Abstract
Macrolides are antimicrobial agents that can be used to treat a variety of infections. Allergic reactions to macrolides occur infrequently but can include minor to severe cutaneous reactions as well as systemic life-threatening reactions such as anaphylaxis. Most reports of allergic reactions occurred in patients without prior exposure to a macrolide. Cross-reactivity among macrolides may occur due to the similarities in their chemical structures; however, some published literature indicates that some patients can tolerate a different macrolide. Most published reports detailed an allergic reaction to erythromycin. Desensitization protocols to clarithromycin and azithromycin have been described in the literature. The purpose of this article is to summarize macrolide-associated allergic reactions reported in published literature. An extensive literature search was conducted to identify publications linking macrolides to hypersensitivity reactions.
Keywords:
macrolides; allergy; azithromycin; erythromycin; clarithromycin; fidaxomicin; desensitization 1. Introduction
One of the most common causes of medication allergies among adults and children is antibiotics [1]. These allergic reactions can range from immediate to non-immediate (delayed) hypersensitivity reactions. Immediate reactions are typically IgE-mediated and can cause clinical manifestations that include urticaria, angioedema, and anaphylaxis [1,2]. Non-immediate reactions are frequently T-cell mediated and can lead to various degrees of cutaneous symptoms in patients [1,2]. Although many of the reported antibiotic allergies are from the beta-lactam class, cases of allergic reactions to macrolide antibiotics have been documented [1,2,3,4]. However, hypersensitivity reactions resulting from macrolide use occurs infrequently (0.4–3%) [4]. Since macrolide allergies are uncommon, there is a lack of recent data reviewing macrolide allergies and management of those allergies. This paper will review the medicinal chemistry, indications, reported allergic reactions, and desensitization protocols associated with macrolide antimicrobials.
2. Medicinal Chemistry
2.1. Macrolide Structure
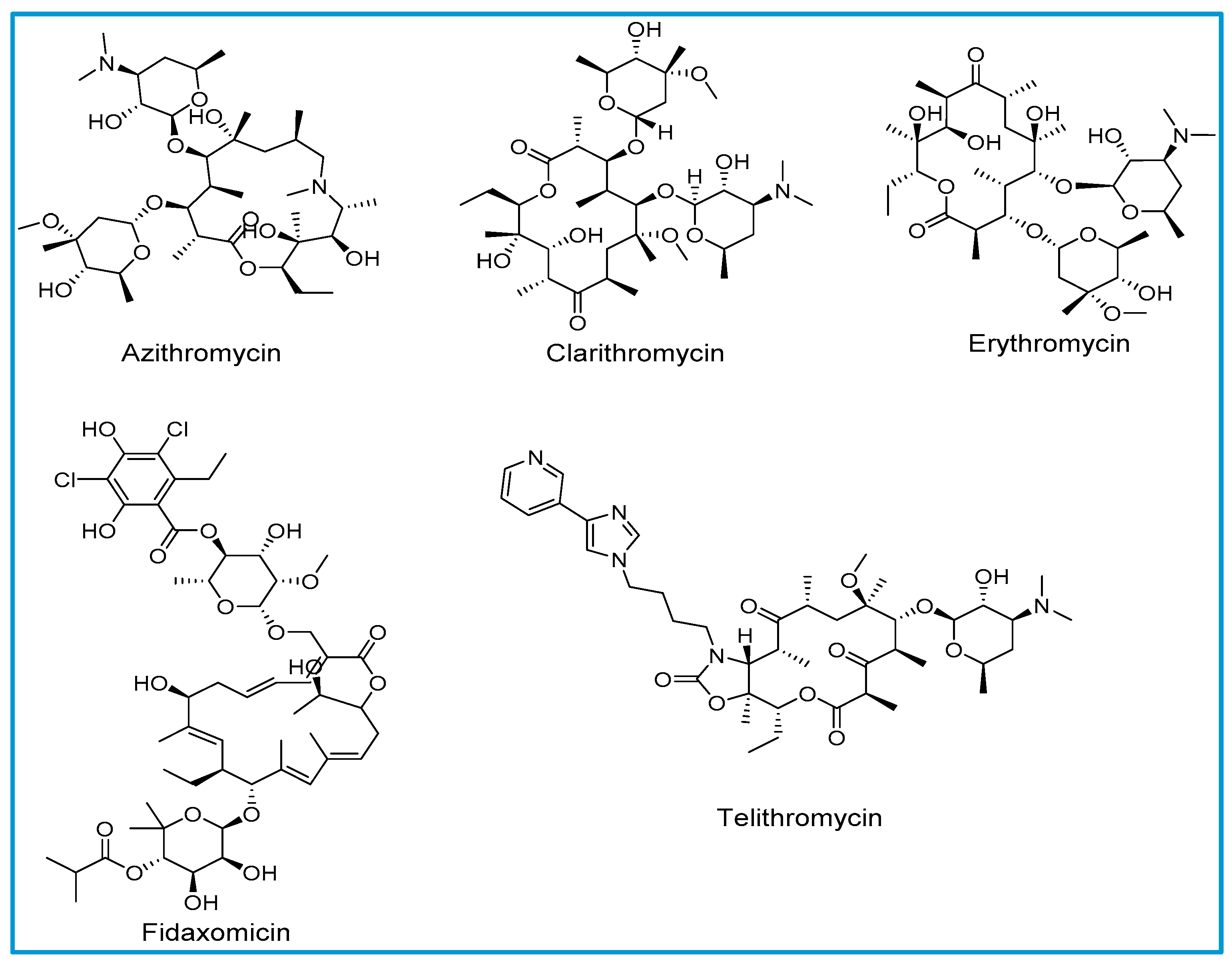
The structure of macrolides consists of a large lactone ring that varies in size from 12 to 18 atoms. Sugar molecules are attached to the lactone ring with glycosidic bonds. Macrolide antibiotics are classified based on the number of atoms in the lactone ring; 14-membered lactones (erythromycin and clarithromycin), 15-membered lactones (azithromycin), ketolide (telithromycin) and 18-membered lactone (fidaxomicin). All clinically available macrolides, with the exception of erythromycin and fidaxomicin, are either synthetically or semi-synthetically generated. Natural macrolides have instability in the gastric environment, resulting in undesired pharmacokinetic properties, such as incomplete absorption resulting in decreased bioavailability [5].
2.2. Mechanism of Action
All macrolides except for fidaxomicin block bacterial protein synthesis by binding reversibly to the 23S ribosomal RNA (rRNA) in the 50S-subunit of prokaryotic ribosomes [6]. Fidaxomicin exerts its bactericidal effects by inhibiting bacterial RNA polymerase at transcription initiation by binding to the DNA template-RNA polymerase complex [7].
2.3. Structural Aspects behind Cross-Reactivity
Allergic reactions to macrolides are relatively less common compared to other classes of antibiotics (Figure 1) [4]. Macrolides with a 14- membered lactone ring such as erythromycin and clarithromycin have been reported to express cross-reactivity in single case reports. The exact mechanism of hypersensitivity due to macrolides is not clearly understood [8]. Azithromycin is a semisynthetic derivative of erythromycin with a 15-membered lactone ring in its structure. Owing to azithromycin’s structural similarity to erythromycin, cross-reactivity with erythromycin has also been reported. There is a lack of scientific evidence to support cross sensitization between various macrolide derivatives [8].

Figure 1.
Chemical structures of macrolides.
3. Place in Therapy
Macrolides are a well-established class of antibiotics. They exhibit bacteriostatic activity against a wide range of gram-positive, gram-negative, and atypical bacteria [9]. Erythromycin, the macrolide with the longest use in practice, has a few remaining indications as the drug of choice given the rise in antibiotic resistance and the availability of more effective and safer antibiotics. Azithromycin and clarithromycin have largely replaced erythromycin in clinical practice because of their broader spectrum of activity, better pharmacokinetics profile, and fewer gastrointestinal adverse effects [9]. In addition, azithromycin is associated with fewer drug–drug interactions than erythromycin and clarithromycin [9].
Macrolides are the drugs of choice for the treatment of various atypical bacteria [9]. Erythromycin is the drug of choice for the treatment of diphtheria caused by Corynebacterium diphtheriae, although antitoxin is the primary treatment. Erythromycin is also the drug of choice for the treatment of infants with pneumonia caused by Chlamydia trachomatis. Azithromycin is the drug of choice for the treatment of trachoma, urethritis, and cervicitis caused by Chlamydia trachomatis and chancroid caused by Haemophilus ducreyi. Azithromycin with or without rifampin is also one of the regimens of choice for the treatment of Legionella pneumophila, which can cause serious atypical pneumonia. Clarithromycin is the drug of choice for the treatment of Helicobacter pylori as part of a combination regimen with amoxicillin and omeprazole. Clarithromycin in addition to amikacin is the regimen of choice for the treatment of infections caused by Mycobacterium fortuitum and Mycobacterium chelonae. Erythromycin and azithromycin are the drugs of choice for the treatment of infections caused by Bartonella henselae (cat scratch fever), Bartonella quintana (trench fever), Campylobacter jejuni (diarrhea), Chlamydia trachomatis (conjunctivitis and urethritis), and Ureaplasma urealyticum (urethritis). Azithromycin and clarithromycin are the drugs of choice for the treatment of infections caused by Mycobacterium avium complex as part of a combination regimen with ethambutol and rifabutin or monotherapy for primary or secondary prophylaxis. Lastly, all three macrolides are the drugs of choice for the treatment of pertussis (whooping cough) caused by Bordetella pertussis and atypical pneumonia caused by Chlamydophila pneumonia and Mycoplasma pneumoniae.
Many clinical practice guidelines recommend the use of macrolides for the empiric treatment of respiratory tract infections although antibiotic resistance among Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis is on the rise [10,11,12,13]. Current guidelines recommend the empiric use of macrolides as the drugs of choice for the treatment of atypical pneumonia in children and community-acquired pneumonia in adults as monotherapy in the outpatient setting and as combination therapy with a beta-lactam in the inpatient setting [10,11]. Current guidelines recommend the empiric use of macrolides as alternative choices for the treatment of acute otitis media and streptococcal pharyngitis [12,13].
Macrolides can also be used as alternative options for the treatment of various infections in patients who are not able to take the drugs of choice because of allergic reaction or intolerance [9]. For example, erythromycin can be used as an alternative to cephamycins as part of a combination regimen to prevent infections associated with colorectal surgeries or as an alternative to penicillins for the prevention of rheumatic fever. In rare circumstances, erythromycin can be used as an alternative to ciprofloxacin, doxycycline, and penicillins for the treatment of anthrax caused by Bacillus anthracis or as an alternative to tetracyclines for the treatment of infections caused by Lymphogranuloma venereum. More commonly, erythromycin can be used as an alternative to tetracyclines for the treatment of acne vulgaris. Azithromycin can be used as an alternative to ceftriaxone or fluoroquinolones for the treatment of typhoid fever caused by Salmonella typhi or as an alternative to fluoroquinolones for the treatment of diarrhea caused by Shigella dysenteriae. Azithromycin can also be used as an alternative to doxycycline and penicllins for the treatment of Lyme disease caused by Borrelia burgdorferi or as an alternative to clindamycin and quinine for the treatment of babesiois caused by Babesia microti. Azithromycin and clarithromycin can be used as an alternative to trimethoprim/sulfamethoxazole for the treatment of respiratory infections caused by Haemophilus influenzae. Lastly, all three macrolides can be used as an alternative to penicillins for the treatment of respiratory and skin and soft tissue infections caused by groups A, C, and G Streptococcus, Streptococcus pneumoniae, and Moraxella catarrhalis.
Fidaxomicin is a unique antibiotic, and represents the latest addition to the macrolides [14]. It exhibits bactericidal activity against Clostridoides difficile. Fidaxomicin is not systemically absorbed, is well tolerated, and is not associated with any known drug interactions. Clinical trials have shown that fidaxomicin is non-inferior to vancomycin for the treatment of Clostridioides difficile infection (CDI) and is associated with lower recurrence rates. Current CDI guidelines recommend the use of fidaxomicin for the treatment of initial severe, non-severe, and recurrent episodes [15].
4. Published Allergic Reactions
To gather relevant information, a literature search was performed using the PubMed, EBSCOhost, and Google Scholar electronic databases for articles published up to 17 May 2019, with restrictions for English language and human subjects. Search terms used to identify the included articles were macrolides, azithromycin, clarithromycin, erythromycin, fidaxomicin, hypersensitivity, allergy, rash, toxic epidermal necrosis, Stevens Johnson Syndrome, fixed drug eruption, maculopapular rash, exanthema, and desensitization. Articles about macrolides used as immunosuppressants (e.g., tacrolimus, everolimus, pimecrolimus, and sirolimus) and uncommonly or commercially unavailable were excluded (e.g., kitasamycin, josamycine midecamycin, roxithromycin, spiramycin, telithromycin, and troleandomycin). References of publications for which the full text was retrieved were also reviewed for additional literature sources.
In general, allergic reactions to macrolides reported in the literature are rare. Macrolides are available in a variety of dosage forms, and of those, topical, oral, intravenous, and ophthalmic formulations have been reported to cause an allergic reaction. The initial search for articles regarding macrolide hypersensitivity yielded 1895 citations. Following completion of all search strategies and terms a total of 120 reports were included and summarized in this review. The types of reactions for erythromycin, clarithromycin, azithromycin, and fidaxomicin are summarized in Table 1, Table 2, Table 3 and Table 4. The included reports were published between 1958 and 2018, with reports from 27 different countries. Reported reactions occurred in a variety of patient populations, such as pediatrics (n = 50), adults (n = 105), and unknown (n = 20). Many providers tested patients to confirm the hypersensitivity (n = 79). Several of the reactions involved patients who had received a prior macrolide (n = 43) and of those 23 patients with repeated reactions. Repeated occupational exposures led to 10 subjects with cutaneous adverse reactions to azithromycin [16,17,18].

Table 1.
Summary of published literature reporting erythromycin hypersensitivity.

Table 2.
Summary of published literature reporting clarithromycin hypersensitivity.

Table 3.
Summary of published literature reporting azithromycin hypersensitivity.

Table 4.
Summary of published literature reporting fidaxomycin hypersensitivity.
The breakdown of studies included 88 case reports, 12 case series, 2 cross-sectional, 1 double-blind, placebo-controlled, 5 prospective, and 12 retrospective. There were 58 publications describing erythromycin-associated allergic reactions [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. There were 33 publications describing clarithromycin as a culprit for allergic reactions [72,73,75,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. There were 31 published reports describing azithromycin associated allergic reactions [16,17,18,95,104,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131]. Uniquely, the ophthalmic formulation of azithromycin was associated with contact dermatitis [119,123]. There was a single publication dedicated to a case series describing 12 patients who had hypersensitivity reactions to fidaxomicin [132]. Maculopapular exanthema eruptions developed in four subjects suffering from mononucleosis who were also on macrolide therapy [43,109,111,122]. Five cases involved the development of contact dermatitis with topical erythromycin [26,35,51,56,58]. Four cases resulted in a fatality secondary to the severe allergic reaction [19,33,88,91]. Fourteen patients were reported to have anaphylactic reactions to a macrolide [19,23,59,66,73,74,81,98,100,108,121,132]. Drug reaction with eosinophilia and systemic symptoms syndrome was reported in four patients who received azithromycin and one patient who received clarithromycin [105,118,120,124,129]. Erythromycin (n = 1), clarithromycin (n = 2), and azithromycin (n = 1) have been implicated in leukocytoclastic vasculitis [60,79,84,113]. Fourteen publications implicated a macrolide as the cause of an allergic reaction with subjects who received concomitant antimicrobials [25,41,44,45,46,58,76,78,90,101,102,105,119,131]. The most common concomitant agents were an aminoglycoside (n = 3), a beta-lactam (n = 3), and a sulfa (n = 4) [41,44,45,46,78,90,101,102,119,131]. It is possible that one of the concomitant antimicrobials could have elicited the allergic reaction. Two publications excluded a concomitant antimicrobial as the cause with allergy testing [45,58].
Macrolide allergies are rare and available desensitization protocols are restricted to case reports, which all demonstrated success [74,100,102]. Three cases involved successful desensitization with clarithromycin, and one of the patients also completed a desensitization for azithromycin desensitization. Four desensitization protocols were identified in the literature: two involving clarithromycin in an adult patient, one for clarithromycin in a pediatric patient, and one for azithromycin in a pediatric patient (Appendix A, Table A1, Table A2, Table A3 and Table A4) [74,100,102]. Holmes et al. reported a case of a 68-year-old female with a history of anaphylaxis with erythromycin and bronchospasms with roxithromycin who underwent oral clarithromycin desensitization for use in a 18 month treatment course for a skin infection caused by Mycobacterium chelonae (Appendix A, Table A1) [74]. Swamy et al. reported a case of a 68-year-old female with a history of anaphylaxis with azithromycin and urticaria and bronchospasms with clarithromycin who underwent oral clarithromycin desensitization for use in a 3 month treatment course for an infection caused by Mycobacterium intracellularae (Appendix A, Table A2) [100]. Repeated intradermal skin testing with azithromycin 1 h after desensitization demonstrated a similar reaction seen at baseline. Six weeks after desensitization, azithromycin and clarithromycin skin prick tests were negative and intradermal skin tests were equivocal to the reactions seen at baseline. Petitto et al. described a case of an 11-year-old female with a history of diffuse urticaria with clarithromycin who underwent successful macrolide desensitization for use in osteomyelitis caused by Mycobacterium fortuitum (Appendix A, Table A3 and Table A4) [102]. Initially, this patient completed an azithromycin desensitization protocol without complication; however, 24 h later, she developed a generalized urticarial rash within 75 min of a treatment dose. One week later, this patient underwent oral clarithromycin desensitization and all subsequent treatment doses were tolerated.
5. Conclusions
Macrolides are infrequently reported to cause various types of allergic reactions, with cutaneous reactions being the most common. Macrolides are similar in chemical structure, and limited reports have demonstrated cross-reactivity. Strategies to overcome hypersensitivity reactions, such as desensitization, or allergy testing for cross-reactivity to another macrolide have been utilized with successful outcomes. Consequently, if a patient has a severe hypersensitivity reaction to a macrolide, then the benefit versus the risk must be evaluated for using an agent in this class. In clinical practice, it may be more convenient and safer to change to an alternative class of medications if the option is available. However, if a macrolide must be used in a patient with a confirmed history of a severe allergic reaction, then allergy testing should be employed to investigate if the patient reacts to other agents in the macrolide class. If so, a desensitization may be conducted while the patient is monitored closely for signs and symptoms of an allergic reaction.
Author Contributions
Conceptualization, K.M.S., J.C., E.B.C.; methodology, K.M.S., J.C.C., E.B.C.; writing—original draft preparation, K.M.S., J.C.C., E.B.C., and S.V.G.; writing—review and editing, K.M.S., J.C.C., E.B.C., and S.V.G.
Funding
This research received no external funding.
Conflicts of Interest
KMS and SVG declare no conflicts of interest. EBC serves on the speakers’ bureau for Merck & Co, Inc. JCCCho serves on the speakers’ bureau for Allergan, Inc.
Appendix A

Table A1.
Oral clarithromycin desensitization protocol in an adult [74] *.
Table A1.
Oral clarithromycin desensitization protocol in an adult [74] *.
| Dose No. | Concentration (mg/mL) | Dose | |
|---|---|---|---|
| mL | mg | ||
| 1. | 0.05 | 0.1 | 0.005 |
| 2. | 0.05 | 0.2 | 0.010 |
| 3. | 0.05 | 0.4 | 0.020 |
| 4. | 0.05 | 1 | 0.050 |
| 5. | 0.05 | 2 | 0.100 |
| 6. | 0.05 | 4 | 0.200 |
| 7. | 0.50 | 0.8 | 0.400 |
| 8. | 0.50 | 1.6 | 0.800 |
| 9. | 0.50 | 3.2 | 1.6 |
| 10. | 0.50 | 6.4 | 3.2 |
| 11. | 5 | 1.2 | 6 |
| 12. | 5 | 2.4 | 12 |
| 13. | 5 | 4.8 | 24 |
| 14. | 50 | 1 | 50 |
| 15. | 50 | 2 | 100 |
| 16. | 50 | 4 | 200 |
| 17. | 50 | 8 | 400 |
| 18. | 50 | 10 | 500 |
| Cumulative dose | 1298.4 | ||
Abbreviations: mg = milligrams; mL = milliliters; no= number. * Serial 10-fold dilutions of a clarithromycin suspension of 125 mg/5 mL (25 mg/mL) were performed to make clarithromycin solutions at 2.5, 0.25, and 0.025 mg/mL. Each dose was administered in 15-min intervals over 4.5 h with close monitoring on the intensive care unit (for 36 h).

Table A2.
Oral clarithromycin desensitization protocol in an adult [100] *.
Table A2.
Oral clarithromycin desensitization protocol in an adult [100] *.
| Dose No. | Concentration (mg/mL) | Dose | |
|---|---|---|---|
| mL | mg | ||
| 1. | 0.025 | 1.25 | 0.030 |
| 2. | 0.025 | 2.50 | 0.060 |
| 3. | 0.025 | 5 | 0.125 |
| 4. | 0.250 | 1 | 0.250 |
| 5. | 0.250 | 2 | 0.5 |
| 6. | 0.250 | 4 | 1 |
| 7. | 2.5 | 0.8 | 2 |
| 8. | 2.5 | 1.6 | 4 |
| 9. | 2.5 | 3.2 | 8 |
| 10. | 2.5 | 6.4 | 16 |
| 11. | 25 | 1.3 | 32 |
| 12. | 25 | 2.5 | 64 |
| 13. | 25 | 5 | 125 |
| 14. | 25 | 10 | 250 |
| Cumulative dose | 503 | ||
Abbreviations: mg = milligrams; mL = milliliters; no = number. * Serial 10-fold dilutions of a clarithromycin suspension of 125 mg/5 mL (25 mg/mL) were performed to make clarithromycin solutions at 2.5, 0.25, and 0.025 mg/mL. Each dose was administered in 15-min intervals over 3.5 h.

Table A3.
Azithromycin oral desensitization protocol in an adolescent [102].
Table A3.
Azithromycin oral desensitization protocol in an adolescent [102].
| Dose No. | Concentration (mg/mL) | Dose | |
|---|---|---|---|
| mL | mg | ||
| 1. | 0.025 | 0.6 | 0.030 |
| 2. | 0.025 | 1.2 | 0.060 |
| 3. | 0.025 | 2.5 | 0.125 |
| 4. | 0.250 | 5 | 0.250 |
| 5. | 0.250 | 1 | 0.500 |
| 6. | 0.250 | 2 | 1 |
| 7. | 2.5 | 4 | 2 |
| 8. | 2.5 | 0.8 | 4 |
| 9. | 2.5 | 1.6 | 8 |
| 10. | 2.5 | 3.2 | 16 |
| 11. | 25 | 6.4 | 32 |
| 12. | 25 | 2.5 | 64 |
| 13. | 25 | 2.5 | 125 |
| Cumulative dose | 253 | ||

Table A4.
Clarithromycin oral desensitization protocol in an adolescent [102].
Table A4.
Clarithromycin oral desensitization protocol in an adolescent [102].
| Dose No. | Concentration (mg/mL) | Dose | |
|---|---|---|---|
| mL | mg | ||
| 1. | 0.05 | 0.60 | 0.030 |
| 2. | 0.05 | 1.20 | 0.060 |
| 3. | 0.05 | 2.50 | 0.125 |
| 4. | 0.05 | 5 | 0.25 |
| 5. | 0.50 | 1 | 0.50 |
| 6. | 0.50 | 2 | 1 |
| 7. | 0.50 | 4 | 2 |
| 8. | 5 | 0.8 | 4 |
| 9. | 5 | 1.6 | 8 |
| 10. | 5 | 3.2 | 16 |
| 11. | 5 | 6.4 | 32 |
| 12. | 50 | 2.5 | 125 |
| 13. | 50 | 2.5 | 125 |
| Cumulative dose | 314 | ||
References
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Sanchez-Borges, M.; Thong, B.; Blanca, M.; Ensina, L.F.; Gonzalez-Diaz, S.; Greenberger, P.A.; Jares, E.; Jee, Y.K.; Kase-Tanno, L.; Khan, D.; et al. Hypersensitivity reactions to non beta-lactam antimicrobial agents, a statement of the WAO special committee on drug allergy. World Allergy Organ J. 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Thong, B.Y. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. 2010, 2, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.; Demoly, P. Macrolides allergy. Curr. Pharm. Des. 2008, 14, 2840–2862. [Google Scholar] [CrossRef] [PubMed]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Brisson-Noel, A.; Trieu-Cuot, P.; Courvalin, P. Mechanism of action of spiramycin and other macrolides. J. Antimicrob. Chemother. 1988, 22 (Suppl. B), 13–23. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Walkty, A.J.; Karlowsky, J.A. Fidaxomicin: A novel agent for the treatment of Clostridium difficile infection. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Caubet, J.C. Antibiotic allergies in children and adults: From clinical symptoms to skin testing diagnosis. J. Allergy Clin. Immunol. Pract. 2014, 2, 3–12. [Google Scholar] [CrossRef]
- Sivapalasingam, S.; Steigbigel, N.H. Macrolides. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Macrolides, Clindamycin, and Ketolides; Churchill Livingstone/Elsevier: Philadelphia, PA, USA, 2010; Available online: http://web.a.ebscohost.com.ezproxy.lib.usf.edu/ehost/detail/detail?vid=0&sid=ab19e82b-d5a8-4468-ab6a-243ed20974fa%40sdc-v-sessmgr04&bdata=JnNpdGU9ZWhvc3QtbGl2ZQ%3d%3d#AN=458761&db=nlebk (accessed on 29 May 2019).
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H., Jr.; Moore, M.R.; et al. Pediatric Infectious Diseases, S. the Infectious Diseases Society of, A. Executive summary: The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, 617–630. [Google Scholar]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), 27–72. [Google Scholar] [CrossRef]
- Lieberthal, A.S.; Carroll, A.E.; Chonmaitree, T.; Ganiats, T.G.; Hoberman, A.; Jackson, M.A.; Joffe, M.D.; Miller, D.T.; Rosenfeld, R.M.; Sevilla, X.D.; et al. The diagnosis and management of acute otitis media. Pediatrics 2013, 131, e964–e999. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Chahine, E.B.; Sucher, A.J.; Mantei, K. Fidaxomicin: A novel macrolide antibiotic for Clostridium difficile infection. Consult. Pharm. 2014, 29, 614–624. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mimesh, S.; Pratt, M. Occupational airborne allergic contact dermatitis from azithromycin. Contact Dermat. 2004, 51, 151. [Google Scholar] [CrossRef] [PubMed]
- Milkovic-Kraus, S.; Macan, J.; Kanceljak-Macan, B. Occupational allergic contact dermatitis from azithromycin in pharmaceutical workers: A case series. Contact Dermat. 2007, 56, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lerma, I.; Romaguera, C.; Vilaplana, J. Occupational airborne contact dermatitis from azithromycin. Clin. Exp. Derm. 2009, 34, e358–e359. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.G. Anaphylactic death from erythromycin. Calif. Med. 1958, 89, 279–280. [Google Scholar]
- Prasad, A.S. Severe urticaria following erythromycin therapy. Acta Med. Iran 1960, 3, 9–11. [Google Scholar] [CrossRef]
- Crawford, L.V.; Roane, J. Use of erythromycin ethyl succinate in allergic children. Ann. Allergy 1969, 27, 18–22. [Google Scholar]
- Grainger, G.J. Erythromycin estolate in general practice. A comparative trial of two dosage forms. Practitioner 1969, 202, 84–86. [Google Scholar] [PubMed]
- Abramov, L.A.; Yust, I.C.; Fierstater, E.M.; Vardinon, N.E. Acute Respiratory Distress Caused by Erythromycin Hypersensitivity. Arch. Intern. Med. 1972, 138, 1156–1158. [Google Scholar] [CrossRef]
- Handa, S.P. The Schonlein-Henoch syndrome: Glomerulonephritis following erythromycin. South Med. J 1972, 65, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.P.; Singh, G. Bullous fixed drug eruption presumably due to erythromycin. Dermatologica 1976, 152, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Van Ketel, W.G. Immediate-and delayed-type allergy to erythromycin. Contact Dermat. 1976, 2, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Cooksley, W.G.; Powell, L.W. Erythromycin jaundice: Diagnosis by an in vitro challenge test. Aust. N. Z. J. Med. 1977, 7, 291–293. [Google Scholar] [CrossRef]
- Lloyd-Still, J.D.; Sherman, J.O.; Boggs, J.; Demers, L.M. Erythromycin estolate hepatotoxicity. Am J Dis. Child 1978, 132, 320. [Google Scholar] [CrossRef]
- Kirby, B.D.; Snyder, K.M.; Meyer, R.D.; Finegold, S.M. Legionnaires’ disease: Clinical features of 24 cases. Ann. Intern Med. 1978, 89, 297–309. [Google Scholar] [CrossRef]
- Harris, R.J.; Harris, R.L. Multiple antibiotic allergies. J. Am. Dent. Assoc. 1978, 97, 994–995. [Google Scholar] [CrossRef]
- Zafrani, E.S.; Ishak, K.G.; Rudzki, C. Cholestatic and hepatocellular injury associated with erythromycin esters: Report of nine cases. Dig Dis. Sci. 1979, 24, 385–396. [Google Scholar] [CrossRef]
- Hartigan, D.A.; Toma, S.; Listgarten, C. Legionnaires’ disease caused by Legionella pneumophila serogroup 3. Lancet 1979, 2, 411. [Google Scholar] [CrossRef]
- Schonheyder, H. Stevens-Johnson syndrome associated with intrahepatic cholestasis and respiratory disease: A case report. Acta Derm. Venereol. 1981, 61, 171–173. [Google Scholar] [PubMed]
- Keeffe, E.B.; Reis, T.C.; Berland, J.E. Hepatotoxicity to both erythromycin estolate and erythromycin ethylsuccinate. Dig. Dis. Sci. 1982, 27, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.; Campolmo, P.; Spallanzani, P.; Sertoli, A. Delayed hypersensitivity to erythromycin. Contact Dermat. 1982, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.; Gura, V.; Boner, G.; Ben-Bassat, M.; Livni, E. Interstitial nephritis with acute renal failure after erythromycin. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 938–939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Furniss, L.D. Rash due to erythromycin. Drug Intell. Clin. Pharm. 1983, 17, 631. [Google Scholar]
- Diehl, A.M.; Latham, P.; Boitnott, J.K.; Mann, J.; Maddrey, W.C. Cholestatic hepatitis from erythromycin ethylsuccinate. Report of two cases. Am. J. Med. 1984, 76, 931–934. [Google Scholar] [CrossRef]
- Pigatto, P.D.; Riboldi, A.; Riva, F.; Altomare, G.F. Fixed drug eruption to erythromycin. Acta Derm. Venereol. 1984, 64, 272–273. [Google Scholar]
- Lund Kofoed, M.; Oxholm, A. Toxic epidermal necrolysis due to erythromycin. Contact Dermat. 1985, 13, 273. [Google Scholar] [CrossRef]
- Singer, D.R.; Simpson, J.G.; Catto, G.R.; Johnston, A.W. Drug hypersensitivity causing granulomatous interstitial nephritis. Am. J. Kidney Dis. 1988, 11, 357–359. [Google Scholar] [CrossRef]
- Rigauts, H.D.; Selleslag, D.L.; Van Eyken, P.L.; Van Damme, B.J.; Fevery, J.M.; Marchal, G.J. Erythromycin-induced hepatitis: Simulator of malignancy. Radiology 1988, 169, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, N.; Mallik, L.J.; Williams, J.G. Erythromycin rash in glandular fever. Br. J. Clin. Pract. 1989, 43, 464–465. [Google Scholar] [PubMed]
- J.acqz-Aigrain, E.; Guillonneau, M.; Brun, P.; Broyard, A.; Bechtel, Y. Toxic epidermal necrolysis due to paediatric erythromycin/sulfisoxazole combination. Lancet 1990, 336, 1010–1011. [Google Scholar] [CrossRef]
- Florido Lopez, J.F.; Lopez Serrano, M.C.; Belchi Hernandez, J.; Estrada Rodriguez, J.L. Fixed eruption due to erythromycin. A case report. Allergy 1991, 46, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.M.; Twarog, F.; Leung, D.Y. Multiple antibiotic sensitivity in a pediatric population. Allergy Proc. 1991, 12, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Igea, J.M.; Quirce, S.; de la Hoz, B.; Fraj, J.; Pola, J.; Diez Gomez, M.L. Adverse cutaneous reactions due to macrolides. Ann. Allergy 1991, 66, 216–218. [Google Scholar] [PubMed]
- Mutalik, S. Fixed drug eruption caused by erythromycin. Int. J. Derm. 1991, 30, 751. [Google Scholar] [CrossRef]
- Shirin, H.; Schapiro, J.M.; Arber, N.; Pinkhas, J.; Sidi, Y.; Salomon, F. Erythromycin base-induced rash and liver function disturbances. Ann. Pharm. 1992, 26, 1522–1523. [Google Scholar] [CrossRef]
- Lopez Serrano, C.; Quiralte Enriquez, J.; Martinez Alzamora, F. Urticaria from erythromycin. Allergol. Immunopathol. 1993, 21, 225–226. [Google Scholar]
- Fernandez Redondo, V.; Casas, L.; Taboada, M.; Toribio, J. Systemic contact dermatitis from erythromycin. Contact Dermat. 1994, 30, 43–44. [Google Scholar] [CrossRef]
- Pandha, H.S.; Dunn, P.J. Stevens-Johnson syndrome associated with erythromycin therapy. N. Z. Med. J. 1995, 108, 13. [Google Scholar] [PubMed]
- Pascual, C.; Crespo, J.F.; Quiralte, J.; Lopez, C.; Wheeler, G.; Martin-Esteban, M. In vitro detection of specific IgE antibodies to erythromycin. J. Allergy Clin. Immunol. 1995, 95, 668–671. [Google Scholar] [CrossRef]
- Moreau, A.; Dompmartin, A.; Castel, B.; Remond, B.; Leroy, D. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int. J. Derm. 1995, 34, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Lestico, M.R.; Smith, A.D. Stevens-Johnson syndrome following erythromycin administration. Am. J. Health Syst. Pharm. 1995, 52, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Freitas, J.D.; Goncalo, M.; Goncalo, S. Allergic contact dermatitis from erythromycin. Contact Dermat. 1995, 33, 360. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lechon, M.J.; Carrasquer, J.; Berenguer, J.; Castell, J.V. Evidence of antibodies to erythromycin in serum of a patient following an episode of acute drug-induced hepatitis. Clin. Exp. Allergy 1996, 26, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, R.; Pansera, B.; Reseghetti, A. Contact allergy to erythromycin. Contact Dermat. 1996, 34, 428. [Google Scholar] [CrossRef]
- J.orro, G.; Morales, C.; Braso, J.V.; Pelaez, A. Anaphylaxis to erythromycin. Ann. Allergy Asthma Immunol. 1996, 77, 456–458. [Google Scholar] [CrossRef]
- Goossens, C.; Sass, U.; Song, M. Baboon syndrome. Dermatology 1997, 194, 421–422. [Google Scholar] [CrossRef]
- Alvarez-Ferrandez, J.A.; Alvarez-Cuesta, E.; Aragoneses, E.; Cuevas, M. Hypersensitivity to erythromycin: Cross Reactivity (A571). J. Allergy Clin. Immunol. 1998, 101, S138. [Google Scholar]
- Quinones, M.D.; Sanchez, I.; Lopez, R.; Rodriguez, F.; Martin-Gil, D.; Santander, J.J. Pustulosis by Erythromycin. Allergy 1998, 53, 104. [Google Scholar]
- Barbaud, A.; Reichert-Penetrat, S.; Trechot, P.; Jacquin-Petit, M.A.; Ehlinger, A.; Noirez, V.; Faure, G.C.; Schmutz, J.L.; Bene, M.C. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br. J. Derm. 1998, 139, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, A.; Haroon, T.S. Drugs causing fixed eruptions: A study of 450 cases. Int. J. Derm. 1998, 37, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Aglas, F.; Horina, J.H. Cholestasis and liver cell damage due to hypersensitivity to erythromycin stearate–recurrence following therapy with erythromycin succinate. Wien. Klin. Wochenschr. 1999, 111, 76–77. [Google Scholar] [PubMed]
- Gallardo, M.A.; Thomas, I. Hypersensitivity reaction to erythromycin. Cutis 1999, 64, 375–376. [Google Scholar]
- Sullivan, S.; Harger, B.; Cleary, J.D. Stevens-Johnson syndrome secondary to erythromycin. Ann. Pharm. 1999, 33, 1369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sethuraman, G.; Kumar, B. Cutaneous adverse drug reactions: Clinical pattern and causative agents–a 6 year series from Chandigarh, India. J. Postgrad. Med. 2001, 47, 95–99. [Google Scholar]
- Williams, D.A. Stevens-Johnson syndrome after erythromycin therapy while deployed at sea. Mil. Med. 2000, 165, 636–637. [Google Scholar] [CrossRef]
- San Pedro de Saenz, B.; Gomez, A.; Quiralte, J.; Florido, J.F.; Martin, E.; Hinojosa, B. FDE to macrolides. Allergy 2002, 57, 55–56. [Google Scholar]
- Forman, R.; Koren, G.; Shear, N.H. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A review of 10 years’ experience. Drug Saf. 2002, 25, 965–972. [Google Scholar] [CrossRef]
- Rallis, E.; Balatsouras, D.G.; Kouskoukis, C.; Verros, C.; Homsioglou, E. Drug eruptions in children with ENT infections. Int. J. Pediatric Otorhinolaryngol. 2006, 70, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Rebelo Gomes, E.; Fonseca, J.; Araujo, L.; Demoly, P. Drug allergy claims in children: From self-reporting to confirmed diagnosis. Clin. Exp. Allergy 2008, 38, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.E.; Hodgkinson, M.; Dendle, C.; Korman, T.M. Report of oral clarithromycin desensitization. Br. J. Clin. Pharmacol. 2008, 66, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.S.; Brocker, E.B.; Trautmann, A. Suspicion of macrolide allergy after treatment of infectious diseases including Helicobacter pylori: Results of allergological testing. Allergol. Immunopathol. 2011, 39, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jakkidi, M.; Basmadjian, C.; Roy, S. An illusion of septic shock: Acute generalised exanthematous pustulosis with multiorgan dysfunction. BMJ Case Rep. 2017, 2017, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, P.D.; van der Lei, J.; Vlug, A.E.; Stricker, B.H. Skin reactions to antibacterial agents in general practice. J. Clin. Epidemiol. 1998, 51, 703–708. [Google Scholar] [CrossRef]
- Price, T.A.; Tuazon, C.U. Clarithromycin-induced thrombocytopenia. Clin. Infect. Dis. 1992, 15, 563–564. [Google Scholar] [CrossRef]
- De Vega, T.; Blanco, S.; Lopez, C.; Pascual, E.; Sanchez, M.; Zamarron, A. Clarithromycin-induced leukocytoclastic vasculitis. Eur J. Clin. Microbiol. Infect. Dis. 1993, 12, 563. [Google Scholar] [CrossRef]
- Oteo, J.A.; Gomez-Cadinanos, R.A.; Rosel, L.; Casas, J.M. Clarithromycin-induced thrombocytopenic purpura. Clin. Infect. Dis. 1994, 19, 1170–1171. [Google Scholar] [CrossRef]
- Vangala, R.; Cernek, P.K. Hypersensitivity reaction to clarithromycin. Ann. Pharm. 1996, 30, 300. [Google Scholar] [CrossRef]
- Igea, J.M.; Lazaro, M. Hypersensitivity reaction to clarithromycin. Allergy 1998, 53, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Rosina, P.; Chieregato, C.; Schena, D. Fixed drug eruption from clarithromycin. Contact Dermat. 1998, 38, 105. [Google Scholar] [CrossRef] [PubMed]
- Gavura, S.R.; Nusinowitz, S. Leukocytoclastic vasculitis associated with clarithromycin. Ann. Pharm. 1998, 32, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.I.; Shoji, T.; Sapadin, A.N. Henoch-Schonlein purpura induced by clarithromycin. Int. J. Derm. 1999, 38, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, Y.; Ohmura, A.; Kinoshita, E.; Muto, M. Fixed drug eruption due to clarithromycin. Clin. Exp. Derm. 2001, 26, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Ricciardi, L.; Fedele, R.; Isola, S.; Purello-D’Ambrosio, F. Immediate reaction to clarithromycin. Allergol. Immunopathol. 2001, 29, 31–32. [Google Scholar] [CrossRef]
- Masia, M.; Gutierrez, F.; Jimeno, A.; Navarro, A.; Borras, J.; Matarredona, J.; Martin-Hidalgo, A. Fulminant hepatitis and fatal toxic epidermal necrolysis (Lyell disease) coincident with clarithromycin administration in an alcoholic patient receiving disulfiram. therapy. Arch. Int. Med. 2002, 162, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Borras-Blasco, J.; Enriquez, R.; Amoros, F.; Cabezuelo, J.B.; Navarro-Ruiz, A.; Perez, M.; Fernandez, J. Henoch-Schonlein purpura associated with clarithromycin. Case report and review of literature. Int. J. Clin. Pharmacol. Ther. 2003, 41, 213–216. [Google Scholar] [CrossRef]
- Terzano, C.; Petroianni, A. Clarithromycin and pulmonary infiltration with eosinophilia. BMJ 2003, 326, 1377–1378. [Google Scholar] [CrossRef]
- Baz, K.; Ikizoglu, G.; Yazici, A.C.; Kokturk, A.; Tiftik, N.; Apa, D.D.; Demirseren, D. Fatal aplastic anaemia in a patient with clarithromycin-induced toxic epidermal necrolysis. J. Eur. Acad. Derm. Venereol. 2004, 18, 104–105. [Google Scholar] [CrossRef]
- Ohnishi, H.; Abe, M.; Yokoyama, A.; Hamada, H.; Ito, R.; Hirayama, T.; Nishimura, K.; Higaki, J. Clarithromycin-induced eosinophilic pneumonia. Int. Med. 2004, 43, 231–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alonso, J.C.; Melgosa, A.C.; Gonzalo, M.J.; Garcia, C.M. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermat. 2005, 53, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, N.; Miras, A.; Gromb, S. Toxic epidermal necrolysis and clarithromycin. Can J. Clin. Pharmcol. 2005, 12, e264–e268. [Google Scholar]
- Dore, J.; Salisbury, R.E. Morbidity and mortality of mucocutaneous diseases in the pediatric population at a tertiary care center. J. Burn Care Res. 2007, 28, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.H.; Barry, J.; Fitzgerald, D.; Watson, R.; Irvine, A.D. Clarithromycin suspension-associated toxic epidermal necrolysis in a 2-year-old girl. Clin. Exp. Derm. 2007, 32, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Koningsbruggen, S.V.; Rietschel, E. Questionnaire-based survey of lifetime-prevalence and character of allergic drug reactions in German children. Pediatr Allergy Immunol. 2008, 19, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Moore, A.; Primeau, M.N. Anaphylactic reaction to clarithromycin in a child. Allergy 2009, 64, 962–963. [Google Scholar] [CrossRef]
- Mori, F.; Barni, S.; Pucci, N.; Rossi, E.; Azzari, C.; de Martino, M.; Novembre, E. Sensitivity and specificity of skin tests in the diagnosis of clarithromycin allergy. Ann. Allergy Asthma Immunol. 2010, 104, 417–419. [Google Scholar] [CrossRef]
- Swamy, N.; Laurie, S.A.; Ruiz-Huidobro, E.; Khan, D.A. Successful clarithromycin desensitization in a multiple macrolide-allergic patient. Ann. Allergy Asthma Immunol. 2010, 105, 489–490. [Google Scholar] [CrossRef]
- Mittmann, N.; Knowles, S.R.; Koo, M.; Shear, N.H.; Rachlis, A.; Rourke, S.B. Incidence of toxic epidermal necrolysis and Stevens-Johnson Syndrome in an HIV cohort: An observational, retrospective case series study. Am. J. Clin. Derm. 2012, 13, 49–54. [Google Scholar] [CrossRef]
- Petitto, J.; Chervinskiy, S.K.; Scurlock, A.M.; Perry, T.T.; Jones, S.M.; Pesek, R.D. Successful clarithromycin desensitization in a macrolide-sensitive pediatric patient. J. Allergy Clin. Immunol. Pract. 2013, 1, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Malkarnekar, S.B.; Naveen, L. Fixed drug eruption due to clarithromycin. J. Res. Pharm. Pract. 2013, 2, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Butti, D.; Mori, F.; Pucci, N.; Rossi, M.E.; Cianferoni, A.; Novembre, E. Azithromycin is more allergenic than clarithromycin in children with suspected hypersensitivity reaction to macrolides. J. Investig. Allergol. Clin. Immunol. 2015, 25, 128–132. [Google Scholar] [PubMed]
- Blair, P.W.; Herrin, D.; Abaalkhail, N.; Fiser, W. DRESS syndrome presenting after initiation of mycobacterium avium complex osteomyelitis treatment. BMJ Case Rep. 2015, 2015. [Google Scholar]
- Guvenir, H.; Dibek Misirlioglu, E.; Capanoglu, M.; Vezir, E.; Toyran, M.; Kocabas, C.N. Proven Non-beta-LactAm. Antibiotic Allergy in Children. Int. Arch. Allergy Immunol. 2016, 169, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Patrizi, A.; Neri, I.; Farina, P. Toxic pustuloderma associated with azithromycin. Clin. Exp. Derm. 1994, 19, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Conforti, A.; Venegoni, M.; Troncon, M.G.; Caputi, A.; Ghiotto, E.; Cocci, A.; Moretti, U.; Velo, G.; Leone, R. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br. J. Clin. Pharmacol. 1999, 48, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Schissel, D.J.; Singer, D.; David-Bajar, K. Azithromycin eruption in infectious mononucleosis: A proposed mechanism of Interaction. Cutis 2000, 65, 163–166. [Google Scholar] [PubMed]
- Cascaval, R.I.; Lancaster, D.J. Hypersensitivity syndrome associated with azithromycin. Am. J. Med. 2001, 110, 330–331. [Google Scholar] [CrossRef]
- Dakdouki, G.K.; Obeid, K.H.; Kanj, S.S. Azithromycin-induced rash in infectious mononucleosis. Scand J. Infect. Dis. 2002, 34, 939–941. [Google Scholar] [CrossRef]
- Taylor, W.R.; Richie, T.L.; Fryauff, D.J.; Ohrt, C.; Picarima, H.; Tang, D.; Murphy, G.S.; Widjaja, H.; Braitman, D.; Tjitra, E.; et al. Tolerability of azithromycin as malaria prophylaxis in adults in northeast papua, indonesia. Antimicrob. Agents Chemother. 2003, 47, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Odemis, E.; Kalyoncu, M.; Okten, A.; Yildiz, K. Azithromycin-induced leukocytoclastic vasculitis. J. Rheumatol. 2003, 30, 2292. [Google Scholar] [PubMed]
- Aihara, Y.; Ito, S.; Kobayashi, Y.; Aihara, M. Stevens-Johnson syndrome associated with azithromycin followed by transient reactivation of herpes simplex virus infection. Allergy 2004, 59, 118. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.V.; Sushma, M.; Guido, S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care center. Indian J. Pharmacol. 2004, 36, 292–295. [Google Scholar]
- Brkljacic, N.; Gracin, S.; Prkacin, I.; Sabljar-Matovinovic, M.; MrzlJak, A.; Nemet, Z. Stevens-Johnson syndrome as an unusual adverse effect of azithromycin. Acta Derm. Croat. 2006, 14, 40–45. [Google Scholar]
- Cummings, J.E.; Snyder, R.R.; Kelly, E.B.; Raimer, S.S. Drug-induced linear immunoglobulin A bullous dermatosis mimicking Stevens-Johnson syndrome: A case report. Cutis 2007, 79, 203–207. [Google Scholar] [PubMed]
- Pursnani, A.; Yee, H.; Slater, W.; Sarswat, N. Hypersensitivity myocarditis associated with azithromycin exposure. Ann. Int. Med. 2009, 150, 225–226. [Google Scholar] [CrossRef]
- Flavia Monteagudo Paz, A.; Francisco Silvestre Salvador, J.; Latorre Martinez, N.; Cuesta Montero, L.; Toledo Alberola, F. Allergic contact dermatitis caused by azithromycin in an eye drop. Contact Dermat. 2011, 64, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Brimhall, A.K.; Chang, T.T. Drug reaction with eosinophilia and Systemic symptoms (DRESS) associated with azithromycin in acute Epstein-Barr virus infection. Pediatric Derm. 2011, 28, 741–743. [Google Scholar] [CrossRef]
- Mori, F.; Pecorari, L.; Pantano, S.; Rossi, M.E.; Pucci, N.; De Martino, M.; Novembre, E. Azithromycin anaphylaxis in children. Int. J. Immunopathol. Pharmacol. 2014, 27, 121–126. [Google Scholar] [CrossRef]
- Banerjee, I.; Mondal, S.; Sen, S.; Tripathi, S.K.; Banerjee, G. Azithromycin-induced rash in a patient of infectious mononucleosis—A case report with review of literature. J. Clin. Diagn. Res. 2014, 8, HD01–HD02. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Bastos, P.; Bras, S.; Amaro, C.; Cardoso, J. Non-occupational allergic contact dermatitis caused by azithromycin in an eye solution. J. Dtsch. Derm. Ges. 2014, 12, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Sriratanaviriyakul, N.; Nguyen, L.P.; Henderson, M.C.; Albertson, T.E. Drug reaction with eosinophilia and Systemic symptoms syndrome (DRESS) syndrome associated with azithromycin presenting like septic shock: A case report. J. Med. Case Rep. 2014, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Filho, R.R.; Bordignon, S.C.; Cassol, M.; Rastelli, G.J. Acute generalized exanthematous pustulosis by azithromycin. Int. J. Derm. 2015, 54, e247–e249. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sancheti, K.; Podder, I.; Das, N.K. Azithromycin induced bullous fixed drug eruption. Indian J. Pharmacol. 2016, 48, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Nappe, T.M.; Goren-Garcia, S.L.; Jacoby, J.L. Stevens-Johnson syndrome after treatment with azithromycin: An uncommon culprit. Am. J. Emerg. Med. 2016, 34, 676 e1-3. [Google Scholar] [CrossRef] [PubMed]
- Campanon-Toro, M.V.; Sierra, O.; Moreno, E.; Sobrino-Garcia, M.; Gracia-Bara, M.T.; Davila, I. Acute generalized exanthematous pustulosis (AGEP) induced by azithromycin. Contact Dermat. 2017, 76, 363–364. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takasawa, R.; Takasawa, K.; Nishioka, M.; Kaneko, M.; Ono, H.; Maekawa, T.; Morio, T.; Shimohira, M. An infant case of severe hypereosinophilia and Systemic symptoms with multiple drug hypersensitivity and reactivation of cytomegalovirus and BK virus. Allergol. Int. 2017, 66, 479–481. [Google Scholar] [CrossRef]
- An, I.; Demir, V.; Akdeniz, S. Fixed drug eruption probably induced by azithromycin. Australas J. Derm. 2017, 58, e253–e254. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Yu, J.; Deng, M.; Zhu, X. Nursing care of a boy seriously infected with Steven-Johnson syndrome after treatment with azithromycin: A case report and literature review. Medicine (Baltimore) 2018, 97, e9112. [Google Scholar] [CrossRef]
- Iarikov, D.E.; Alexander, J.; Nambiar, S. Hypersensitivity reactions associated with fidaxomicin use. Clin. Infect. Dis. 2014, 58, 537–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.; Matsui, D.; Rieder, M.J. Multiple antibiotic sensitivity syndrome in children. Can. J. Clin. Pharmacol. 2000, 7, 38–41. [Google Scholar] [PubMed]
- Messaad, D.; Sahla, H.; Benahmed, S.; Godard, P.; Bousquet, J.; Demoly, P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann. Int. Med. 2004, 140, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).