Addressing the Issue of Chronic, Inappropriate Benzodiazepine Use: How Can Pharmacists Play a Role?
Abstract
:1. Introduction
1.1. Risk—Benefit Analysis of Chronic Benzodiazepine Use
1.2. Prescribing Guidelines for Benzodiazepines
1.3. Surveys of Benzodiazepine Usage Patterns
1.4. Epidemiology of Long-Term Benzodiazepine Use
1.5. Aims of This Study
2. Experimental Section
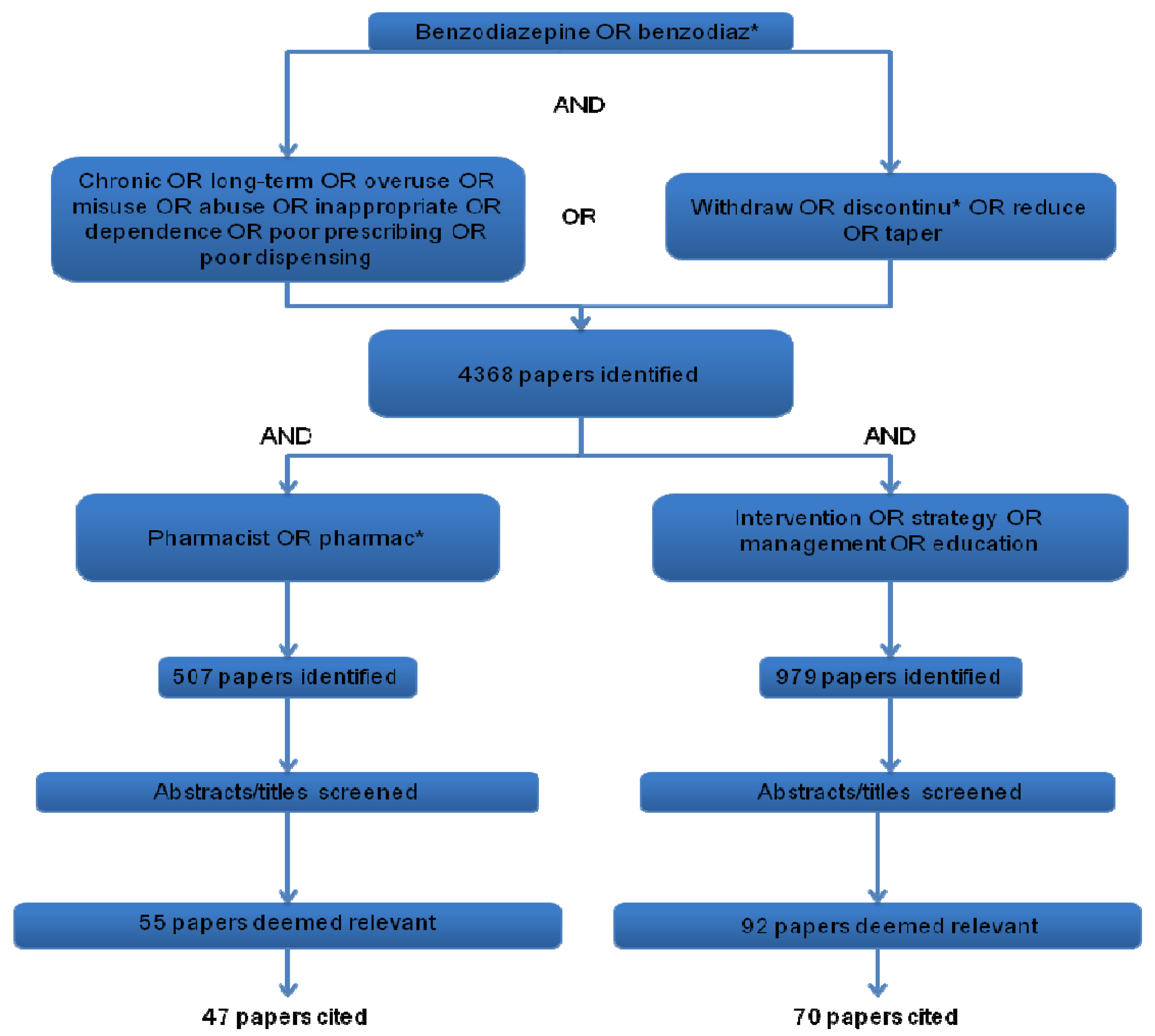
3. Results and Discussion
3.1. The Ongoing Challenge of Optimising Drug Prescribing and Drug Use
| Study | Location | Pharmacist-Specific Intervention Details | Type/size & setting of study | Summary of results/Comments |
|---|---|---|---|---|
| Dollman et al., 2005 | Australia | Community pharmacists included in initial educational programme and pharmacies were used to provide information to consumers to discourage benzodiazepine use. | Before and after study (dispensing records examined in one rural area of population 20,000). | 19% reduction in benzodiazepine dispensing (defined daily dose/1,000 population/day) achieved 2 years post-intervention compared with 6% nationally. Dispensing of anti-depressants increased by 33%. |
| Elde and Schjott, 2001 | Norway | Pharmacist conducted an audit on benzodiazepine use and provided feedback and academic education to all staff. | Controlled before and after study (not randomised; 10 long-term care facilities, 5 intervention, 5 control). | Significant decrease in % of patients using long-acting benzodiazepines in intervention group (24%) vs. controls (44%). Improved drug administration practices. 5 year follow-up. |
| Lerat et al., 2010 | France | Pharmacist and psychistrists held monthly meetings to develop prescribing guidelines and discuss those patients receiving high-dose BDZs. | Retrospective study design. 473 prisoners (222 control and 251 intervention) | Daily dose of benzodiazepines decreased significantly from 46 mg diazepam equivalent to 34 mg in the intervention group. |
| McClaugherty et al., 1997 | USA | Pharmacist conducted an audit on benzodiazepine use and provided feedback to nurses & doctors. | Quasi-experimental design across 10 nursing homes. | Percentage of patients prescribed routine benzodiazepines decreased from 4.5% to 1.6% post intervention. 3 month follow-up |
| Martin et al., 2013 | Canada | Pharmacists provided educational material directly to patients to improve knowledge of risks of benzodiazepine misuse | Before and after study with 144 participants | 45% of participants had increased risk perception after the intervention. Intent to discontinue or self-taper appeared higher in the intervention group. |
| Midlov et al., 2006 | Sweden | Educational visits from pharmacist and physician focussing on “avoidance of confusion in the elderly”. | RCT design. (15 GP practices, 8 intervention and 7 control) | Significant decrease of ~25% in both total benzodiazepine prescribing and medium-long-acting benzodiazepines in intervention vs. control groups. One year follow-up. |
| Monane et al., 1998 | USA | Alert system established by pharmacist using computerised drug usage review system. Telephone conferences between pharmacist and geriatrician. | Population-based cohort design. 23,269 patients over 65 years from benefits manager database. | 43,007 alerts triggered by system regarding suboptimal prescribing/dispensing. 24,266 alerts discussed with physician. Rate of change to a more appropriate therapeutic agent was 40% for long-acting benzodiazepines. |
| Roberts et al., 2001 | Australia | Clinical pharmacy program involving development of professional relationships, nurse education on medication issues, and individualized medication reviews. | Cluster RCT. (52 nursing homes, 13 intervention and 39 control) | Percentage of patients being prescribed benzodiazepines reduced by ~17% in intervention vs. control groups. Overall reduction in numbers of prescribed items per resident in intervention groups. |
| Schmidt et al., 1998 | Sweden | Monthly multidisciplinary meetings led by pharmacist, including medication review of benzodiazepine use. | RCT design. (33 nursing homes; 15 intervention, 18 control) | Improved prescribing of appropriate hypnotics (+70%) and anxiolytics (+50%) in intervention versus control groups. One month follow-up. |
| Smith and Tett, 2010b | Australia | Community pharmacists included in educational emails about benzodiazepines. Consumers received simpler information from pharmacist when filling benzodiazepine prescriptions directing them to a website. | Controlled before and after study based on regional participation. (136 pharmacies involved; 69 intervention, 67 control) | Significant reduction in % of residents of aged care homes taking benzodiazepines after the intervention but no difference in overall benzodiazepine consumption in whole population. |
| Soo et al., 2010 | Australia | Benzodiazepine voluntary undertaking by patients enrolled with one pharmacy. Pharmaceutical care delivered. | Audit study of 129 doctors, 68 pharmacies, 606 patients. | Goals of the undertaking not currently measurable. |
| Towle and Adams, 2006 | Scotland | Letter to repeat benzodiazepine users telling them that they have been enrolled in a step-down programme. Invited patients to attend GP for medication review. Inactivated repeat benzodiazepine prescriptions. | Sampling study (206 patients; 1 pharmacy) | At three years number of tablets prescribed down by 64% and only 23% remained on a repeat prescription for benzodiazepines. No statistical analysis performed. |
| Van de Steeg-van Gompel et al., 2009 | The Netherlands | Community pharmacists were asked to run a benzodiazepine discontinuation letter service with either written instructions alone or intensive support. | Cluster randomized controlled trial. 90 pharmacies, 47 intervention (support) 43 control (written manual) | More pharmacies in the intervention group sent discontinuation letters to long-term benzodiazepine users (70% vs. 40%), but this did not impact on the % of GPs willing to identify suitable patients to target for benzodiazepine reduction. |
| Westbury et al., 2010 | Tasmania | Community pharmacist provided audit and feedback, educational sessions and an interdisciplinary sedative review to intervention nursing homes. | Controlled trial. (25 nursing homes, 13 intervention and 12 control) | Significant reduction in the percentage of intervention home residents regularly taking benzodiazepines (31.8% to 26.9%, p < 0.005). No change in controls.6-month follow-up. |
3.2. Strategies for Reducing Inappropriate Benzodiazepine Use
3.2.1. Role of the Pharmacist in Referring Patients to Psychological Support Services
3.2.2. Role of the Pharmacist in Patient-Directed Educational Interventions for Reducing Benzodiazepine Use
3.2.3. Involvement of Pharmacists in Educational Interventions Directed at Healthcare Professionals
3.3. Interdisciplinary Collaboration between Doctors and Pharmacists is Key to Reducing Benzodiazepine Use
3.4. The Use of Technology in Reducing Inappropriate Benzodiazepine Use
3.5. Policy and Legal Measures
3.6. Pharmacological Guidelines Surrounding Benzodiazepine Withdrawal
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Donoghue, J.; Lader, M. Usage of benzodiazepines: A review. Int. J. Psychiatr. Clin. Prac. 2010, 14, 78–87. [Google Scholar] [CrossRef]
- Lader, M.; Tylee, A.; Donoghue, J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009, 23, 19–34. [Google Scholar] [CrossRef]
- Arbanas, G.; Arbanas, D.; Dujam, K. Adverse effects of benzodiazepines in psychiatric outpatients. Psychiatr. Danub. 2009, 21, 103–107. [Google Scholar]
- Spanemberg, L.; Nogueira, E.L.; Belem da Silva, C.T.; Dargel, A.A.; Menezes, F.S.; Neto, A.C. High prevalence and prescription of benzodiazepines for elderly: Data from psychiatric consultation to patients from an emergency room of a general hospital. Gen. Hosp. Psychiatry 2011, 33, 45–50. [Google Scholar] [CrossRef]
- Curran, H.V. Tranquillising memories: A review of the effects of benzodiazepines on human memory. Biol. Psychol. 1986, 23, 179–213. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Paquette, A.; Hilmer, S.; Holroyd-Leduc, J.; Carnahan, R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, gabaergic and opioid drugs. Drugs Aging 2012, 29, 639–658. [Google Scholar]
- Swift, C. Postural instability as a measure of sedative drug response. Br. J. Clin. Pharmacol. 1984, 18, 87S. [Google Scholar] [CrossRef]
- Wu, C.S.; Wang, S.C.; Chang, I. The association between dementia and long-term use of benzodiazepine in the elderly: Nested case-control study using claims data. Am. J. Geriatr. Psychiatry 2009, 17, 614–620. [Google Scholar] [CrossRef]
- Nurmi-Luthje, I.; Kaukonen, J.P.; Luthje, P.; Naboulsi, H.; Tanninen, S.; Kataja, M.; Kallio, M.L.; Leppilampi, M. Use of benzodiazepines and benzodiazepine-related drugs among 223 patients with an acute hip fracture in Finland: Comparison of benzodiazepine findings in medical records and laboratory assays. Drugs Aging 2006, 23, 27–37. [Google Scholar] [CrossRef]
- Leufkens, T.R.M.; Vermeeren, A. Highway driving in the elderly the morning after bedtime use of hypnotics: A comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J. Clin. Psychopharmacol. 2009, 29, 432–438. [Google Scholar] [CrossRef]
- Woolcott, J.C.; Richardson, K.J.; Wiens, M.O.; Patel, B.; Marin, J.; Khan, K.M.; Marra, C.A. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 2009, 169, 1952–1960. [Google Scholar] [CrossRef]
- Zint, K.; Haefeli, W.E.; Glynn, R.J.; Mogun, H.; Avorn, J.; Sturmer, T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol. Drug Saf. 2010, 19, 1248–1255. [Google Scholar] [CrossRef]
- Kirby, M.; Denihan, A.; Bruce, I.; Radic, A.; Coakley, D.; Lawlor, B.A. Benzodiazepine use among the elderly in the community. Int. J. Geriatr. Psychiatry 1999, 14, 280–284. [Google Scholar] [CrossRef]
- Puustinen, J.; Nurminen, J.; Kukola, M.; Vahlberg, T.; Laine, K.; Kivela, S.L. Associations between use of benzodiazepines or related drugs and health, physical abilities and cognitive function: A non-randomised clinical study in the elderly. Drugs Aging 2007, 24, 1045–1059. [Google Scholar] [CrossRef]
- Obiora, E.; Hubbard, R.; Sanders, R.D.; Myles, P.R. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: A nested case-control and survival analysis in a population-based cohort. Thorax 2013, 68, 163–170. [Google Scholar] [CrossRef]
- El-Aneed, A.; Alaghehbandan, R.; Gladney, N.; Collins, K.; Macdonald, D.; Fischer, B. Prescription drug abuse and methods of diversion: The potential role of a pharmacy network. J. Subst. Use 2009, 14, 75–83. [Google Scholar] [CrossRef]
- Charlson, F.; Degenhardt, L.; McLaren, J.; Hall, W.; Lynskey, M. A systematic review of research examining benzodiazepine related mortality. Pharmacoepidemiol. Drug Saf. 2009, 18, 93–103. [Google Scholar] [CrossRef]
- Mallon, L.; Broman, J.E.; Hetta, J. Is usage of hypnotics associated with mortality? Sleep Med. 2009, 10, 279–286. [Google Scholar] [CrossRef]
- Bellerose, D.; Lyons, S.; Carew, A.; Walsh, S.; Long, J. Problem Benzodiazapine Use in Ireland: Treatment (2003 to 2008) and Deaths (1998 to 2007). Available online: http://www.hrb.ie/publications/hrb-publication/publications//532/ (accessed on 24 June 2013).
- Krska, J.; MacLeod, T. Sleep quality and the use of benzodiazepine hypnotics in general practice. J. Clin. Pharm. Ther. 1995, 20, 91–96. [Google Scholar] [CrossRef]
- Martin, J.L.R.; Sainz-Pardo, M.; Furukawa, T.A.; Martin-Sanchez, E.; Seoane, T.; Galan, C. Review: Benzodiazepines in generalized anxiety disorder: Heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J. Psychopharmacol. 2007, 21, 774–782. [Google Scholar]
- Glass, J.; Lanctôt, K.L.; Herrmann, N.; Sproule, B.A.; Busto, U.E. Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. BMJ 2005, 331, 1169. [Google Scholar] [CrossRef]
- Kasper, S.; Resinger, E. Panic disorder: The place of benzodiazepines and selective serotonin reuptake inhibitors. Eur. Neuropsychopharmacol. 2001, 11, 307–321. [Google Scholar] [CrossRef]
- Otto, M.W.; Pollack, M.H.; Gould, R.A.; WorthingtonIII, J.J.; McArdle, E.T.; Rosenbaum, J.F.; Heimberg, R.G. A comparison of the efficacy of clonazepam and cognitive-behavioral group therapy for the treatment of social phobia. J. Anxiety Disord. 2000, 14, 345–358. [Google Scholar] [CrossRef]
- Otto, M.W.; McHugh, R.K.; Simon, N.M.; Farach, F.J.; Worthington, J.J.; Pollack, M.H. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behav. Res. Ther. 2010, 48, 720–727. [Google Scholar] [CrossRef]
- Zandstra, S.; van Rijswijk, E.; Rijnders, C.A.T.; van de Lisdonk, E.; Bor, J.; van Weel, C.; Zitman, F. Long-term benzodiazepine users in family practice: Differences from short-term users in mental health, coping behaviour and psychological characteristics. Fam. Pract. 2004, 21, 266–269. [Google Scholar] [CrossRef]
- Rickels, K.; Case, W.G.; Schweizer, E.; Garcia-Espana, F.; Fridman, R. Long-term benzodiazepine users 3 years after participation in a discontinuation program. Am. J. Psychiatry 1991, 148, 757–761. [Google Scholar]
- Salzman, C.; Fisher, J.; Nobel, K.; Glassman, R.; Wolfson, A.; Kelley, M. Cognitive improvementfollowing benzodiazepine discontinuation in elderly nursing home residents. Int. J. Geriatr. Psychiatry 1992, 7, 89–93. [Google Scholar] [CrossRef]
- Curran, H.; Collins, R.; Fletcher, S.; Kee, S.; Woods, B.; Iliffe, S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: Effects on cognitive function, sleep, mood and quality of life. Psychol. Med. 2003, 33, 1223–1237. [Google Scholar] [CrossRef]
- Cahir, C.; Fahey, T.; Teeling, M.; Teljeur, C.; Feely, J.; Bennett, K. Potentially inappropriate prescribing and cost outcomes for older people: A national population study. Br. J. Clin. Pharmacol. 2010, 69, 543–552. [Google Scholar] [CrossRef]
- Benzodiazepines: Good Practice Guidelines for Clinicians. Available online: http://www.dohc.ie/publications/benzodiazepines_good_practice_guidelines.html (accessed on 24 June 2013).
- Committee on Safety of Medicines. Benzodiazepines, dependence and withdrawal symptoms. Curr. Probl. 1988, 21, 1–2.
- Jorgensen, V.R. Benzodiazepine and cyclopyrrolone reduction in general practice—does this lead to concomitant change in the use of antipsychotics? A study based on a Danish population. J. Affect. Disord. 2010, 126, 293–298. [Google Scholar] [CrossRef]
- Salzman, C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am. J. Psychiatry 1991, 148, 151. [Google Scholar]
- Smith, A.J.; Tett, S.E. Interventions to improve benzodiazepine prescribing, lessons from the past 20 years to guide future interventions. BMC Health Serv. Res. 2010. [Google Scholar] [CrossRef]
- Royal Australian College of General Practioners. Benzodiazepine Guidelines. Available online: http://www.racgp.org.au/guidelines/benzodiazepines (accessed on 24 June 2013).
- Straand, J.; Rokstad, K. General practitioners’ prescribing patterns of benzodiazepine hypnotics: Are elderly patients at particular risk for overprescribing? Scand. J. Prim. Health Care Suppl. 1997, 15, 16–21. [Google Scholar] [CrossRef]
- Hartikainen, S.; Rahkonen, T.; Kautiainen, H.; Sulkava, R. Kuopio 75+ study: Does advanced age predict more common use of psychotropics among the elderly? Int. Clin. Psychopharmacol. 2003, 18, 163–167. [Google Scholar]
- National Institute for Health and Clinical Excellence. Clinical Guideline 113: Management of anxiety (panic disorder, with or without agoraphobia, and generalised anxiety disorder) in adults in primary, secondary and community care. Available online: http://www.nice.org.uk/nicemedia/live/13314/52599/52599.pdf (accessed on 24 June 2013).
- Ciuna, A.; Andretta, M.; Corbari, L.; Levi, D.; Mirandola, M.; Sorio, A.; Barbui, C. Are we going to increase the use of antidepressants up to that of benzodiazepines? Eur. J. Clin. Pharmacol. 2004, 60, 629–634. [Google Scholar] [CrossRef]
- Veronese, A.; Garatti, M.; Cipriani, A.; Barbui, C. Benzodiazepine use in the real world of psychiatric practice: Low-dose, long-term drug taking and low rates of treatment discontinuation. Eur. J. Clin. Pharmacol. 2007, 63, 867–873. [Google Scholar] [CrossRef]
- Magrini, N.; Vaccheri, A.; Parma, E.; DíAlessandro, R.; Bottoni, A.; Occhionero, M.; Montanaro, N. Use of benzodiazepines in the Italian general population: Prevalence, pattern of use and risk factors for use. Eur. J. Clin. Pharmacol. 1996, 50, 19–25. [Google Scholar] [CrossRef]
- Balestrieri, M.; Marcon, G.; Samani, F.; Marini, M.; Sessa, E.; Gelatti, U.; Donato, F. Mental disorders associated with benzodiazepine use among older primary care attenders. Soc. Psychiatry Psychiatr. Epidemiol. 2005, 40, 308–315. [Google Scholar] [CrossRef]
- Lagnaoui, R.; Depont, F.; Fourrier, A.; Abouelfath, A.; Begaud, B.; Verdoux, H.; Moore, N. Patterns and correlates of benzodiazepine use in the French general population. Eur. J. Clin. Pharmacol. 2004, 60, 523–529. [Google Scholar] [CrossRef]
- Lasserre, A.; Younes, N.; Blanchon, T.; Cantegreil-Kallen, I.; Passerieux, C.; Thomas, G.; Chan-Chee, C.; Hanslik, T. Psychotropic drug use among older people in general practice: Discrepancies between opinion and practice. Br. J. Gen. Pract. 2010, 60, e156–e162. [Google Scholar] [CrossRef]
- Cunningham, C.M.; Hanley, G.E.; Morgan, S. Patterns in the use of benzodiazepines in British Columbia: Examining the impact of increasing research and guideline cautions against long-term use. Health Policy 2010, 97, 122–129. [Google Scholar] [CrossRef]
- Simon, G.E.; Ludman, E.J. Outcome of new benzodiazepine prescriptions to older adults in primary care. Gen. Hosp. Psychiatry 2006, 28, 374–378. [Google Scholar] [CrossRef]
- Vasile, R.G.; Bruce, S.E.; Goisman, R.M.; Pagano, M.; Keller, M.B. Results of a naturalistic longitudinal study of benzodiazepine and SSRI use in the treatment of generalized anxiety disorder and social phobia. Depress. Anxiety 2005, 22, 59–67. [Google Scholar] [CrossRef]
- Uchida, H.; Suzuki, T.; Mamo, D.C.; Mulsant, B.H.; Kikuchi, T.; Takeuchi, H.; Tomita, M.; Watanabe, K.; Yagi, G. Benzodiazepine and antidepressant use in elderly patients with anxiety disorders: A survey of 796 outpatients in Japan. J. Anxiety Disord. 2009, 23, 477–481. [Google Scholar] [CrossRef]
- Alonso, J.; Angermeyer, M.; Bernert, S.; Bruffaerts, R.; Brugha, T.; Bryson, H.; Girolamo, G.; Graaf, R.; Demyttenaere, K.; Gasquet, I. Psychotropic drug utilization in europe: Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr. Scand. 2004, 109, 55–64. [Google Scholar]
- Ohayon, M.M.; Lader, M.H. Use of psychotropic medication in the general population of France, Germany, Italy, and the United Kingdom. J. Clin. Psychiatry 2002, 63, 817–825. [Google Scholar] [CrossRef]
- Smith, A.J.; Sketris, I.; Cooke, C.; Gardner, D.; Kisely, S.; Tett, S.E. A comparison of benzodiazepine and related drug use in Nova Scotia and Australia. Can. J. Psychiatry 2008, 53, 545–552. [Google Scholar]
- O’Mhaolin, A.; Gallagher, D.; O’Connell, H.; Chin, A.; Bruce, I.; Hamilton, F.; Tehee, E.; Coen, R.; Coakley, D.; Walsh, B. Benzodiazepine use amongst community dwelling elderly: 10 years on. Int. J. Geriatr. Psychiatry 2010, 25, 650–651. [Google Scholar] [CrossRef]
- Hallahan, B.; Murray, I.; McDonald, C. Benzodiazepine and hypnotic prescribing in an acute adult psychiatric in-patient unit. Psychiatr. Bull. 2009, 33, 12. [Google Scholar] [CrossRef]
- Zandstra, S.M.; Furer, J.W.; van de Lisdonk, E.H.; Bor, J.H.; Zitman, F.G.; van Weel, C. Differences in health status between long-term and short-term benzodiazepine users. Br. J. Gen. Pract. 2002, 52, 805–808. [Google Scholar]
- Neutel, C.I. The epidemiology of long-term benzodiazepine use. Int Rev Psychiatry 2005, 17, 189–197. [Google Scholar] [CrossRef]
- Mant, A.; Duncan-Jones, P.; Saltman, D.; Bridges-Webb, C.; Kehoe, L.; Lansbury, G.; Chancellor, A.H. Development of long term use of psychotropic drugs by general practice patients. BMJ 1988, 296, 251–254. [Google Scholar] [CrossRef]
- Van Hulten, R.; Teeuw, K.B.; Bakker, A.; Leufkens, H.G. Initial 3-month usage characteristics predict long-term use of benzodiazepines: An 8-year follow-up. Eur. J. Clin. Pharmacol. 2003, 58, 689–694. [Google Scholar]
- Arroll, B. GPs should prescribe more benzodiazepines for the elderly. J. Prim. Health Care 2009, 1, 57. [Google Scholar]
- Parr, J.M.; Kavanagh, D.J.; Young, R.M.D.; McCafferty, K. Views of general practitioners and benzodiazepine users on benzodiazepines: A qualitative analysis. Soc. Sci. Med. 2006, 62, 1237–1249. [Google Scholar] [CrossRef]
- Dempsey, O.P.; Moore, H. Psychotropic prescribing for older people in residential care in the UK, are guidelines being followed? Primary Care Commun. 2005, 10, 13–18. [Google Scholar] [CrossRef]
- Rogers, A.; Pilgrim, D.; Brennan, S.; Sulaiman, I.; Watson, G.; Chew-Graham, C. Prescribing benzodiazepines in general practice: A new view of an old problem. Health 2007, 11, 181–198. [Google Scholar]
- Srisurapanont, M.; Garner, P.; Critchley, J.; Wongpakaran, N. Benzodiazepine prescribing behaviour and attitudes: A survey among general practitioners practicing in Northern Thailand. BMC Fam. Pract. 2005. [Google Scholar] [CrossRef] [Green Version]
- Anthierens, S.; Habraken, H.; Petrovic, M.; Christiaens, T. The lesser evil? Initiating a benzodiazepine prescription in General Practice. Scand. J. Prim. Health 2007, 25, 214–219. [Google Scholar] [CrossRef]
- Siriwardena, A.N.; Qureshi, Z.; Gibson, S.; Collier, S.; Latham, M. GPs’ attitudes to benzodiazepine and ‘z-drug’ prescribing: A barrier to implementation of evidence and guidance on hypnotics. Br. J. Gen. Pract. 2006, 56, 964–967. [Google Scholar]
- Boixet, M.; Batlle, E.; Bolibar, I. Benzodiazepines in primary health care: A survey of general practitioners prescribing patterns. Addiction 1996, 91, 549–556. [Google Scholar] [CrossRef]
- Ten Wolde, G.B.; Dijkstra, A.; van Empelen, P.; Knuistingh Neven, A.; Zitman, F. Psychological determinants of the intention to educate patients about benzodiazepines. Pharm. World Sci. 2008, 30, 336–342. [Google Scholar] [CrossRef]
- O’Reilly, C.L.; Simon Bell, J.; Chen, T.F. Pharmacists’ beliefs about treatments and outcomes of mental disorders: A mental health literacy survey. Aust. NZ J. Psychiat. 2010, 44, 1089–1096. [Google Scholar] [CrossRef]
- Oxman, A.D.; Thomson, M.A.; Davis, D.A.; Haynes, R.B. No magic bullets: A systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995, 153, 1423–1431. [Google Scholar]
- Van Grootheest, A.; Edwards, I.R. Labelling and ‘Dear Doctor’ letters: Are they noncommittal? Drug Saf. 2002, 25, 1051–1055. [Google Scholar] [CrossRef]
- Batty, G.; Oborne, C.A.; Swift, C.; Jackson, S.H.D. Development of an indicator to identify inappropriate use of benzodiazepines in elderly medical in-patients. Int. J. Geriatr. Psychiatry 2000, 15, 892–896. [Google Scholar] [CrossRef]
- Batty, G.M.; Hooper, R.; Oborne, C.A.; Jackson, S. Investigating intervention strategies to increase the appropriate use of benzodiazepines in elderly medical in-patients. Br. J. Clin. Govern. 2001, 6, 252–258. [Google Scholar] [CrossRef]
- Wan Md Adnan, W.A.H.; Zaharan, N.L.; Bennett, K.; Wall, C.A. Trends in co prescribing of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in ireland. Br. J. Clin. Pharmacol. 2010, 458–466. [Google Scholar]
- Olsson, J.; Bergman, A.; Carlsten, A.; Oke, T.; Bernsten, C.; Schmidt, I.K.; Fastbom, J. Quality of drug prescribing in elderly people in nursing homes and special care units for dementia: A cross-sectional computerized pharmacy register analysis. Clin. Drug Investig. 2010, 30, 289–300. [Google Scholar] [CrossRef]
- Bateman, D.; Eccles, M.; Campbell, M.; Soutter, J.; Roberts, S.; Smith, J. Setting standards of prescribing performance in primary care: Use of a consensus group of general practitioners and application of standards to practices in the North of England. Br. J. Gen. Pract. 1996, 46, 20–25. [Google Scholar]
- Lipton, H.L.; Byrns, P.J.; Soumerai, S.B.; Chrischilles, E.A. Pharmacists as agents of change for rational drug therapy. Int. J. Technol. Assess. Health Care 1995, 11, 485–508. [Google Scholar] [CrossRef]
- Winslade, N.; Taylor, L.; Shi, S.; Schuwirth, L.; van der Vleuten, C.; Tamblyn, R. Monitoring community pharmacist's quality of care: A feasibility study of using pharmacy claims data to assess performance. BMC Health Serv. Res. 2011. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.D.; Wright, D.J.; Chrystyn, H. Pharmacoeconomic evaluation of a patient education letter aimed at reducing long-term prescribing of benzodiazepines. Pharm. World Sci. 2002, 24, 231–235. [Google Scholar] [CrossRef]
- Baillargeon, L.; Landreville, P.; Verreault, R.; Beauchemin, J.P.; Gregoire, J.P.; Morin, C.M. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: A randomized trial. CMAJ 2003, 169, 1015–1020. [Google Scholar]
- Morin, C.M.; Bastien, C.; Guay, B.; Radouco-Thomas, M.; Leblanc, J.; Vallieres, A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am. J. Psychiatry 2004, 161, 332–342. [Google Scholar] [CrossRef]
- Oude Voshaar, R.C.; Gorgels, W.J.; Mol, A.J.; van Balkom, A.J.; van de Lisdonk, E.H.; Breteler, M.H.; van den Hoogen, H.J.; Zitman, F.G. Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: Three-condition, randomised controlled trial. Br. J. Psychiatry 2003, 182, 498–504. [Google Scholar] [CrossRef]
- Gosselin, P.; Ladouceur, R.; Morin, C.M.; Dugas, M.J.; Baillargeon, L. Benzodiazepine discontinuation among adults with GAD: A randomized trial of cognitive-behavioral therapy. J. Consult. Clin. Psychol. 2006, 74, 908–919. [Google Scholar] [CrossRef]
- Parr, J.M.; Kavanagh, D.J.; Cahill, L.; Mitchell, G.; Young, R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: A meta-analysis. Addiction 2009, 104, 13–24. [Google Scholar]
- Kippist, C.; Wong, K.; Bartlett, D.; Saini, B. How do pharmacists respond to complaints of acute insomnia? A simulated patient study. Int. J. Clin. Pharm. 2011, 33, 237–245. [Google Scholar] [CrossRef]
- Cormack, M.A.; Owens, R.G.; Dewey, M.E. The effect of minimal interventions by general practitioners on long-term benzodiazepine use. J. R. Coll. Gen. Pract. 1989, 39, 408–411. [Google Scholar]
- Heather, N.; Bowie, A.; Ashton, H.; McAvoy, B.; Spencer, I.; Brodie, J.; Giddings, D. Randomised controlled trial of two brief interventions against long-term benzodiazepine use: Outcome of intervention. Addict. Res. Theory 2004, 12, 141–154. [Google Scholar] [CrossRef]
- Cormack, M.; Sweeney, K.; Hughes-Jones, H.; Foot, G. Evaluation of an easy, cost-effective strategy for cutting benzodiazepine use in general practice. Br. J. Gen. Pract. 1994, 44, 5. [Google Scholar]
- Gorgels, W.J.; Oude Voshaar, R.C.; Mol, A.J.; van de Lisdonk, E.H.; van Balkom, A.J.; van den Hoogen, H.J.; Mulder, J.; Breteler, M.H.; Zitman, F.G. Discontinuation of long-term benzodiazepine use by sending a letter to users in family practice: A prospective controlled intervention study. Drug Alcohol Depend. 2005, 78, 49–56. [Google Scholar] [CrossRef]
- Towle, I.; Adams, J. A novel, pharmacist-led strategy to reduce the prescribing of benzodiazepines in paisley. Pharm. J. 2006, 276, 136–138. [Google Scholar]
- Dollman, W.B.; Leblanc, V.; Stevens, L.; O’Connor, P.; Roughead, E.E.; Gilbert, A.L. Achieving a sustained reduction in benzodiazepine use through implementation of an area-wide multi-strategic approach. J. Clin. Pharm. Ther. 2005, 30, 425–432. [Google Scholar] [CrossRef]
- Van de Steeg-van Gompel, C.; Wensing, M.; de Smet, P. Implementation of a discontinuation letter to reduce long-term benzodiazepine use—a cluster randomized trial. Drug Alcohol Depend. 2009, 99, 105–114. [Google Scholar] [CrossRef]
- Martin, P.; Tamblyn, R.; Ahmed, S.; Tannenbaum, C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ. Couns. 2013, 92, 81–87. [Google Scholar] [CrossRef]
- Thomson, O.; Oxman, A.; Davis, D.; Haynes, R.; Freemantle, N.; Harvey, E. Educational outreach visits: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2000, 2, CD000409. [Google Scholar]
- Westbury, J.; Jackson, S.; Gee, P.; Peterson, G. An effective approach to decrease antipsychotic and benzodiazepine use in nursing homes: The REDUSE project. Int. Psychogeriatr. 2010, 22, 26–36. [Google Scholar] [CrossRef]
- Roberts, M.S.; Stokes, J.A.; King, M.A.; Lynne, T.A.; Purdie, D.M.; Glasziou, P.P.; Wilson, D.A.J.; McCarthy, S.T.; Brooks, G.E.; de Looze, F.J. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br. J. Clin. Pharmacol. 2001, 51, 257–265. [Google Scholar]
- Schmidt, I.K.; Claesson, C.B.; Westerholm, B.; Nilsson, L.G. Physician and staff assessments of drug interventions and outcomes in swedish nursing homes. Ann. Pharmacother. 1998, 32, 27–32. [Google Scholar] [CrossRef]
- Crotty, M.; Halbert, J.; Rowett, D.; Giles, L.; Birks, R.; Williams, H.; Whitehead, C. An outreach geriatric medication advisory service in residential aged care: A randomised controlled trial of case conferencing. Age Ageing 2004, 33, 612–617. [Google Scholar] [CrossRef]
- Opedal, K.; Schjott, J.; Eide, E. Use of hypnotics among patients in geriatric institutions. Int. J. Geriatr. Psychiatry 1998, 13, 846–851. [Google Scholar] [CrossRef]
- Eide, E.; Schjott, J. Assessing the effects of an intervention by a pharmacist on prescribing and administration of hypnotics in nursing homes. Pharm. World Sci. 2001, 23, 227–231. [Google Scholar] [CrossRef]
- Gallagher, R.M.; Gallagher, H.C. Improving the working relationship between doctors and pharmacists: Is inter-professional education the answer? Adv. Health Sci. Educ. 2012, 17, 247–257. [Google Scholar] [CrossRef]
- Soo, T.M.; Kljakovic, M.; Bishop, L.; Baxfield, N.; Dwan, K.; Toyne, H.; Zwikael, M.; Strang, J.; Bech, A. The benzodiazepine voluntary undertaking. A data audit and retrospective evaluation in the act. Available online: http://directionsact.com/pdf/drug_news/BenzoVolUndertaking.pdf (accessed on 24 June 2013).
- Midlov, P.; Bondesson, A.; Eriksson, T.; Nerbrand, C.; Hoglund, P. Effects of educational outreach visits on prescribing of benzodiazepines and antipsychotic drugs to elderly patients in primary health care in southern sweden. Fam. Pract. 2006, 23, 60–64. [Google Scholar] [CrossRef]
- Lerat, M.C.; Cabelguenne, D.; Lassia, J.; Meunier, F.; Zimmer, L. Impact of pharmacist and clinician dual intervention on prescribed benzodiazepines in prisoner patients: A retrospective study. Fundam. Clin. Pharmacol. 2010, 25, 762–767. [Google Scholar]
- Henman, M.; Vivero, L.; Gustaffson, A.; Mulvenna, K. Benzodiazepine Usage in the North Eastern Health Board Region of the Republic of Ireland. Available online: http://www.drugsandalcohol.ie/12699/ (accessed on 24 June 2013).
- Monane, M.; Matthias, D.M.; Nagle, B.A.; Kelly, M.A. Improving prescribing patterns for the elderly through an online drug utilization review intervention: A system linking the physician, pharmacist, and computer. JAMA 1998, 280, 1249–1252. [Google Scholar] [CrossRef]
- Smith, A.J.; Tett, S.E. An intervention to improve benzodiazepine use—a new approach. Fam. Pract. 2010, 27, 320–327. [Google Scholar] [CrossRef]
- Government of Ireland. Misuse of Drugs Act. Available online: http://www.irishstatutebook.ie/1984/en/act/pub/0018/index.html (accessed on 24 June 2013).
- Butler, S. The making of the methadone protocol: The Irish system? Drugs Educ. Prev. Pol. 2002, 9, 311–324. [Google Scholar] [CrossRef]
- Roberts, K. Revised drug misuse guidelines pharmacists must get more involved. Pharm. J. 1999, 263, 203–205. [Google Scholar]
- Chung, K. Benzodiazepine prescribing trend after its inclusion as a dangerous drug under the Hong Kong dangerous drugs ordinance. Hong Kong Med. J. 1997, 3, 16–20. [Google Scholar]
- Briesacher, B.A.; Soumerai, S.B.; Field, T.S.; Fouayzi, H.; Gurwitz, J.H. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch. Intern. Med. 2010, 170, 693–698. [Google Scholar] [CrossRef]
- Weintraub, M.; Singh, S.; Byrne, L.; Maharaj, K.; Guttmacher, L. Consequences of the 1989 New York State triplicate benzodiazepine prescription regulations. JAMA 1991, 266, 2392–2397. [Google Scholar] [CrossRef]
- De Gier, N.; Gorgels, W.; Lucassen, P.; Oude Voshaar, R.; Mulder, J.; Zitman, F. Discontinuation of long-term benzodiazepine use: 10-year follow-up. Fam. Pract. 2011, 28, 253–259. [Google Scholar] [CrossRef]
- Godfrey, C.; Heather, N.; Bowie, A.; Brodie, J.; Parrott, S.; Ashton, H.; McAvoy, B. Randomised controlled trial of two brief interventions against long-term benzodiazepine use: Cost-effectiveness. Addict. Res. Theory 2008, 16, 309–317. [Google Scholar] [CrossRef]
- Busto, U.; Sellers, E.M.; Naranjo, C.A.; Cappell, H.; Sanchez-Craig, M.; Sykora, K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. NEJM 1986, 315, 854–859. [Google Scholar] [CrossRef]
- Beier, M.T.; Martin, C.M.H. Gradual dose reduction and medication tapering: A clinical perspective. Consult. Pharm. 2007, 22, 628–644. [Google Scholar] [CrossRef]
- Denis, C.; Fatsväas, M.; Lavie, E.; Auriacombe, M. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst. Rev. 2006, 3, CD005194. [Google Scholar]
- Murphy, S.M.; Tyrer, P. A double-blind comparison of the effects of gradual withdrawal of lorazepam, diazepam and bromazepam in benzodiazepine dependence. Br. J. Psychiatry 1991, 158, 511–516. [Google Scholar] [CrossRef]
- Lemoine, P.; Ohayon, M.M. Is hypnotic withdrawal facilitated by the transitory use of a substitute drug? Prog. NeuroPsychopharmacol. Biol. Psychiatry 1997, 21, 111–124. [Google Scholar] [CrossRef]
- Cantopher, T.; Olivieri, S.; Cleave, N.; Edwards, J.G. Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. Br. J. Psychiatry 1990, 156, 406–411. [Google Scholar] [CrossRef]
- Schweizer, E.; Rickels, K.; Case, W.G.; Greenblatt, D.J. Carbamazepine treatment in patients discontinuing long-term benzodiazepine therapy. Effects on withdrawal severity and outcome. Arch. Gen. Psychiatry 1991, 48, 448–452. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallagher, H.C. Addressing the Issue of Chronic, Inappropriate Benzodiazepine Use: How Can Pharmacists Play a Role? Pharmacy 2013, 1, 65-93. https://doi.org/10.3390/pharmacy1020065
Gallagher HC. Addressing the Issue of Chronic, Inappropriate Benzodiazepine Use: How Can Pharmacists Play a Role? Pharmacy. 2013; 1(2):65-93. https://doi.org/10.3390/pharmacy1020065
Chicago/Turabian StyleGallagher, Helen C. 2013. "Addressing the Issue of Chronic, Inappropriate Benzodiazepine Use: How Can Pharmacists Play a Role?" Pharmacy 1, no. 2: 65-93. https://doi.org/10.3390/pharmacy1020065
APA StyleGallagher, H. C. (2013). Addressing the Issue of Chronic, Inappropriate Benzodiazepine Use: How Can Pharmacists Play a Role? Pharmacy, 1(2), 65-93. https://doi.org/10.3390/pharmacy1020065




