Opioids, Polypharmacy, and Drug Interactions: A Technological Paradigm Shift Is Needed to Ameliorate the Ongoing Opioid Epidemic
Abstract
:1. Introduction
2. Opioid Users and Polypharmacy: Defining the Scope of the Problem
3. Polypharmacy and Drug Interactions: Explaining Their Relationship
4. Opioid-Related DDIs: Mechanisms and Consequences
4.1. Pharmacodynamic
4.2. Pharmacokinetic
4.2.1. CYP2D6
4.2.2. CYP3A4/5
5. Current State of CDSS for Opioid DDI Management
6. Features of an Optimal Opioid CDSS: An Expert Opinion with a Case Discussion
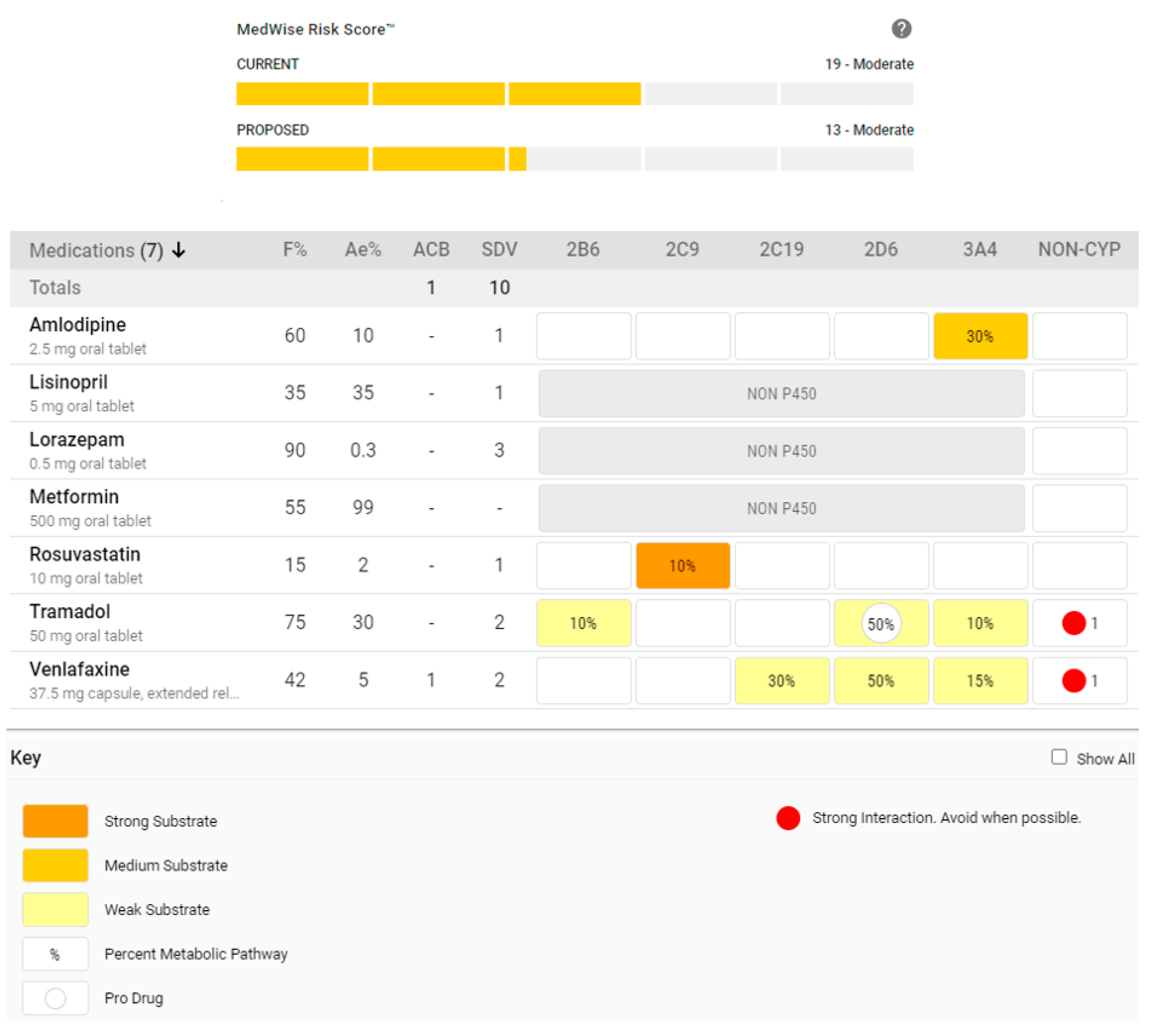
6.1. Content-Related Consideration: Embrace Simultaneous Assessments with a Comprehensive Visualization of Pertinent DDI Mechanisms
6.2. Content-Related Consideration: Simulate, Quantify, and Estimate Risk Associated with the Interactions Present in the Current Regimen
6.3. System-Related Consideration: EHR Interoperability
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinha, S.; Jensen, M.; Mullin, S.; Elkin, P.L. Safe Opioid Prescripting: A SMART on FHIR Approach to Clinical Decision Support. Online J. Public Health Inform. 2017, 9, e193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, L.B.; Olfson, M.; Kales, H.C.; Maust, D.T. Opioids and Other Central Nervous System-Active Polypharmacy in Older Adults in the United States. J. Am. Geriatr. Soc. 2017, 65, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Fullington, H.M.; Alvarez, C.A.; Betts, A.C.; Lee, S.J.C.; Haggstrom, D.A.; Halm, E.A. Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer 2018, 124, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Medicare Payment Advisory Committee. Polypharmacy and opioid use among Medicare Part D enrollees. In Relationship between Polypharmacy, Adherence, and Patient Confusion: Report to the Congress: Medicare and the Health Care Delivery System; Medicare Payment Advisory Commission: Washington, DC, USA, 2014. Available online: http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf (accessed on 11 June 2020).
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keine, D.; Zelek, M.; Walker, J.Q.; Sabbagh, M.N. Polypharmacy in an Elderly Population: Enhancing Medication Management Through the Use of Clinical Decision Support Software Platforms. Neurol. Ther. 2019, 8, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Doan, J.; Zakrzewski-Jakubiak, H.; Roy, J.; Turgeon, J.; Tannenbaum, C. Prevalence and Risk of Potential Cytochrome P450–Mediated Drug-Drug Interactions in Older Hospitalized Patients with Polypharmacy. Ann. Pharmacother. 2013, 47, 324–332. [Google Scholar] [CrossRef]
- Bain, K.T.; Knowlton, C.H. Role of Opioid-Involved Drug Interactions in Chronic Pain Management. J. Am. Osteopat. Assoc. 2019, 119, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Pergolizzi, J.V.; Labhsetwar, S.A.; Puenpatom, R.A.; Ben-Joseph, R.; Ohsfeldt, R.; Summers, K.H. Economic Impact of Potential CYP450 Pharmacokinetic Drug-Drug Interactions Among Chronic Low Back Pain Patients Taking Opioids. Pain Pract. 2011, 12, 45–56. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Labhsetwar, S.A.; Puenpatom, R.A.; Ben-Joseph, R.; Ohsfeldt, R.; Summers, K.H. Economic impact of potential drug-drug interactions among osteoarthritis patients taking opioids. Pain Pract. 2012, 12, 33–44. [Google Scholar] [CrossRef]
- Turgeon, J.; Michaud, V. Clinical decision support systems: Great promises for better management of patients’ drug therapy. Expert Opin. Drug Metab. Toxicol. 2016, 12, 993–995. [Google Scholar] [CrossRef] [Green Version]
- Saverno, K.R.; E Hines, L.; Warholak, T.L.; Grizzle, A.J.; Babits, L.; Clark, C.; Taylor, A.M.; Malone, D.C. Ability of pharmacy clinical decision-support software to alert users about clinically important drug–drug interactions. J. Am. Med. Inform. Assoc. 2010, 18, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassman, P.A.; Simon, B.; Belperio, P.; Lanto, A. Improving recognition of drug interactions: Benefits and barriers to using automated drug alerts. Med. Care 2002, 40, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Malone, D.C.; Skrepnek, G.H.; Armstrong, E.P.; Murphy, J.E.; Abarca, J.; Rehfeld, R.A.; Reel, S.J.; Woosley, R.L.; Malone, D.C. Prescribers’ Knowledge of and Sources of Information for Potential drug-drug Interactions: A postal survey of US prescribers. Drug Saf. 2008, 31, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Weideman, R.A.; Bernstein, I.H.; McKinney, W.P. Pharmacist recognition of potential drug interactions. Am. J. Health Pharm. 1999, 56, 1524–1529. [Google Scholar] [CrossRef]
- Abarca, J.; Colon, L.R.; Wang, V.S.; Malone, D.; Murphy, J.E.; Armstrong, E.P. Evaluation of the Performance of Drug-Drug Interaction Screening Software in Community and Hospital Pharmacies. J. Manag. Care Pharm. 2006, 12, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Hazlet, T.K.; Lee, T.A.; Hansten, P.D.; Horn, J.R. Performance of community pharmacy drug interaction software. J. Am. Pharm. Assoc. 2001, 41, 200–204. [Google Scholar] [CrossRef]
- Van Der Sijs, H.; Aarts, J.; Vulto, A.; Berg, M. Overriding of Drug Safety Alerts in Computerized Physician Order Entry. J. Am. Med. Inform. Assoc. 2006, 13, 138–147. [Google Scholar] [CrossRef]
- Genco, E.K.; Forster, J.E.; Flaten, H.; Goss, F.; Heard, K.J.; Hoppe, J.; Monte, A.A. Clinically Inconsequential Alerts: The Characteristics of Opioid Drug Alerts and Their Utility in Preventing Adverse Drug Events in the Emergency Department. Ann. Emerg. Med. 2015, 67, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Jena, A.B.; Goldman, D.; Weaver, L.; Karaca-Mandic, P. Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ 2014, 348, g1393. [Google Scholar] [CrossRef] [Green Version]
- By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Boudreau, R.M.; Roumani, Y.F.; Newman, A.B.; Ruby, C.M.; Wright, R.M.; Hilmer, S.N.; Shorr, R.I.; Bauer, D.C.; Simonsick, E.M.; et al. Number and Dosage of Central Nervous System Medications on Recurrent Falls in Community Elders: The Health, Aging and Body Composition Study. J. Gerontol Ser. A Boil. Sci. Med. Sci. 2009, 64, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.M.; Roumani, Y.F.; Boudreau, R.M.; Newman, A.B.; Ruby, C.M.; Studenski, S.A.; Shorr, R.I.; Bauer, U.C.; Simonsick, E.M.; Hilmer, S.N.; et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: Findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspinall, S.L.; Springer, S.P.; Zhao, X.; Cunningham, F.E.; Thorpe, C.T.; Semla, T.P.; Shorr, R.I.; Hanlon, J.T. Central Nervous System Medication Burden and Risk of Recurrent Serious Falls and Hip Fractures in Veterans Affairs Nursing Home Residents. J. Am. Geriatr. Soc. 2018, 67, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Park, T.W.; Saitz, R.; Ganoczy, D.; Ilgen, M.A.; Bohnert, A.S.B. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: Case-cohort study. BMJ 2015, 350, h2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA Warns about Serious Risks and Death When Combining Opioid Pain and Cough Medicines with Benzodiazepines; Requires Its Strongest Warning; FDA: Washington, DC, USA, 2017.
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2014, 44, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski-Jakubiak, H.; Doan, J.; Lamoureux, P.; Singh, D.; Turgeon, J.; Tannenbaum, C. Detection and Prevention of Drug–Drug Interactions in the Hospitalized Elderly: Utility of New Cytochrome P450–Based Software. Am. J. Geriatr. Pharmacother. 2011, 9, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Labhsetwar, S.A.; Puenpatom, R.A.; Joo, S.; Summers, K.H.; Ben-Joseph, R.H.; Pergolizzi, J. Prevalence of Exposure to Potential CYP450 Pharmacokinetic Drug-Drug Interactions among Patients with Chronic Low Back Pain Taking Opioids. Pain Pract. 2010, 11, 230–239. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Puenpatom, R.A.; Joo, S.; Summers, K.H.; Ben-Joseph, R.; Labhsetwar, S.A. Exposure to Potential CYP450 Pharmacokinetic Drug–Drug Interactions among Osteoarthritis Patients: Incremental Risk of Multiple Prescriptions. Pain Pract. 2010, 11, 325–336. [Google Scholar] [CrossRef]
- Nightingale, G.; Pizzi, L.T.; Barlow, A.; Barlow, B.; Jacisin, T.; McGuire, M.; Swartz, K.; Chapman, A. The prevalence of major drug-drug interactions in older adults with cancer and the role of clinical decision support software. J. Geriatr. Oncol. 2018, 9, 526–533. [Google Scholar] [CrossRef]
- Bankes, D.L.; Amin, N.S.; Bardolia, C.; Awadalla, M.S.; Knowlton, C.H.; Bain, K.T. Medication-related problems encountered in the Program of All-Inclusive Care for the Elderly: An observational study. J. Am. Pharm. Assoc. 2020, 60, 319–327. [Google Scholar] [CrossRef]
- Kotlinska-Lemieszek, A.; Klepstad, P.; Haugen, D.F. Clinically significant drug–drug interactions involving opioid analgesics used for pain treatment in patients with cancer: A systematic review. Drug Des. Dev. Ther. 2015, 9, 5255–5267. [Google Scholar] [CrossRef] [Green Version]
- Bingham, J.M.; Taylor, A.M.; Boesen, K.; Axon, D.R. Preliminary Investigation of Pharmacist-Delivered, Direct-to-Provider Interventions to Reduce Co-Prescribing of Opioids and Benzodiazepines among a Medicare Population. Pharmacy 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng, A.O.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1105–1121. [Google Scholar] [CrossRef]
- Zagaria, M.A.E. Central Sleep Apnea: Potential Impact of Benzodiazepines, Opioids, and CYP3A4 Inhibitors. USA Pharm. 2015, 40, 21–24. Available online: https://www.uspharmacist.com/article/central-sleep-apnea-potential-impact-of-benzodiazepines-opioids-and-cyp3a4-inhibitors (accessed on 21 July 2020).
- Dolinak, D. Opioid Toxicity. Acad. Forensic Pathol. 2017, 7, 19–35. [Google Scholar] [CrossRef]
- Gavidia, M. Chronic Opioid Therapy, Prescription Drug Use Linked With Increased Risk of Sleep Disorders, Frailty. Am. J. Manag. Care. 2019. Available online: https://www.ajmc.com/focus-of-the-week/chronic-opioid-therapy-prescription-drug-use-linked-with-increased-risk-of-sleep-disorders-frailty (accessed on 21 July 2020).
- Beakley, B.D.; Kaye, A.M.; Kaye, A.D. Tramadol, Pharmacology, Side Effects, and Serotonin Syndrome: A Review. Pain Physician 2015, 18, 395–400. [Google Scholar]
- Tannenbaum, C.; Sheehan, N.L. Understanding and preventing drug–drug and drug–gene interactions. Expert Rev. Clin. Pharmacol. 2014, 7, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanch, B.; Degenhardt, L.; Buckley, N.A.; Gisev, N.; A Dobbins, T.; A Karanges, E.; Larance, B.; Larney, S.; Pearson, S. Prescription Opioid Access Patterns and Factors Associated with Increasing Number of Prescribers, Pharmacies, and Dispensings: An Observational Study Using Pharmaceutical Claims. Pain Med. 2017, 19, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Paulozzi, L.J.; Strickler, G.K.; Kreiner, P.W.; Koris, C.M. Controlled Substance Prescribing Patterns—Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill. Summ. 2015, 64, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.C.; Carlson, K.; Izrael, D. Geographic Variation in Opioid Prescribing in the USA. J. Pain 2012, 13, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Bell, G.C.; Donovan, K.A.; McLeod, H.L. Clinical Implications of Opioid Pharmacogenomics in Patients with Cancer. Cancer Control. 2015, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; E Klein, T.; E Caudle, K.; E Haidar, C.; Shen, D.D.; Callaghan, J.T.; Sadhasivam, S.; et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450 2D6 Genotype and Codeine Therapy: 2014 Update. Clin. Pharmacol. Ther. 2014, 95, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollason, V.; Samer, C.; Piguet, V.; Dayer, P.; Desmeules, J. Pharmacogenetics of analgesics: Toward the individualization of prescription. Pharmacogenomics 2008, 9, 905–933. [Google Scholar] [CrossRef] [PubMed]
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP 2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2019, 13, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Stamer, U.M.; Lehnen, K.; Höthker, F.; Bayerer, B.; Wolf, S.; Hoeft, A.; Stuber, F. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain 2003, 105, 231–238. [Google Scholar] [CrossRef]
- Stamer, U.M.; Musshoff, F.; Kobilay, M.; Madea, B.; Hoeft, A.; Stüber, F. Concentrations of Tramadol and O-desmethyltramadol Enantiomers in Different CYP2D6 Genotypes. Clin. Pharmacol. Ther. 2007, 82, 41–47. [Google Scholar] [CrossRef]
- Hajj, A.; Khabbaz, L.; Laplanche, J.-L.; Peoc’H, K. Pharmacogenetics of opiates in clinical practice: The visible tip of the iceberg. Pharmacogenomics 2013, 14, 575–585. [Google Scholar] [CrossRef]
- Samer, C.F.; Daali, Y.; Wagner, M.; Hopfgartner, G.; Eap, C.B.; Rebsamen, M.; Rossier, M.; Hochstrasser, D.; Dayer, P.; Desmeules, J. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br. J. Pharmacol. 2010, 160, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Kummer, O.; Hammann, F.; Moser, C.; Schaller, O.; Drewe, J.; Krähenbühl, S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur. J. Clin. Pharmacol. 2010, 67, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Lurcott, G. The effects of the genetic absence and inhibition of CYP2D6 on the metabolism of codeine and its derivatives, hydrocodone and oxycodone. Anesthesia Prog. 1998, 45, 154–156. [Google Scholar]
- Zhou, S.-F.; Liu, J.-P.; Lai, X.-S. Substrate specificity, inhibitors and regulation of human cytochrome P450 2D6 and implications in drug development. Curr. Med. Chem. 2009, 16, 2661–2805. [Google Scholar] [CrossRef] [PubMed]
- Monte, A.A.; Heard, K.J.; Campbell, J.; Hamamura, D.; Weinshilboum, R.M.; Vasiliou, V.; Heard, K.J. The Effect of CYP2D6 Drug-Drug Interactions on Hydrocodone Effectiveness. Acad. Emerg. Med. 2014, 21, 879–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, K.T.; McGain, D.; Cicali, E.J.; Knowlton, C.H.; Michaud, V.; Turgeon, J. Precision medication: An illustrative case series guiding the clinical application of multi-drug interactions and pharmacogenomics. Clin. Case Rep. 2019, 8, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnert, A.S.; Valenstein, M.; Bair, M.J.; Ganoczy, D.; McCarthy, J.F.; Ilgen, M.A.; Blow, F. Association Between Opioid Prescribing Patterns and Opioid Overdose-Related Deaths. JAMA 2011, 305, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, T.; Mamdani, M.; A Dhalla, I.; Paterson, J.M.; Juurlink, D.N. Opioid Dose and Drug-Related Mortality in Patients with Nonmalignant Pain. Arch. Intern. Med. 2011, 171. [Google Scholar] [CrossRef] [Green Version]
- Franklin, G.M.; Stover, B.D.; Turner, J.A.; Fulton-Kehoe, D.; Wickizer, T.M. Disability Risk Identification Study C. Early opioid prescription and subsequent disability among workers with back injuries: The Disability Risk Identification Study Cohort. Spine 2008, 33, 199–204. [Google Scholar] [CrossRef]
- Gomes, T.; Redelmeier, D.A.; Juurlink, D.N.; Dhalla, I.A.; Camacho, X.; Mamdani, M.M. Opioid Dose and Risk of Road Trauma in Canada. JAMA Intern. Med. 2013, 173, 196–201. [Google Scholar] [CrossRef] [Green Version]
- McCance-Katz, E.F.; Sullivan, L.E.; Nallani, S. Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and Other Frequently Prescribed Medications: A Review. Am. J. Addict. 2010, 19, 4–16. [Google Scholar] [CrossRef]
- Naito, T.; Takashina, Y.; Yamamoto, K.; Tashiro, M.; Ohnishi, K.; Kagawa, Y.; Kawakami, J. CYP3A5*3 Affects Plasma Disposition of Noroxycodone and Dose Escalation in Cancer Patients Receiving Oxycodone. J. Clin. Pharmacol. 2011, 51, 1529–1538. [Google Scholar] [CrossRef]
- Pon, D.; Hwang, C.J.; Lo, C.T.; Van Zyl, C. Decreased responsiveness to oxycodone: A case of a pharmacokinetic drug interaction? J. Opioid Manag. 2015, 11, 357–361. [Google Scholar] [CrossRef]
- Kawamoto, K.; A Houlihan, C.; Balas, E.A.; Lobach, D.F. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. BMJ 2005, 330, 765. [Google Scholar] [CrossRef] [Green Version]
- Hunt, D.L.; Haynes, R.B.; Hanna, S.E.; Smith, K. Effects of Computer-Based Clinical Decision Support Systems on Physician Performance and Patient Outcomes. JAMA 1998, 280, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Blum, K. Genetic addiction risk score GARS trade a predictor of vulnerability to opioid dependence. Front. Biosci. 2018, 10, 175–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenton, A.; Richeimer, S.; Sharma, M.; Lee, C.; Kantorovich, S.; Blanchard, J.; Meshkin, B. Observational study to calculate addictive risk to opioids: A validation study of a predictive algorithm to evaluate opioid use disorder. Pharmacogenomics Pers. Med. 2017, 10, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Guideline Resources. CDC Opioid Guideline Mobile App. Available online: https://www.cdc.gov/drugoverdose/prescribing/app.html (accessed on 19 August 2020).
- Christ, T.N.; Villadolid, J.J.; Choksi, A.; Malec, M.; Knoebel, R.W. Impact of a Clinical Decision Support Tool on Cancer Pain Management in Opioid-Tolerant Inpatients. Hosp. Pharm. 2017, 53, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Malte, C.A.; Berger, D.; Saxon, A.J.; Hagedorn, H.J.; Achtmeyer, C.E.; Mariano, A.J.; Hawkins, E.J. Electronic Medical Record Alert Associated With Reduced Opioid and Benzodiazepine Coprescribing in High-risk Veteran Patients. Med. Care 2018, 56, 171–178. [Google Scholar] [CrossRef]
- Maurer, D. Safe Opioids App Aims to Prescribe narcotics More Appropriately with Patients. Available online: https://www.imedicalapps.com/2016/02/safe-opioids-prescribing-narcotics-patients/ (accessed on 19 August 2020).
- American Pharmacist Association. OpioidCalc: A Tool to Address the Opioid Analgesic Overdose Epidemic. 2014. Available online: https://www.pharmacist.com/article/opioidcalc-tool-address-opioid-analgesic-overdose-epidemic (accessed on 19 August 2020).
- Oliva, E.M.; Bowe, T.; Tavakoli, S.; Martins, S.; Lewis, E.T.; Paik, M.; Wiechers, I.; Henderson, P.; Harvey, M.; Avoundjian, T.; et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol. Serv. 2017, 14, 34–49. [Google Scholar] [CrossRef]
- Patel, S.; Carmichael, J.M.; Taylor, J.M.; Bounthavong, M.; Higgins, D.T. Evaluating the Impact of a Clinical Decision Support Tool to Reduce Chronic Opioid Dose and Decrease Risk Classification in a Veteran Population. Ann. Pharmacother. 2017, 52, 325–331. [Google Scholar] [CrossRef]
- pH—Medical Opioid Converter by Philip Eagan. Available online: https://itunes.apple.com/us/app/ph-medical-opioid-converter/id1082147868?mt=8 (accessed on 19 August 2020).
- Ponton, R.; Sawyer, R. Opioid prescribing in general practice: Use of a two-stage review tool to identify and assess high-dose prescribing. Br. J. Pain 2017, 12, 171–182. [Google Scholar] [CrossRef]
- Price-Haywood, E.G.; Robinson, W.; Harden-Barrios, J.; Burton, J.; Burstain, T. Intelligent Clinical Decision Support to Improve Safe Opioid Management of Chronic Noncancer Pain in Primary Care. Ochsner J. 2018, 18, 30–35. [Google Scholar] [PubMed]
- Soto, R.; Yaldou, B. The Michigan Opioid Safety Score (MOSS): A Patient Safety and Nurse Empowerment Tool. J. PeriAnesthesia Nurs. 2015, 30, 196–200. [Google Scholar] [CrossRef]
- Trafton, J.A.; Martins, S.B.; Michel, M.C.; Wang, D.; Tu, S.W.; Clark, J.D.; Elliott, J.; Vucic, B.; Balt, S.; E Clark, M.; et al. Designing an automated clinical decision support system to match clinical practice guidelines for opioid therapy for chronic pain. Implement. Sci. 2010, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Wilsey, B.L.; Fishman, S.M.; Casamalhuapa, B.C.; Gupta, A. Documenting and Improving Opioid Treatment: The Prescription Opioid Documentation and Surveillance (PODS) System. Pain Med. 2009, 10, 866–877. [Google Scholar] [CrossRef]
- Manchikanti, L. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017, 2, s3–s92. [Google Scholar] [CrossRef]
- Haffey, F.; Brady, R.R.; Maxwell, S. A Comparison of the Reliability of Smartphone Apps for Opioid Conversion. Drug Saf. 2013, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bain, K.T.; Knowlton, C.H.; Turgeon, J. Medication Risk Mitigation. Clin. Geriatr. Med. 2017, 33, 257–281. [Google Scholar] [CrossRef]
- Schwartz, E.J.; Turgeon, J.; Patel, J.; Shah, H.; Issa, A.M.; Knowlton, O.V.; Bain, K.T.; Patel, P.; Knowlton, C.H. Implementation of a Standardized Medication Therapy Management Plus Approach within Primary Care. J. Am. Board Fam. Med. 2017, 30, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Smithburger, P.L.; Buckley, M.S.; Bejian, S.; Burenheide, K.; Kane-Gill, S.L. A critical evaluation of clinical decision support for the detection of drug–drug interactions. Expert Opin. Drug Saf. 2011, 10, 871–882. [Google Scholar] [CrossRef]
- Sweidan, M.; Reeve, J.F.; Brien, J.A.; Jayasuriya, P.; Martin, J.H.; Vernon, G.M. Quality of drug interaction alerts in prescribing and dispensing software. Med. J. Aust. 2009, 190, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Alterovitz, G.; Warner, J.L.; Zhang, P.; Chen, Y.; Ullman-Cullere, M.; Kreda, D.; Kohane, I.S. SMART on FHIR Genomics: Facilitating standardized clinico-genomic apps. J. Am. Med. Inform. Assoc. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, S.A.; Inflexxion, I.; Black, R.A.; Butler, S.F. Psychometric evaluation of the PainCAS Interference with Daily Activities, Psychological/Emotional Distress, and Pain scales. Qual. Life Res. 2017, 27, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; Bain, K.T.; Bankes, D.L.; Furman, A.; Skalski, B.; Verzicco, J.; Turgeon, J. Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia. Pharmacy 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linjakumpu, T.; Hartikainen, S.; Klaukka, T.; Koponen, H.; Kivelä, S.-L.; Isoaho, R. A model to classify the sedative load of drugs. Int. J. Geriatr. Psychiatry 2003, 18, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Lantz, M.S.; Buchalter, E.N.; Giambanco, V. Serotonin syndrome following the administration of tramadol with paroxetine. Int. J. Geriatr. Psychiatry 1998, 13, 343–345. [Google Scholar] [CrossRef]
- Seree, E.J.; Pisano, P.J.; Placidi, M.; Rahmani, R.; A Barra, Y. Identification of the human and animal hepatic cytochromes P450 involved in clonazepam metabolism. Fundam. Clin. Pharmacol. 1993, 7, 69–75. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Li, Q.; Zhu, M.; Du, A.; Tang, W.; Chen, W. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab. Dispos. 2014, 42, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Schmider, J.; Greenblatt, D.J.; Von Moltke, L.L.; Karsov, D.; I Shader, R. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: Studies of phenytoin p-hydroxylation. Br. J. Clin. Pharmacol. 1997, 44, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Parthipan, A.; Banerjee, I.; Humphreys, K.; Asch, S.M.; Curtin, C.; Carroll, I.; Hernandez-Boussard, T. Predicting inadequate postoperative pain management in depressed patients: A machine learning approach. PLoS ONE 2019, 14, e0210575. [Google Scholar] [CrossRef]
- Frost, D.A.; Soric, M.M.; Kaiser, R.; Neugebauer, R.E. Efficacy of Tramadol for Pain Management in Patients Receiving Strong Cytochrome P450 2D6 Inhibitors. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 724–729. [Google Scholar] [CrossRef]
- Brady, A.; Curtis, C.E.; Jalal, Z. Screening Tools Used by Clinical Pharmacists to Identify Elderly Patients at Risk of Drug-Related Problems on Hospital Admission: A Systematic Review. Pharmacy 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankes, D.L.; Jin, H.; Finnel, S.; Michaud, V.; Knowlton, C.H.; Turgeon, J.; Stein, A. Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, E.L.; Iverson, D.J.; Perkins, B.; Russell, J.W.; et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011, 76, 1758–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, J.C.; Kreda, D.A.; Mandl, K.D.; Kohane, I.S.; Ramoni, R.B. SMART on FHIR: A standards-based, interoperable apps platform for electronic health records. J. Am. Med. Inform. Assoc. 2016, 23, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- J2 Global. 3 Health IT Standards Driving Healthcare Interoperability in the US. Health IT Security. Available online: https://healthitsecurity.com/news/3-health-it-standards-driving-healthcare-interoperability-in-the-us (accessed on 21 July 2020).


| Source | Country | Setting | Sample Size, N | Name of CDSS | Feature(s) of CDSS |
|---|---|---|---|---|---|
| Blum, 2018 [68] | Multinational | N/A | N/A | Genetic Addiction Risk Score™ (GARS) |
|
| Brenton, 2017 [69] | US | 24 study sites | 908 | Proove Opioid Risk (POR) algorithm |
|
| CDC [70] | US | N/A | N/A | CDC Opioid Guideline App |
|
| Christ, 2018 [71] | US | University of Chicago Medicine | 30 (pre-enactment); 32 (post-enactment) | Pain Clinical Decision Support Tool (PCDST) |
|
| Genco, 2016 [19] | US | ED | 4581 | Epic electronic health record and computerized provider order entry system (Epic Systems Corporation, Verona, WI) with the First Databank drug information plug-in (First Databank, Inc., San Francisco, CA) |
|
| Malte, 2018 [72] | US | Veteran Affairs healthcare system | 1332 | No name provided |
|
| Maurer, 2016 [73] | US | N/A | N/A | Safe Opioids application |
|
| NYC Department of Health and Mental Hygiene [74] | US | N/A | N/A | OpioidCalc NYC |
|
| Oliva, 2017 [75] | US | Veteran Affairs healthcare system | 1,135,601 | StratificationTool for Opioid Risk Mitigation (STORM) |
|
| Patel, 2018 [76] | US | Veteran Affairs healthcare system | 7602 | Chronic Opioid Therapy–Clinical Reminder (COT-CR) |
|
| Philip Eagan [77] | US | N/A | N/A | pH-Medical Opioid Converter App |
|
| Ponton, 2018 [78] | UK | 41 general practitioner practices | 1881 | No name provided |
|
| Price-Haywood, 2018 [79] | US | Primary care providers, Ochsner Health System | 2640 | Opioid Risk Tool (ORT) |
|
| Sinha, 2017 [1] | US | N/A | N/A | Substitutable Medical Applications and Reusable Technologies (SMART) for CDSS app development |
|
| Soto, 2015 [80] | US | Inpatient | N/A | Michigan Opioid Safety Score (MOSS) |
|
| Trafton, 2010 [81] | US | Veteran Affairs healthcare system | N/A | No name provided |
|
| Wilsey, 2009 [82] | US | Veteran Affairs Pain Clinic | 1400 | The Prescription Opioid Documentation and Surveillance (PODS) System |
|
| Common Features | Description |
| Opioid prescription aides | Guiding the practice of prescribing opioids, such as quantity and days’ supply limitations. |
| Opioid conversion calculators | Determining the equianalgesic dose between opioids by calculating the total daily MME, taking into consideration the specific opioid, strength, and quantity. |
| Opioid drug alerts | Alerting clinicians to opioid-related factors that may pose a risk to the patient.
|
| Opioid prescribing guidelines | Referencing clinical practice guidelines to assist clinicians with opioid medication management. |
| Pain assessment tools | Utilizing applications and/or scoring methods for assessing the patient’s pain. |
| Common Shortcomings | Description |
| System-related |
|
| Content-related |
|
| Shortcomings of Drug Interaction Alert Software | |
| System-related |
|
| Content-related |
|
| Ideal Characteristics of Drug Interaction Alert Software | |
| System-related |
|
| Content-related |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.; Bankes, D.L.; Bain, K.T.; Ballinghoff, T.; Turgeon, J. Opioids, Polypharmacy, and Drug Interactions: A Technological Paradigm Shift Is Needed to Ameliorate the Ongoing Opioid Epidemic. Pharmacy 2020, 8, 154. https://doi.org/10.3390/pharmacy8030154
Matos A, Bankes DL, Bain KT, Ballinghoff T, Turgeon J. Opioids, Polypharmacy, and Drug Interactions: A Technological Paradigm Shift Is Needed to Ameliorate the Ongoing Opioid Epidemic. Pharmacy. 2020; 8(3):154. https://doi.org/10.3390/pharmacy8030154
Chicago/Turabian StyleMatos, Adriana, David L. Bankes, Kevin T. Bain, Tyler Ballinghoff, and Jacques Turgeon. 2020. "Opioids, Polypharmacy, and Drug Interactions: A Technological Paradigm Shift Is Needed to Ameliorate the Ongoing Opioid Epidemic" Pharmacy 8, no. 3: 154. https://doi.org/10.3390/pharmacy8030154
APA StyleMatos, A., Bankes, D. L., Bain, K. T., Ballinghoff, T., & Turgeon, J. (2020). Opioids, Polypharmacy, and Drug Interactions: A Technological Paradigm Shift Is Needed to Ameliorate the Ongoing Opioid Epidemic. Pharmacy, 8(3), 154. https://doi.org/10.3390/pharmacy8030154






