Reduction in Use of Risperidone for Dementia in Australia Following Changed Guidelines
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Changes in the Rate of Use of Risperidone in Veteran Residents of Aged Care Facilities (DVA Data)
2.2. Comparison Cohorts: Assessment of Changes in the Rate of Use of Risperidone in Community Dwelling Older Veterans, and the General Australian Population
2.3. Statistical Analysis of Changes in the Rate of Use of Risperidone
2.4. Assessment of Changes in the Duration of Use of Risperidone in the DVA Aged Care Cohort
3. Results
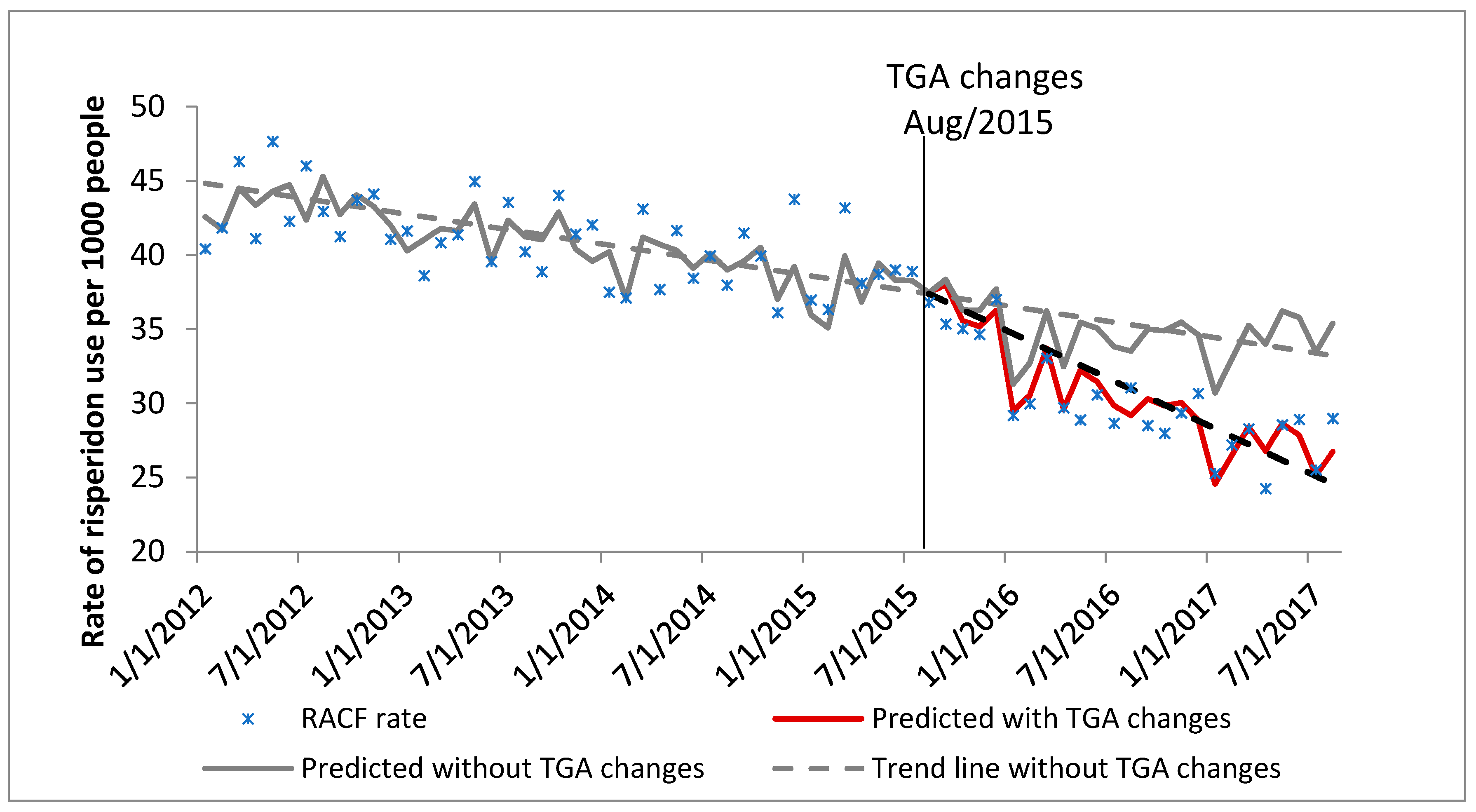
3.1. Changes in the Rate of Use of Risperidone
3.2. Changes in the Duration of Use of Risperidone in DVA Aged Care Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, L.; Hansnata, E.; La, H. Economic Cost of Dementia in Australia 2016–2056; National Centre for Social and Economic Modelling, Alzheimer’s Australia: Canberra, Australia, 2017. [Google Scholar]
- Selbæk, G.; Engedal, K.; Bergh, S. The Prevalence and Course of Neuropsychiatric Symptoms in Nursing Home Patients With Dementia: A Systematic Review. J. Am. Med. Dir. Assoc. 2013, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, R.B.; Zuidema, S.U.; De Jonghe, J.F.; Verhey, F.R.; Koopmans, R.T. Course of Neuropsychiatric Symptoms in Residents with Dementia in Nursing Homes Over 2-Year Period. Am. J. Geriatr. Psychiatry 2010, 18, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Liperoti, R.; Pedone, C.; Corsonello, A. Antipsychotics for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD). Curr. Neuropharmacol. 2008, 6, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-Y.; Gadzhanova, S.; Roughead, E.E.; Ward, M.B.; Pont, L.G. The use of antipsychotics among people treated with medications for dementia in residential aged care facilities. Int. Psychogeriatr. 2016, 28, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Welfare AIoHa. Dementia in Australia; AIHW: Canberra, Australia, 2012.
- Brodaty, H.; Draper, B.; Saab, D.; Low, L.-F.; Richards, V.; Paton, H.; Lie, D. Psychosis, depression and behavioural disturbances in Sydney nursing home residents: Prevalence and predictors. Int. J. Geriatr. Psychiatry 2001, 16, 504–512. [Google Scholar] [CrossRef]
- Yunusa, I.; Alsumali, A.; Garba, A.; Regestein, Q.; Eguale, T. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia A network meta-analysis. JAMA Netw. Open 2019, 2, e190828. [Google Scholar] [CrossRef] [PubMed]
- Pratt, N.; Ramsay, E.; Salter, A.; Pratt, D.N.; Roughead, E.E.; Ryan, P. Risk of Hospitalization for Hip Fracture and Pneumonia Associated with Antipsychotic Prescribing in the Elderly. Drug Saf. 2011, 34, 567–575. [Google Scholar] [CrossRef]
- Pratt, N.; Roughead, E.E.; Ryan, P.; Salter, A. Antipsychotics and the risk of death in the elderly: An instrumental variable analysis using two preference based instruments. Pharmacoepidemiol. Drug Saf. 2010, 19, 699–707. [Google Scholar] [CrossRef]
- Guideline Adaptation Committee. Clinical Practice Guidelines and Principles of Care for People with Dementia; NHMRC Guideline Adaptation Committee: Sydney, Australia, 2016.
- Snowdon, J.; Galanos, D.; Vaswani, D. Patterns of psychotropic medication use in nursing homes: Surveys in Sydney, allowing comparisons over time and between countries. Int. Psychogeriatr. 2011, 23, 1520–1525. [Google Scholar] [CrossRef]
- Westbury, J.; Jackson, S.; Peterson, G. Psycholeptic use in aged care homes in Tasmania, Australia. J. Clin. Pharm. Ther. 2010, 35, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health TGA. Risperidone and Risk of Cerebrovascular Adverse Events in Dementia Patients. Medicines Safety Update (Cited 7 April 2017); 2015. Available online: https://www.tga.gov.au/publication-issue/medicines-safety-update-volume-6-number-4-august-2015 (accessed on 22 July 2019).
- Magarey, J. TGA Restricts Dementia Drug Risperidone Following Stroke Link. The Australian, 12 August 2015. [Google Scholar]
- Prosser Scully, R. TGA Restricst Prescribing of Risperidone. Medical Observer, 4 August 2015. [Google Scholar]
- Ozturk, S. Tougher Rules on Use of Antipsychotic in Dementia. Australian Doctor, 4 August 2015. [Google Scholar]
- Veterans’ Medicines Advice and Therapeutics Education Services (MATES). Antipsychotic Use in BPSD: Limited Benefits, High Risks; Australian Government Department of Veterans’ Affairs: Canberra, Australia, 2016. Available online: https://www.veteransmates.net.au/documents/10184/23464/VeteransMates_TherapeuticBrief_Topic-1-2016_web_F3.pdf/f55314eb-c5fb-4c3b-9ead-3e45e3e4ad9b?version=1.0 (accessed on 22 July 2019).
- Australian Government Department of Veterans’ Affairs. Treatment Population Statistics; Quarterly Report—June 2017; DVA: Canberra, Australia, 2017. Available online: https://www.dva.gov.au/sites/default/files/files/publications/datastatistical/treatmentpop/TPopJun2017.pdf (accessed on 17 April 2019).
- Anatomical Therapeutic Chemical Classification Index with Defined Daily Doses; World Health Organisation Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2016.
- Ageing AGDoHa. Schedule of Pharmaceutical Benefits. PBS for Health Professionals; PBS: Arlington, TX, USA, 2016. [Google Scholar]
- Paige, E.; Kemp-Casey, A.; Korda, R.; Banks, E. Using Australian Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: Challenges and approaches. Public Health Res. Pract. 2015, 25, e2541546. [Google Scholar] [CrossRef] [PubMed]
- Australian Medicines Handbook; Australian Medicines Handbook Pty Ltd.: Adelaide, Australia, 2018.
- Gallini, A.; Andrieu, S.; Donohue, J.; Oumouhou, N.; Lapeyre-Mestre, M.; Gardette, V. Trends in use of antipsychotics in elderly patients with dementia: Impact of national safety warnings. Eur. Neuropsychopharmacol. 2014, 24, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.; Clark, S.A.; Reynish, E.L.; McCowan, C.; Morales, D.R. Differential Impact of Two Risk Communications on Antipsychotic Prescribing to People with Dementia in Scotland: Segmented Regression Time Series Analysis 2001–2011. PLoS ONE 2013, 8, e68976. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Fontana, A.; Giorgianni, F.; Pasqua, A.; Cricelli, C.; Spina, E.; Gambassi, G.; Ivanovic, J.; Ferrajolo, C.; Molokhia, M.; et al. The Effect of Safety Warnings on Antipsychotic Drug Prescribing in Elderly Persons with Dementia in the United Kingdom and Italy: A Population-Based Study. CNS Drugs 2016, 30, 1097–1109. [Google Scholar] [CrossRef]
- Valiyeva, E.; Herrmann, N.; Rochon, P.A.; Gill, S.S.; Anderson, G.M. Effect of regulatory warnings on antipsychotic prescription rates among elderly patients with dementia: A population-based time-series analysis. Can. Med. Assoc. J. 2008, 179, 438–446. [Google Scholar] [CrossRef]
- Roughead, E.; Gilbert, A. Development, delivery and evaluation of implementation programmes. In Drug Utilization Research; Wiley: Hoboken, NJ, USA, 2016; pp. 468–476. [Google Scholar]
- Mittal, V.; Kurup, L.; Williamson, D.; Muralee, S.; Tampi, R.R. Review: Risk of Cerebrovascular Adverse Events and Death in Elderly Patients With Dementia When Treated With Antipsychotic Medications: A Literature Review of Evidence. Am. J. Alzheimers Dis. Other Dement. 2011, 26, 10–28. [Google Scholar] [CrossRef]
- Sink, K.M.; Holden, K.F.; Yaffe, K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA 2005, 293, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Margallo-Lana, M.; O’Brien, J.T.; James, I.; Howard, R.; Fossey, J. Quality of life for people with dementia living in residential and nursing home care: The impact of performance on activities of daily living, behavioral and psychological symptoms, language skills, and psychotropic drugs. Int. Psychogeriatr. 2009, 21, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.A.; Dennehy, R.; Sinnott, C.; Browne, J.; Byrne, S.; McSharry, J.; Coughlan, E.; Timmons, S. Influences on Decision-Making Regarding Antipsychotic Prescribing in Nursing Home Residents with Dementia: A Systematic Review and Synthesis of Qualitative Evidence. J. Am. Med. Dir. Assoc. 2017, 18, 897.e1–897.e12. [Google Scholar] [CrossRef] [PubMed]
- Kalisch Ellett, L.M.; Pratt, N.; Sluggett, J.K.; Ramsay, E.N. Sustaining practice change in health care: The impact of a national quality improvement program on the uptake of collaborative medicines reviews. J. Pharm. Pract. Res. 2018, 48, 222–230. [Google Scholar] [CrossRef]



| Veterans Aged 65 and over Living in Aged Care * | Veterans Aged 65 and over Living in the Community * | General Australian Cohort Aged 65 and over # | ||
|---|---|---|---|---|
| Study start date: 1 January 2012 | Total N | 28,949 | 160,282 | 411,546 |
| N Men (%) | 10,734 (37.1%) | 85,089 (53.1%) | 195,305 (47.5%) | |
| Median age (IQR) | 89 (86–91) | 85 (77–88) | 74 (69–82) | |
| Date of intervention: 1 August 2015 | Total N | 23,141 | 136,196 | 490,757 |
| N Men (%) | 7283 (31.5%) | 77,759 (57.1%) | 235,869 (48.1%) | |
| Median age (IQR) | 91 (88–93) | 83 (70–90) | 75 (69–83) | |
| Study end date: 31 August 2017 | Total N | 19,350 | 122,072 | 544,858 |
| N Men (%) | 5549 (28.7%) | 72,533 (59.4%) | 263,376 (48.3%) | |
| Median age (IQR) | 92 (89–94) | 80 (70–90) | 75 (69–83) |
| Month-to-Month Change (%) in the Trends | Relative Effect (95% CI) at 24 Months (August 2017) Post the TGA Changes | ||
|---|---|---|---|
| Trend without TGA Changes | Trend with TGA Changes | Trend with Versus Trend without TGA Changes | |
| Veterans aged 65 and over living in aged care * | −0.49% | −1.74% | −26.07% (−10.89% to −41.26%) |
| Veterans aged 65 and over living in the community * | 0.45% | −0.50% | −20.44% (−7.98% to −32.90%) |
| General Australian cohort aged 65 and over # | −0.10% | −1.49% | −28.42% (−11.55% to −45.30%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalisch Ellett, L.M.; Moffat, A.K.; Gadzhanova, S.; Pratt, N.L.; Apajee, J.; Woodward, M.; Roughead, E.E. Reduction in Use of Risperidone for Dementia in Australia Following Changed Guidelines. Pharmacy 2019, 7, 100. https://doi.org/10.3390/pharmacy7030100
Kalisch Ellett LM, Moffat AK, Gadzhanova S, Pratt NL, Apajee J, Woodward M, Roughead EE. Reduction in Use of Risperidone for Dementia in Australia Following Changed Guidelines. Pharmacy. 2019; 7(3):100. https://doi.org/10.3390/pharmacy7030100
Chicago/Turabian StyleKalisch Ellett, Lisa M, Anna K Moffat, Svetla Gadzhanova, Nicole L Pratt, Jemisha Apajee, Michael Woodward, and Elizabeth E Roughead. 2019. "Reduction in Use of Risperidone for Dementia in Australia Following Changed Guidelines" Pharmacy 7, no. 3: 100. https://doi.org/10.3390/pharmacy7030100
APA StyleKalisch Ellett, L. M., Moffat, A. K., Gadzhanova, S., Pratt, N. L., Apajee, J., Woodward, M., & Roughead, E. E. (2019). Reduction in Use of Risperidone for Dementia in Australia Following Changed Guidelines. Pharmacy, 7(3), 100. https://doi.org/10.3390/pharmacy7030100





