effects of CA storage, 1-Methylcyclopropene and Methyl Jasmonate on quality of apple fruit. Acta Hortic. 2010, 857, 259–266. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.F.; Zhang, Y.; Watkins, C.B. Physiological disorder development of ‘Honeycrisp’ apples after pre- and post-harvest 1-methycyclopropene (1-MCP) treatments. Postharvest Biol. Technol. 2021, 182, 111703. [Google Scholar] [CrossRef]
- De Castro, H.E.; Biasi, W.; Mitcham, E. Controlled atmosphere-induced internal browning in ‘Pink Lady’ apples. Acta Hortic. 2005, 687, 63–70. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ayres, J.T.; Murr, D.P. 1-Methylcyclopropene influences ‘Empire’ and ‘Delicious’ apple quality during long-term commercial storage. HortTechnology 2007, 17, 46–51. [Google Scholar] [CrossRef] [Green Version]
- DeEll, J.R.; Lum, G.B. Storage regimes to allow softening in a processing apple treated with 1-methylcyclopropene. Can. J. Plant Sci. 2020, 100, 226–238. [Google Scholar] [CrossRef]
- Lee, J.; Rudell, D.R.; Watkins, C.B. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple at different storage temperatures. Acta Hortic. 2014, 1048, 113–119. [Google Scholar] [CrossRef]
- Jung, S.-K.; Choi, H.-S. Browning of ‘Empire’ and ‘Fuji’ apples as affected by antioxidant activities. Agronomy 2020, 10, 1883. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Nock, J.F.; Miller, W.B.; Watkins, C.B. Preharvest aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) effects on ethylene and starch concentrations of ‘Empire’ and ‘McIntosh’ apples. Sci. Hortic. 2019, 244, 134–140. [Google Scholar] [CrossRef]
- Sakaldas, M.; Gundogdu, M.A. The effects of preharvest 1-methylcyclopropene (Harvista) treatments on harvest maturity of ‘Golden Delicious’ apple cultivar. Acta Hortic. 2016, 1139, 601–608. [Google Scholar] [CrossRef]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. Analysis of possibility to apply preharvest 1-Methylcyclopropene (1-MCP) treatment to delay harvesting of Red Jonaprince apples. Sustainability 2020, 12, 4575. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Robledo, P. Use of 1-Methylcyclopropene (1-MCP) as a strategy to improve post-harvest life of ‘Abate Fetel’ pears. Acta Hortic. 2011, 909, 739–744. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ehsani-Moghaddam, B. Preharvest 1-Methylcyclopropene treatment reduces Soft Scald in ‘Honeycrisp’ apples during storage. HortScience 2010, 45, 414–417. [Google Scholar] [CrossRef] [Green Version]
- Nock, J.; Doerflinger, F.; Sutanto, G.; Al-Shoffe, Y.; Gunes, N.; Zhang, Y.; Wright, A.; Delong, J.; Watkins, C. Managing stem-end flesh browning, a physiological disorder of ‘Gala’ apples. Acta Hortic. 2019, 1256, 163–168. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Sutanto, G.; Nock, J.F.; Shoffe, Y.; Zhang, Y.; Watkins, C.B. Stem-end flesh browning of ‘Gala’ apples is decreased by preharvest 1-MCP (Harvista) and conditioning treatments. Fruit Q. 2017, 25, 9–14. [Google Scholar]
- DeEll, J.R.; Lum, G.B.; Mostofi, Y.; Lesage, S.K. Timing of ethylene inhibition affects internal browning and quality of ‘Gala’ apples in long-term low oxygen storage. Front. Plant Sci. 2022, 13, 914441. [Google Scholar] [CrossRef]
- Lurie, S. Regulation of ethylene biosynthesis in fruits by Aminoethoxyvinyl Glycine and 1-Methylcyclopropene. Acta Hortic. 2008, 796, 31–41. [Google Scholar] [CrossRef]
- Robinson, T.L.; Watkins, C.B.; Hoying, S.A.; Nock, J.F.; Iungermann, K.I. Aminoethoxyvinylglycine and 1-Methylcyclopropene effects on ’McIntosh´ preharvest drop, fruit maturation and fruit quality after storage. Acta Hortic. 2006, 727, 473–780. [Google Scholar] [CrossRef]
- Costa, G. Bioregulators application in pear production. In Proceedings of the 6th Conference ‘Innovation in Fruit Growing’, Belgrade, Serbia, 2 February 2017; pp. 37–50. [Google Scholar]
- Amarante, C.V.T.D.; Simioni, A.; Megguer, C.A.; Blum, L.E.B. Effect of aminoethoxyvinilglycine (AVG) on preharvest fruit drop and maturity of apples. Rev. Bras. Frutic. 2002, 24, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Argenta, L.C.; Vieira, M.J.; Krammes, J.G.; Petri, L.; Basso, C. AVG and 1-MCP effects on maturity and quality of apple fruit at harvest and after storage. Acta Hortic. 2006, 727, 495–504. [Google Scholar] [CrossRef]
- Do Amarante, C.V.T.; Argenta, L.C.; De Freitas, S.T.; Steffens, C.A. Efficiency of pre-harvest application of 1-MCP (Harvista™ 1.3 SC) to delay maturation of ‘Cripps Pink’ apple fruit. Sci. Hortic. 2022, 293, 110715. [Google Scholar] [CrossRef]
- Bramlage, W. Effect of aminoethoxyvinylglycine on internal ethylene concentrations and storage of apples. J. Am. Soc. Hortic. Sci. 1980, 105, 847–851. [Google Scholar] [CrossRef]
- Masia, A.; Ventura, M.; Gemma, H.; Sansavini, S. Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Regul. 1998, 25, 127–134. [Google Scholar] [CrossRef]
- Wargo, J.M.; Merwin, I.A.; Watkins, C.B. Nitrogen fertilization, mid-summer trunk girdling, and AVG treatments affect maturity and quality of ‘Jonagold’ apple. HortScience 2004, 39, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Amarante, C.V.T.d.; Steffens, C.A.; Blum, L.E.B. Fruit color, physiological disorders and diseases of ‘Gala’ and ‘Fuji’ apples sprayed with aminoethoxyvinylglycine. Rev. Bras. Frutic. 2010, 32, 009–018. [Google Scholar]
- Schupp, J.R.; Greene, D.W. Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of ‘McIntosh’ apples. I. Concentration and timing of dilute applications of AVG. HortScience 2004, 39, 1030–1035. [Google Scholar] [CrossRef] [Green Version]
- Whale, S.K.; Singh, Z.; Behboudian, M.H.; Janes, J.; Dhaliwal, S.S. Fruit quality in ‘Cripp’s Pink’ apple, especially colour, as affected by preharvest sprays of aminoethoxyvinylglycine and ethephon. Sci. Hortic. 2008, 115, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Argenta, L.C.; Scolaro, A.M.T.; do Amarante, C.V.T.; Vieira, M.J. Preharvest treatment of ‘Gala’ apples with 1-MCP and AVG-II: Effects on fruit quality after storage. Acta Hortic. 2018, 1194, 127–134. [Google Scholar] [CrossRef]
- Dias, C.; Amaro, A.L.; Salvador, Â.C.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Strategies to preserve postharvest quality of horticultural crops and superficial scald control: From Diphenylamine antioxidant usage to more recent approaches. Antioxidants 2020, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Gamalero, E.; Glick, B.R. Ethylene and abiotic stress tolerance in plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Parvaiz Ahmad, M.N.V.P., Ed.; Springer: New York, NY, USA, 2012; pp. 395–412. [Google Scholar]
- Meheriuk, M.; Lau, O.; Hall, J. Effects of some postharvest and storage treatments on the incidence of flesh browning in controlled-atmosphere-stored ‘Delicious’ apples. J. Am. Soc. Hortic. Sci. 1984, 109, 290–293. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P.; Nock, J.F.; Watkins, C.B. Superficial scald, carbon dioxide injury, and changes of fermentation products and organic acids in ‘Cortland’ and ‘Law Rome’ apples after high carbon dioxide stress treatment. J. Am. Soc. Hortic. Sci. 2001, 126, 235–241. [Google Scholar] [CrossRef]
- Argenta, L.C.; Fan, X.; Mattheis, J.P. Responses of ‘Fuji’ apples to short and long duration exposure to elevated CO2 concentration. Postharvest Biol. Technol. 2002, 24, 13–24. [Google Scholar] [CrossRef]
- Felicetti, E.; Mattheis, J.P.; Zhu, Y.; Fellman, J.K. Dynamics of ascorbic acid in ‘Braeburn’ and ‘Gala’ apples during on-tree development and storage in atmospheres conducive to internal browning development. Postharvest Biol. Technol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Lee, J.; Mattheis, J.P.; Rudell, D.R. Antioxidant treatment alters metabolism associated with internal browning in ‘Braeburn’ apples during controlled atmosphere storage. Postharvest Biol. Technol. 2012, 68, 32–42. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Rudell, D.R. Diphenylamine metabolism in ‘Braeburn’ apples stored under conditions conducive to the development of internal browning. J. Agric. Food Chem. 2008, 56, 3381–3385. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.F.; Zhang, Y.; Watkins, C.B. Pre- and post-harvest γ-aminobutyric acid application in relation to fruit quality and physiological disorder development in ‘Honeycrisp’ apples. Sci. Hortic. 2021, 289, 110431. [Google Scholar] [CrossRef]
- Buts, K.; Hertog, M.L.; Ho, Q.T.; America, A.H.; Cordewener, J.; Vercammen, J.; Carpentier, S.C.; Nicolai, B. Influence of pre-harvest calcium, potassium and triazole application on the proteome of apple at harvest. J. Sci. Food Agric. 2016, 96, 4984–4993. [Google Scholar] [CrossRef]

| Pre-Harvest Factor | Intensity of Factor | IFB Risk/Incidence | Cultivar | Reference |
|---|---|---|---|---|
| Tree/fruit factors | ||||
| Fruit size | Large fruit | ↑ RFB & DFB | ‘Cripps Pink’ | Butler [35] |
| Large fruit 250 to 350 g | ↑ Senescent breakdown | ‘Honeycrisp’ ‘Royal Gala’ | Prange et al. [100] Lee et al. [108] | |
| Medium/average fruit (173–176 g) | ↑ CO2 injury | ‘Cripps Pink’ | De Castro et al. [40] | |
| Crop load | Light (3.1 to 3.8 fruit cm−2 TCSA) | ↑ BBD | ‘Braeburn’ | Elgar et al. [38] |
| Light | ↑ RFB | ‘Cripps Pink’ | Jobling et al. [29] James [37], Bergman et al. [36] | |
| Light | ↑ IFB related disorders & BBD | _ | Little and Holmes [65] Volz et al. [127] | |
| Heavy (36 to 44 tonnes/ha) | ↑ CO2 injury | ‘Cripps Pink’ | De Castro et al. [40] | |
| No effect of crop load | ↔ CO2 injury | ‘Cripps Pink’ | Brown et al. [25] | |
| Light (50% of standard crop load) | ↑ BBD | ‘Braeburn’ | McCormick et al. [34] | |
| Maturity at harvest | Late harvest (1 week after normal) | ↑ BBD | ‘Braeburn’ | Lau [32] Streif [45] |
| Late harvest (1 week after commercial) | ↑ RFB & DFB | ‘Cripps Pink’ | Brown et al. [26] | |
| Late harvest (>50% starch breakdown) | ↑ RFB & DFB | ‘Rosy Glow’ | Doe et al. [43] | |
| Late harvest | ↑ RFB | ‘Cripps Pink’ | Moggia et al. [42] | |
| Late harvest (210 DAFB) | ↑ CO2 injury | ‘Fuji’ | Volz et al. [46] | |
| Late harvest (2 to 3 weeks after commercial or 72% starch breakdown) | ↑ DFB | ‘Cripps Pink’ | Elgar et al. [38], Crouch et al. [44] | |
| Both early and late harvest | ↑ CO2 injury | ‘Braeburn’ ‘Cripps Pink’ | Elgar et al. [38] De Castro et al. [40] | |
| Early harvesting | ↓ FFB | ‘Empire’ | James et al. [47] Doerflinger et al. [134] Doerflinger et al. [68] | |
| Cultivar | Susceptible | ↑ IFB related disorders | ‘Jonagold’ ‘Cox’s Orange Pippin’ ‘Jonathan’ ‘Delicious’ | Meheriuk et al. [78] |
| Less susceptible | ↓ CO2 injury | ‘Jonagold’, ‘Kanzi’ ‘Fuji Superma’ ‘Golden Delicious’ | Saquet et al. [141] Ho et al. [89] Hatoum et al. [24] Argenta et al. [144] | |
| Highly susceptible | ↑ BBD | ‘Braeburn’ | Ho et al. [89] Hatoum et al. [24] | |
| Highly susceptible | ↑ CI | ‘McIntosh’ ‘Boskoop’ ‘Elstar’ | Little and Holmes [65] Watkins [101] | |
| Less susceptible | ↓ CI | ‘Delicious’ ‘Granny Smith’ | Little and Holmes [65] Watkins [101] | |
| Susceptible | ↑ CO2 injury | ‘Braeburn’ ‘Fuji’ ‘Rosy Glow’ ‘Han Fu’ | Elgar et al. [31] Lau [32] Volz et al. [83] Little and Holmes [65] Watkins [101] Williamson et al. [143] Li et al. [145] | |
| Climate | ||||
| Seasonal temperatures | Warmer climate 4–6 weeks before harvest | ↓ LTB & CB | _ | Bramlage [39] |
| Higher temperatures before harvest | ↑ RFB & DFB | ‘Cripps Pink’ | Brown et al. [26] | |
| Cooler climate 50 days post flowering | ↑ CO2 injury | ‘Braeburn’ | Lau [32] Little and Holmes [65] | |
| Warmer average daily temperatures from 90 to 210 DAFB | ↓ CO2 injury | ‘Fuji’ | Corrêa et al. [150] | |
| Warmer night temperatures (>10 °C) four weeks before harvest | ↓ BBD | ‘Braeburn’ | McCormick et al. [34] | |
| Cold growing region | ↑ CO2 injury | ‘Fuji’ | Argenta et al. [149] | |
| Growing degree days | GDD>10 °C below 1100 | ↑ DFB | ‘Cripps Pink’ | James and Jobling [28] James et al. [103] |
| GDD>10 °C between 1100 to 1700 | ↑ RFB | |||
| GDD>10 °C below 500 (during 50 to 60 DAFB) | ↑ DFB | ‘Honeycrisp’ | Tong et al. [64] | |
| Mineral nutrition | ||||
| Nitrogen | Higher fruit N | ↑ Internal breakdown & CB | _ | Sharples [157] Bramlage [39] |
| High fruit N (>400 mg kg−1 fresh weight) | Senescent & internal breakdown | ‘Lobo’ | Dris et al. [159] | |
| Internal breakdown & CB | ‘Raike’ ‘Red Atlas’ | |||
| Phosphorus | Low fruit P (<110 ppm) at whole fruit less seeds and stem basis | ↑ LTB | ‘Cox’s Orange Pippin’ | Sharples [157] |
| Low fruit P (<0.011%) | ↑ LTB & senescent breakdown | _ | Bergmann [171] Bramlage [39] Marcelle [155] | |
| Higher fruit P (>90 ppm) at whole fruit less seeds and stem basis | ↓ LTB | ‘McIntosh’ | Bramlage et al. [163] Webster and Lidster [77] | |
| Higher fruit P (18–19 mg 100 g−1 fresh weight) | ↓ LTB | ‘Cox’s Orange Pippin’ ‘McIntosh’ | Johnson and Yogaratnam [76] | |
| Low fruit P (<85 ppm) at whole fruit less seeds and stem basis | ↑ LTB | ‘McIntosh’ | Webster and Lidster [77] | |
| High fruit P | ↑ BBD | ‘Braeburn’ | Neuwald et al. [165] | |
| Potassium | Low fruit K | ↑ LTB | _ | Sharples [157] Bergmann [171] Bramlage [39] |
| Higher fruit K | ↓ CI & LTB (induces resistance to CI and LTB) | _ | Hakerlerler et al. [168] Singer and El-Tohamy [169] | |
| Higher fruit K (>0.12–0.15%) | ↑ CB or Core flush | Sharples [157] Bergmann [171] | ||
| Higher fruit K | ↑ CO2 injury & BBD | ‘Santana’ ‘Braeburn’ | Neuwald et al. [165,170] | |
| Calcium | Higher fruit Ca (~80 mg per gram fresh weight | ↓ CO2 injury | ‘Cripps Pink’ | De Castro et al. [40] |
| High Ca (~110 mg kg−1 fresh weight) or low Ca:K ratio (15.8) | ↓ DFB | ‘Cripps Pink’ | James and Jobling [57] | |
| Higher fruit Ca (>4 mg 100 mg−1 fresh weight) | ↑ CO2 injury | ‘Braeburn’ | Wood et al. [181] | |
| Lower fruit Ca | ↑ CO2 injury | ‘Cripps Pink’ | De Castro et al. [27] | |
| Lower fruit Ca (<40–60 mg kg−1 fresh weight) | ↑ flesh breakdown & other disorders | _ | Dris et al. [159] Suzuki and Basso [182] | |
| Lower fruit Ca (<80 mg kg−1 fresh weight) | ↑ CO2 injury | ‘Fuji’ | Corrêa et al. [175] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, R.S.; Bound, S.A.; Swarts, N.D. Internal Flesh Browning in Apple and Its Predisposing Factors—A Review. Physiologia 2023, 3, 145-172. https://doi.org/10.3390/physiologia3020012
Sidhu RS, Bound SA, Swarts ND. Internal Flesh Browning in Apple and Its Predisposing Factors—A Review. Physiologia. 2023; 3(2):145-172. https://doi.org/10.3390/physiologia3020012
Chicago/Turabian StyleSidhu, Ramandeep Singh, Sally A. Bound, and Nigel D. Swarts. 2023. "Internal Flesh Browning in Apple and Its Predisposing Factors—A Review" Physiologia 3, no. 2: 145-172. https://doi.org/10.3390/physiologia3020012
APA StyleSidhu, R. S., Bound, S. A., & Swarts, N. D. (2023). Internal Flesh Browning in Apple and Its Predisposing Factors—A Review. Physiologia, 3(2), 145-172. https://doi.org/10.3390/physiologia3020012







