Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Context
2.2. Study Sample
2.3. Definitions, Terms, and Measures
2.3.1. The Novel MRS
2.3.2. ADEs
2.3.3. Pertinent Risk Outcomes
2.4. Statistical Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ICD Code | Description | n (%) |
|---|---|---|
| T38.0 | Glucocorticoids and synthetic analogues | 4 (2.4) |
| F15.1 | Mental and behavioral disorders due to use of other stimulants, including caffeine: harmful use | 4 (2.4) |
| T38.3 | Insulin and oral hypoglycemic (antidiabetic) drugs | 4 (2.4) |
| F15.9 | Mental and behavioral disorders due to use of other stimulants, including caffeine: unspecified mental and behavioral disorder | 4 (2.4) |
| I95.2 | Hypotension due to drugs | 3 (1.8) |
| E16.0 | Drug-induced hypoglycemia without coma | 3 (1.8) |
| F15.2 | Mental and behavioral disorders due to use of other stimulants, including caffeine: dependence syndrome | 3 (1.8) |
| T42.6 | Other antiepileptic and sedative-hypnotic drugs | 3 (1.8) |
| T40.4 | Poisoning by other synthetic narcotics (buprenorphine, pethidine, dextropropoxyphene, etc.) | 3 (1.8) |
| T42.8 | Antiparkinsonism drugs and other central muscle-tone depressants | 2 (1.2) |
| G25.1 | Drug-induced tremor | 2 (1.2) |
| F11.1 | Mental and behavioral disorders due to use of opioids: harmful use | 2 (1.2) |
| F19.9 | Mental and behavioral disorders due to multiple drug use and use of other psychoactive substances: unspecified mental and behavioral disorder | 2 (1.2) |
| M87.1 | Osteonecrosis due to drugs | 2 (1.2) |
| R50.2 | Drug-induced fever | 2 (1.2) |
| T45.1 | Antineoplastic and immunosuppressive drugs | 2 (1.2) |
| E03.2 | Hypothyroidism due to medicaments and other exogenous substances | 2 (1.2) |
| T78.3 | Angioneurotic edema | 2 (1.2) |
| T50.3 | Electrolytic, caloric, and water-balance agents | 2 (1.2) |
| T42.0 | Hydantoin derivatives | 2 (1.2) |
| E27.3 | Drug-induced adrenocortical insufficiency | 1 (0.6) |
| T50.2 | Carbonic-anhydrase inhibitors, benzothiadiazides and other diuretics | 1 (0.6) |
| L56.0 | Drug phototoxic response | 1 (0.6) |
| Z03.6 | Observation for suspected toxic effect from ingested substance | 1 (0.6) |
| T43.5 | Other and unspecified antipsychotics and neuroleptics | 1 (0.6) |
| T37.1 | Antimycobacterial drugs | 1 (0.6) |
| D61.1 | Drug-induced aplastic anemia due to chemotherapy | 1 (0.6) |
| T39.3 | Other nonsteroidal anti-inflammatory drugs (NSAID) | 1 (0.6) |
| T43.6 | Poisoning by psychostimulants with abuse potential | 1 (0.6) |
| H40.6 | Glaucoma secondary to drugs | 1 (0.6) |
| T42.4 | Poisoning by benzodiazepines | 1 (0.6) |
| T38.1 | Thyroid hormones and substitutes | 1 (0.6) |
| T37.8 | Other specified systemic anti-infectives and antiparasitics (poisoning) | 1 (0.6) |
| F13.2 | Mental and behavioral disorders due to use of sedatives or hypnotics: dependence syndrome | 1 (0.6) |
| T46.2 | Other antidysrhythmic drugs, not elsewhere classified | 1 (0.6) |
| T46.1 | Calcium-channel blockers (poisoning) | 1 (0.6) |
| T88.7 | Unspecified adverse event of drug or medicament | 1 (0.6) |
| L64.0 | Drug-induced androgenic alopecia | 1 (0.6) |
| T42.3 | Barbiturates (poisoning) | 1 (0.6) |
| G44.4 | Drug-induced headache, not elsewhere classified | 1 (0.6) |
| T44.7 | Beta-adrenoreceptor antagonists, not elsewhere classified | 1 (0.6) |
| N14.2 | Nephropathy induced by unspecified drug, medicament or biological substance | 1 (0.6) |
| T37.0 | Sulfonamides (poisoning) | 1 (0.6) |

References
- Hanlon, J.T.; Schmader, K.E.; Koronkowski, M.J.; Weinberger, M.; Landsman, P.B.; Samsa, G.P.; Lewis, I.K. Adverse drug events in high risk older outpatients. J. Am. Geriatr. Soc. 1997, 45, 945–948. [Google Scholar] [CrossRef]
- Parameswaran Nair, N.; Chalmers, L.; Peterson, G.M.; Bereznicki, B.J.; Castelino, R.L.; Bereznicki, L.R. Hospitalization in Older Patients Due to Adverse Drug Reactions—The Need for a Prediction Tool. Clin. Interv. Aging 2016, 11, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.H.; McInnis, T.; Hirsch, J.D. Cost of Prescription Drug-Related Morbidity and Mortality. Ann. Pharmacother. 2018, 52, 829–837. [Google Scholar] [CrossRef]
- Hakkarainen, K.M.; Hedna, K.; Petzold, M.; Hagg, S. Percentage of Patients with Preventable Adverse Drug Reactions and Preventability of Adverse Drug Reactions—A Meta-Analysis. PLoS ONE 2012, 7, e33236. [Google Scholar] [CrossRef]
- Parameswaran Nair, N.; Chalmers, L.; Connolly, M.; Bereznicki, B.J.; Peterson, G.M.; Curtain, C.; Castelino, R.L.; Bereznicki, L.R. Prediction of Hospitalization due to Adverse Drug Reactions in Elderly Community-Dwelling Patients (The PADR-EC Score). PLoS ONE 2016, 11, e0165757. [Google Scholar] [CrossRef] [Green Version]
- Brady, A.; Curtis, C.E.; Jalal, Z. Screening Tools Used by Clinical Pharmacists to Identify Elderly Patients at Risk of Drug-Related Problems on Hospital Admission: A Systematic Review. Pharmacy 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Tangiisuran, B.; Scutt, G.; Stevenson, J.; Wright, J.; Onder, G.; Petrovic, M.; van der Cammen, T.J.; Rajkumar, C.; Davies, G. Development and Validation of a Risk Model for Predicting Adverse Drug Reactions in Older People During Hospital Stay: Brighton Adverse Drug Reactions Risk (BADRI) Model. PLoS ONE 2014, 9, e111254. [Google Scholar] [CrossRef] [Green Version]
- Onder, G.; Petrovic, M.; Tangiisuran, B.; Meinardi, M.C.; Markito-Notenboom, W.P.; Somers, A.; Rajkumar, C.; Bernabei, R.; van der Cammen, T.J. Development and Validation of a Score to Assess Risk of Adverse Drug Reactions Among In-Hospital Patients 65 Years or Older: The GerontoNet ADR Risk Score. Arch. Intern. Med. 2010, 170, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Cicali, B.; Michaud, V.; Knowlton, C.H.; Turgeon, J. Application of a Novel Medication-Related Risk Stratification Strategy to a Self-Funded Employer Population. Benef. Q. 2018, 34, 49–55. [Google Scholar]
- Bouwmeester, C. The PACE program: Home-Based Long-Term Care. Consult. Pharm. 2012, 27, 24–30. [Google Scholar] [CrossRef]
- PACE by the Numbers. Available online: https://www.npaonline.org/sites/default/files/PDFs/pace_infographic_update_final_0719.pdf (accessed on 22 April 2020).
- Bankes, D.L.; Amin, N.S.; Bardolia, C.; Awadalla, M.S.; Knowlton, C.H.; Bain, K.T. Medication-Related Problems Encountered in the Program of All-Inclusive Care for the Elderly: An Observational Study. J. Am. Pharm. Assoc. 2020, 60, 319–327. [Google Scholar] [CrossRef] [PubMed]
- McCarrell, J. PACE: An Interdisciplinary Community-Based Practice Opportunity for Pharmacists. Sr. Care Pharm. 2019, 34, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Vouri, S.M.; Tiemeier, A. The Ins and Outs of Pharmacy Services at a Program of All-Inclusive Care for the Elderly. Consult. Pharm. 2012, 27, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Bain, K.T.; Matos, A.; Knowlton, C.H.; McGain, D. Genetic Variants and Interactions from a Pharmacist-Led Pharmacogenomics Service for PACE. Pharmacogenomics 2019, 20, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Bankes, D.L.; Schamp, R.O.; Knowlton, C.H.; Bain, K.T. Prescriber-Initiated Engagement of Pharmacists for Information and Intervention in Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Boustani, M.; Campbell, N.; Munger, S.; Maidment, I.; Fox, C. Impact of Anticholinergics on the Aging Brain: A Review and Practical Application. Aging Health 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Duffull, S.B.; Nishtala, P.S. Anticholinergic Burden Quantified by Anticholinergic Risk Scales and Adverse Outcomes in Older People: A Systematic Review. BMC Geriatr. 2015, 15, 31. [Google Scholar] [CrossRef] [Green Version]
- Campbell, N.L.; Perkins, A.J.; Bradt, P.; Perk, S.; Wielage, R.C.; Boustani, M.A.; Ng, D.B. Association of Anticholinergic Burden with Cognitive Impairment and Health Care Utilization Among a Diverse Ambulatory Older Adult Population. Pharmacotherapy 2016, 36, 1123–1131. [Google Scholar] [CrossRef]
- Paul, K.J.; Walker, R.L.; Dublin, S. Anticholinergic Medications and Risk of Community-Acquired Pneumonia in Elderly Adults: A Population-Based Case-Control Study. J. Am. Geriatr. Soc. 2015, 63, 476–485. [Google Scholar] [CrossRef]
- West, T.; Pruchnicki, M.C.; Porter, K.; Emptage, R. Evaluation of Anticholinergic Burden of Medications in Older Adults. J. Am. Pharm. Assoc. 2013, 53, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Gerretsen, P.; Pollock, B.G. Rediscovering Adverse Anticholinergic Effects. J. Clin. Psychiatry 2011, 72, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, K.; Woodman, R.J.; Mangoni, A.A. Drugs with Anticholinergic Effects and Cognitive Impairment, Falls and All-Cause Mortality in Older Adults: A Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2015, 80, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Hanlon, J.T. Anticholinergic Drugs and Dementia in Older Adults. BMJ 2018, 361, k1722. [Google Scholar] [CrossRef]
- Szabo, S.M.; Gooch, K.; Schermer, C.; Walker, D.; Lozano-Ortega, G.; Rogula, B.; Deighton, A.; Vonesh, E.; Campbell, N. Association Between Cumulative Anticholinergic Burden and Falls and Fractures in Patients with Overactive Bladder: US-based Retrospective Cohort Study. BMJ Open 2019, 9, e026391. [Google Scholar] [CrossRef] [Green Version]
- Linjakumpu, T.; Hartikainen, S.; Klaukka, T.; Koponen, H.; Kivela, S.L.; Isoaho, R. A Model to Classify the Sedative Load of Drugs. Int. J. Geriatr. Psychiatry 2003, 18, 542–544. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Boudreau, R.M.; Roumani, Y.F.; Newman, A.B.; Ruby, C.M.; Wright, R.M.; Hilmer, S.N.; Shorr, R.I.; Bauer, D.C.; Simonsick, E.M.; et al. Number and Dosage of Central Nervous System Medications on Recurrent Falls in Community Elders: The Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 492–498. [Google Scholar] [CrossRef]
- Wright, R.M.; Roumani, Y.F.; Boudreau, R.; Newman, A.B.; Ruby, C.M.; Studenski, S.A.; Shorr, R.I.; Bauer, D.C.; Simonsick, E.M.; Hilmer, S.N.; et al. Effect of Central Nervous System Medication Use on Decline in Cognition in Community-Dwelling Older Adults: Findings from the Health, Aging And Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Aspinall, S.L.; Springer, S.P.; Zhao, X.; Cunningham, F.E.; Thorpe, C.T.; Semla, T.P.; Shorr, R.I.; Hanlon, J.T. Central Nervous System Medication Burden and Risk of Recurrent Serious Falls and Hip Fractures in Veterans Affairs Nursing Home Residents. J. Am. Geriatr. Soc. 2019, 67, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Hart, L.A.; Marcum, Z.A.; Gray, S.L.; Walker, R.L.; Crane, P.K.; Larson, E.B. The Association Between Central Nervous System-Active Medication Use and Fall-Related Injury in Community-Dwelling Older Adults with Dementia. Pharmacotherapy 2019, 39, 530–543. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Bos, J.M.; Tarrell, R.F.; Morlan, B.W.; Caraballo, P.J.; Ackerman, M.J. Institution-wide QT Alert System Identifies Patients with a High Risk of Mortality. Mayo Clin. Proc. 2013, 88, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Post, W.S.; Blasco-Colmenares, E.; Dalal, D.; Tomaselli, G.F.; Guallar, E. Electrocardiographic QT Interval and Mortality: A Meta-Analysis. Epidemiology 2011, 22, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, M.; Scarcelli, C.; Niro, V.; Seripa, D.; Pazienza, A.M.; Pepe, G.; Colusso, A.M.; Pacilli, L.; Pilotto, A. Prevalence, Clinical Features and Avoidability of Adverse Drug Reactions as Cause of Admission to a Geriatric Unit: A Prospective Study of 1756 Patients. Drug Saf. 2008, 31, 545–556. [Google Scholar] [CrossRef]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital Admissions/Visits Associated with Drug-Drug Interactions: A Systematic Review and Meta-Analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Verbeurgt, P.; Mamiya, T.; Oesterheld, J. How Common Are Drug and Gene Interactions? Prevalence in a Sample of 1143 Patients with CYP2C9, CYP2C19 and CYP2D6 Genotyping. Pharmacogenomics 2014, 15, 655–665. [Google Scholar] [CrossRef]
- Fischer, H.D.; Juurlink, D.N.; Mamdani, M.M.; Kopp, A.; Laupacis, A. Hemorrhage During Warfarin Therapy Associated with Cotrimoxazole and Other Urinary Tract Anti-Infective Agents: A Population-Based Study. Arch. Intern. Med. 2010, 170, 617–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohl, C.M.; Karpov, A.; Reddekopp, L.; Stausberg, J. ICD-10 Codes Used to Identify Adverse Drug Events in Administrative Data: A Systematic Review. J. Am. Med. Inf. Assoc. 2013, 21, 547–557. [Google Scholar] [CrossRef]
- Hazell, L.; Shakir, S.A. Under-reporting of Adverse Drug Reactions: A Systematic Review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- Keehan, S.P.; Stone, D.A.; Poisal, J.A.; Cuckler, G.A.; Sisko, A.M.; Smith, S.D.; Madison, A.J.; Wolfe, C.J.; Lizonitz, J.M. National Health Expenditure Projections, 2016-25: Price Increases, Aging Push Sector To 20 Percent oOf Economy. Health Aff. (Millwood) 2017, 36, 553–563. [Google Scholar] [CrossRef]
- Hough, A.; Vartan, C.M.; Groppi, J.A.; Reyes, S.; Beckey, N.P. Evaluation of Clinical Pharmacy Interventions in a Veterans Affairs Medical Center Primary Care Clinic. Am. J. Health Syst. Pharm. 2013, 70, 1168–1172. [Google Scholar] [CrossRef]
- Sloane, P.D.; Oudenhoven, M.D.; Broyles, I.; McNabney, M. Challenges to Cost-Effective Care of Older Adults with Multiple Chronic Conditions: Perspectives of Program of All-Inclusive Care for the Elderly Medical Directors. J. Am. Geriatr. Soc. 2014, 62, 564–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, W.L.; McIlvain, H.E.; Lacy, N.L.; Magsi, H.; Crabtree, B.F.; Yenny, S.K.; Sitorius, M.A. Primary Care for Elderly People: Why Do Doctors Find It So Hard? Gerontologist 2002, 42, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riordan, D.O.; Byrne, S.; Fleming, A.; Kearney, P.M.; Galvin, R.; Sinnott, C. GPs’ Perspectives on Prescribing for Older People in Primary Care: A Qualitative Study. Br. J. Clin. Pharmacol. 2017, 83, 1521–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailabouni, N.J.; Nishtala, P.S.; Tordoff, D.; Mangin, J.M. Challenges and Enablers of Deprescribing: A General Practitioner Perspective. PLoS ONE 2016, 11, e0151066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippoli, J.; Bottos, K.; Knowlton, O.V. How Does an Evidence-Based Medication Risk Score Affect Clinicians’ Attitudes about De-Prescribing and Medication Changes for Older Adults? In Proceedings of the American Public Health Association Annual Meeting and Expo, Philadelphia, PA, USA, 5 November 2019. [Google Scholar]
- Hohl, C.M.; Kuramoto, L.; Yu, E.; Rogula, B.; Stausberg, J.; Sobolev, B. Evaluating Adverse Drug Event Reporting in Administrative Data from Emergency Departments: A Validation Study. BMC Health Serv. Res. 2013, 13, 473. [Google Scholar] [CrossRef] [Green Version]





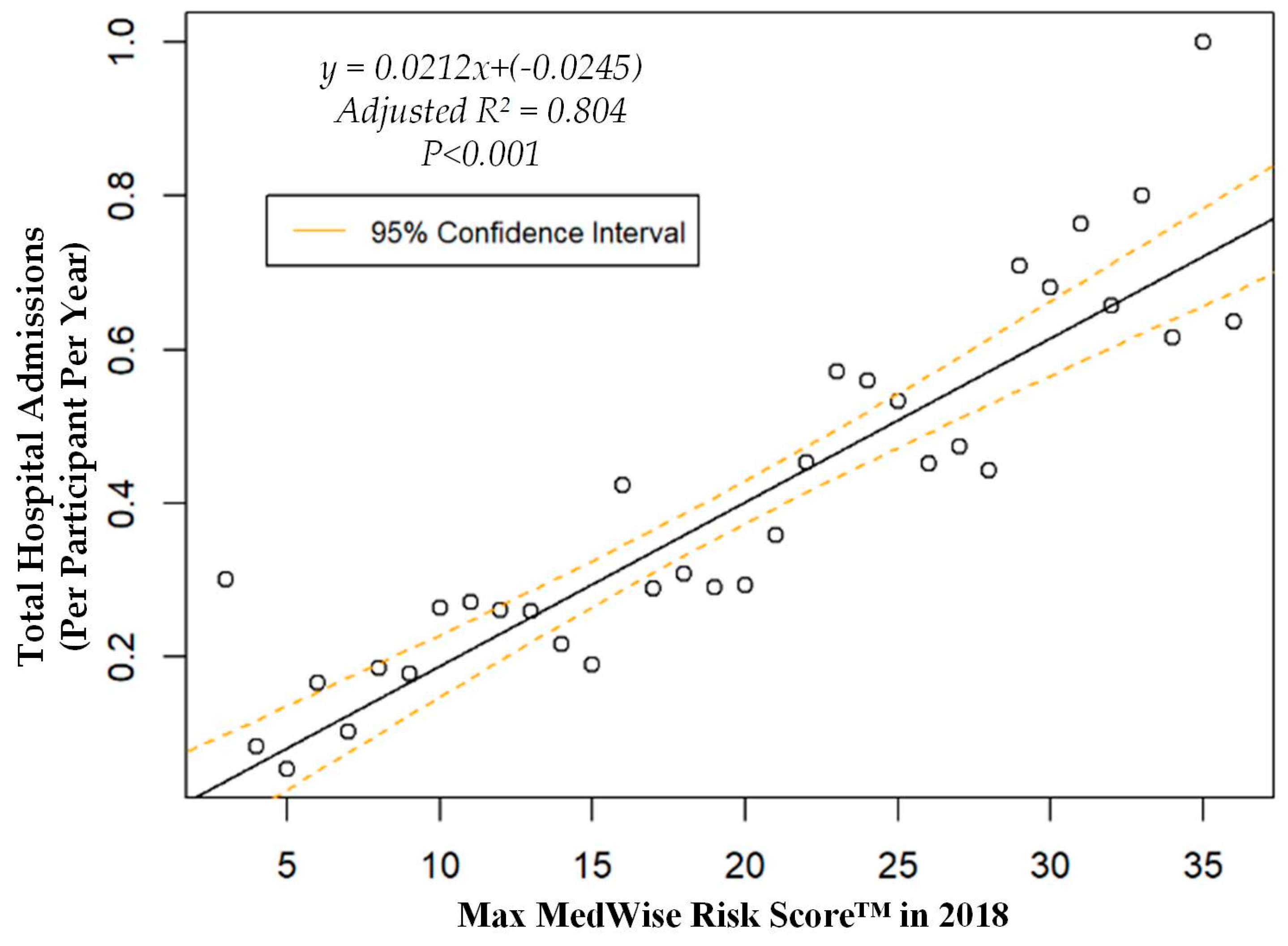
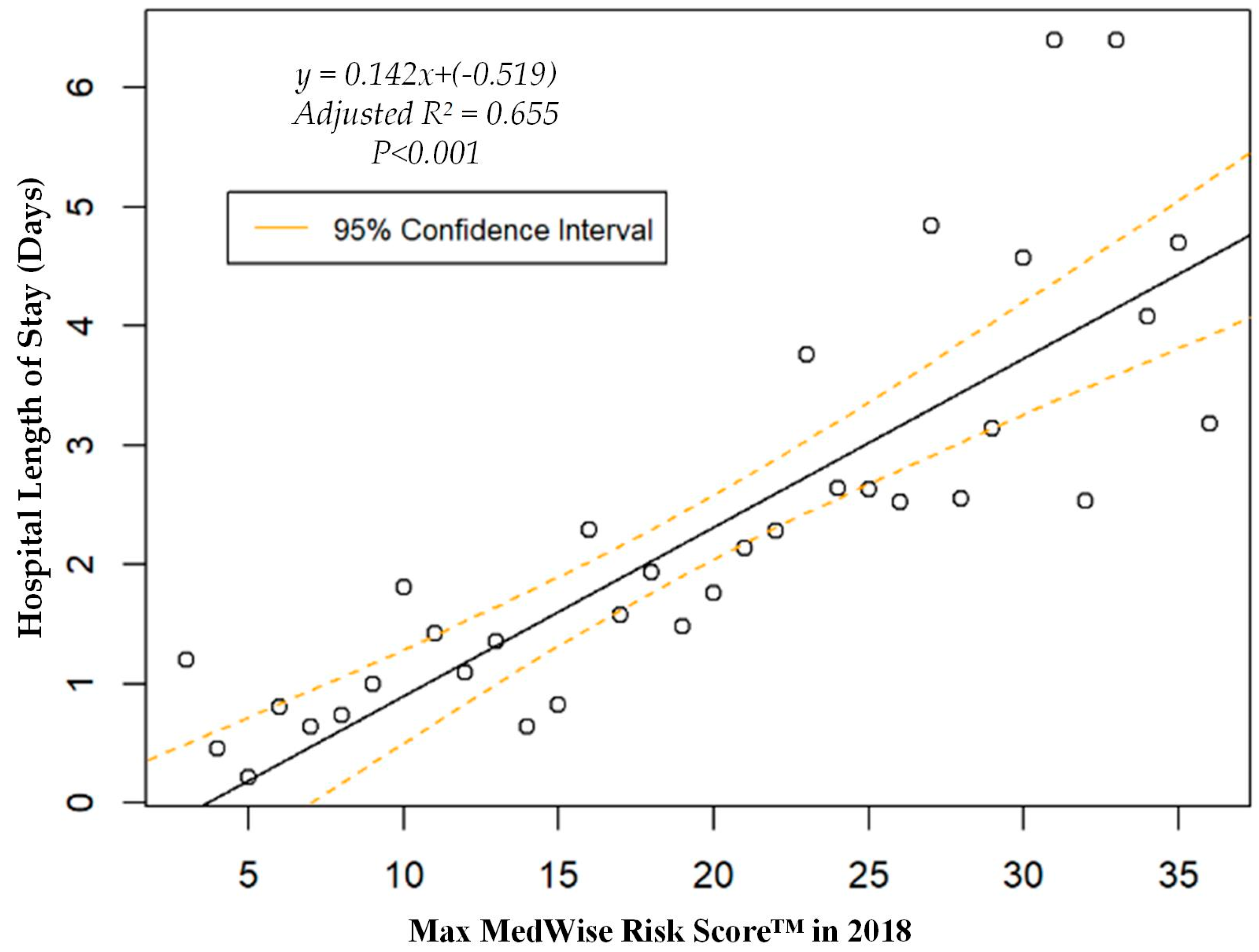
| Characteristic | Total | Participants without ADEs | Participants with ADEs |
|---|---|---|---|
| PACE participants, n (%) | 1965 (100) | 1837 (93.5) | 128 (6.5) |
| Age, mean ± SD (range) | 76.8 ± 9.9 (56–104) | 77.1 ± 9.9 (56–104) | 72.9 ± 9.7 (56–96) |
| Age, median (IQR) | 77 (69, 84) | 77 (69, 84) | 71 (66, 80) |
| Female, n (%) | 1282 (65.2) | 1206 (65.7) | 76 (59.4) |
| Number of profiled medications, mean ± SD (range) 1 | 13.4 ± 5.8 (1–42) | 13.2 ± 5.8 (1–42) | 16.2 ± 6.1 (4–37) |
| Number of profiled medications, median (IQR) 1 | 13 (9, 17) | 13 (9, 17) | 15.5 (12, 20) |
| MedWise Risk Score™, mean ± SD (range) | 18.5 ±7.8 (2–40) | 18.2 ± 7.7 (2–40) | 23.1 ± 7.8 (3–40) |
| MedWise Risk Score™, median (IQR) | 18 (12, 25) | 18 (12, 24) | 23 (17.75, 29) |
| Ethnicity, n (%) | |||
| Non-Hispanic White | 1140 (58.0) | 1061 (57.8) | 79 (61.7) |
| Black | 454 (23.1) | 422 (23.0) | 32 (25.0) |
| Hispanic | 142 (7.2) | 135 (7.3) | 7 (5.5) |
| Asian or Pacific Islander | 59 (3.0) | 58 (3.2) | 1 (0.8) |
| American Indian/Alaskan Native | 7 (0.4) | 7 (0.4) | 0 (0.0) |
| Unknown or other | 163 (8.3) | 154 (8.4) | 9 (7.0) |
| Top chronic comorbidities (by diagnosis code), n (%) | |||
| Essential (primary) hypertension | 1233 (62.7) | 1135 (61.8) | 98 (76.6) |
| Type 2 diabetes mellitus without complications | 790 (40.2) | 727 (39.6) | 63 (49.2) |
| Hyperlipidemia, unspecified | 622 (31.7) | 573 (31.2) | 49 (38.3) |
| Atherosclerotic heart disease of native coronary artery without angina pectoris | 428 (21.8) | 388 (21.1) | 40 (31.2) |
| Hypothyroidism, unspecified | 421 (21.4) | 385 (21.0) | 36 (28.1) |
| Unspecified dementia without behavioral disturbance | 406 (20.7) | 374 (20.4) | 32 (25.0) |
| Chronic obstructive pulmonary disease, unspecified | 373 (19.0) | 335 (18.2) | 38 (29.7) |
| Chronic kidney disease, stage 3 | 371 (18.9) | 335 (18.2) | 36 (28.1) |
| Heart failure, unspecified | 349 (17.8) | 314 (17.1) | 35 (27.3) |
| Pertinent risk outcomes | |||
| Total medical expenditures, mean (95% CI), USD | $26,299.97 (24,917.54, 27,682.39) | $25,087.07 (23,720.95, 26,453.19) | $43,706.95 (36,149.34, 51,264.56) |
| Hospital admissions, mean (95% CI), n per participant per year | 0.37 (0.33, 0.41) | 0.34 (0.3, 0.37) | 0.84 (0.62, 1.05) |
| Hospital length of stay, mean (95% CI), days | 2.1 (1.8, 2.4) | 1.9 (1.7, 2.2) | 4.8 (3.3, 6.2) |
| ED visits, mean (95% CI), n per participant per year | 0.63 (0.57, 0.69) | 0.59 (0.53, 0.65) | 1.21 (0.88, 1.54) |
| PACE organizations, n | 12 | ||
| Geographic location of PACE, n (%) | |||
| Northeast | 3 (25.0) | ||
| South | 4 (33.3) | ||
| Midwest | 1 (8.3) | ||
| West | 4 (33.3) | ||
| PACE census size, n (%) | |||
| <120 participants | 1 (8.3) | ||
| 120–220 participants | 7 (58.3) | ||
| >220 participants | 4 (33.3) |
| ICD Code | Description | n (%) |
|---|---|---|
| F11.2 | Mental and behavioral disorders due to use of opioids: dependence syndrome | 20 (11.8) |
| F11.9 | Mental and behavioral disorders due to use of opioids: unspecified mental and behavioral disorder | 12 (7.1) |
| D68.3 | Hemorrhagic disorder due to circulating anticoagulants | 11 (6.5) |
| F19.1 | Mental and behavioral disorders due to multiple drug use and use of other psychoactive substances: harmful use | 9 (5.3) |
| T50.9 | Poisoning: other and unspecified drugs, medicaments, and biological substances | 9 (5.3) |
| G24.0 | Drug-induced dystonia | 7 (4.1) |
| L27.0 | Generalized skin eruption due to drugs and medicaments | 6 (3.5) |
| T40.2 | Poisoning by other opioids (includes morphine and codeine) | 5 (2.9) |
| G21.1 | Other drug-induced secondary parkinsonism | 5 (2.9) |
| T45.5 | Anticoagulants (poisoning) | 5 (2.9) |
| F19.2 | Mental and behavioral disorders due to multiple drug use and use of other psychoactive substances: dependence syndrome | 5 (2.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankes, D.L.; Jin, H.; Finnel, S.; Michaud, V.; Knowlton, C.H.; Turgeon, J.; Stein, A. Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 87. https://doi.org/10.3390/pharmacy8020087
Bankes DL, Jin H, Finnel S, Michaud V, Knowlton CH, Turgeon J, Stein A. Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy. 2020; 8(2):87. https://doi.org/10.3390/pharmacy8020087
Chicago/Turabian StyleBankes, David L., Hubert Jin, Stephanie Finnel, Veronique Michaud, Calvin H. Knowlton, Jacques Turgeon, and Alan Stein. 2020. "Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly" Pharmacy 8, no. 2: 87. https://doi.org/10.3390/pharmacy8020087
APA StyleBankes, D. L., Jin, H., Finnel, S., Michaud, V., Knowlton, C. H., Turgeon, J., & Stein, A. (2020). Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy, 8(2), 87. https://doi.org/10.3390/pharmacy8020087







