Addressing Barriers to HIV Point-of-Care Testing in Community Pharmacies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Development
2.2. Participants
2.3. Data Collection and Analysis
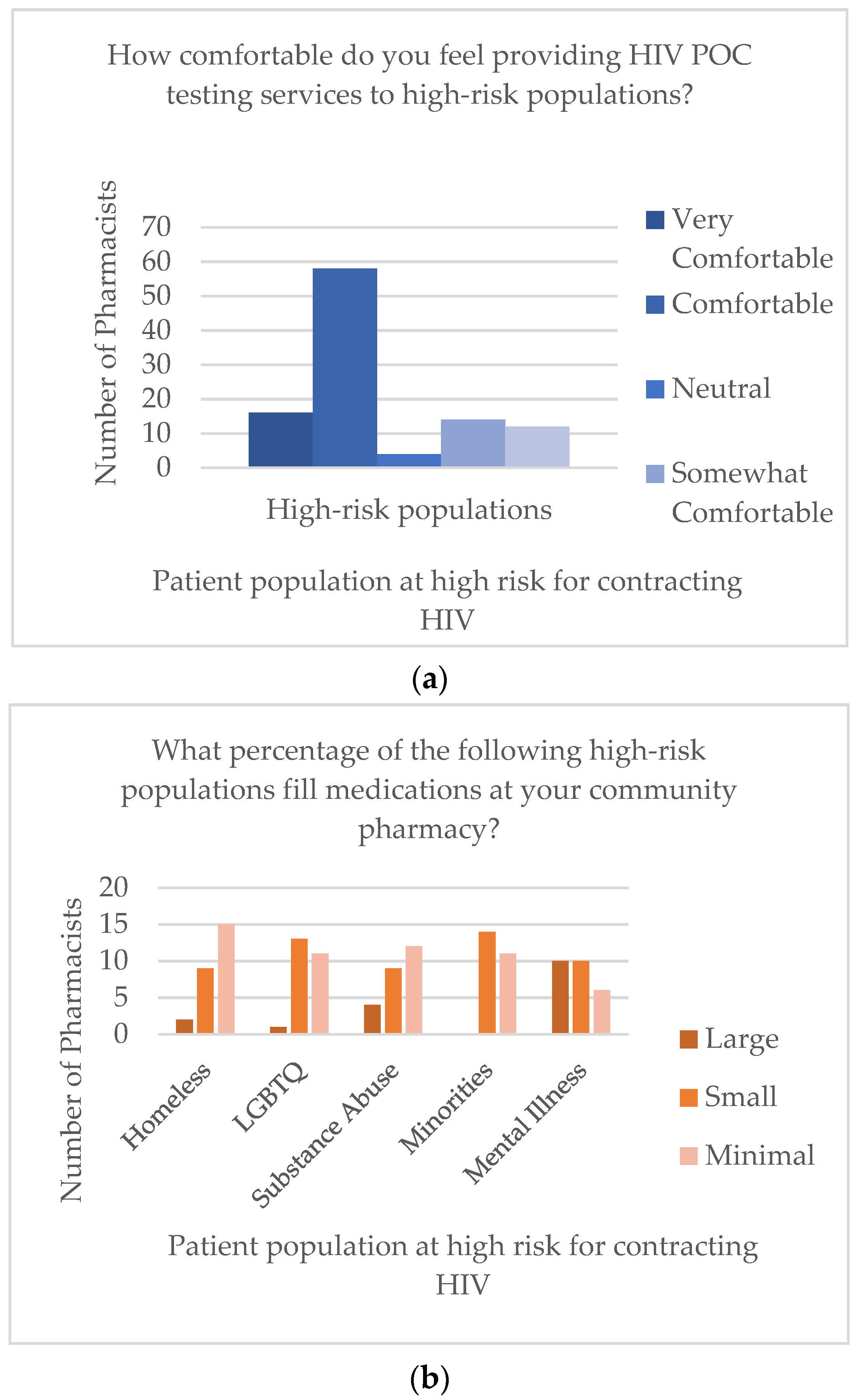
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HIV Surveillance. Available online: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 23 September 2020).
- HIV. Available online: https://www.cdc.gov/hiv/default.html (accessed on 30 March 2020).
- Li, Z.; Purcell, D.W.; Sansom, S.L.; Hayes, D.; Hall, H.I. Vital Signs: HIV Transmission Along the Continuum of Care—United States, 2016. Morb. Mortal. Wkly. Rep. 2019, 68, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HIV/AIDS and Homelessness. Available online: https://www.nationalhomeless.org/factsheets/hiv.html (accessed on 23 September 2020).
- Baral, S.D.; Poteat, T.; Strömdahl, S.; Wirtz, A.L.; Guadamuz, T.E.; Beyrer, C. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 214–222. [Google Scholar] [CrossRef]
- Who Is at Risk for HIV? Available online: https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/who-is-at-risk-for-hiv#:~:text=Blacks%2FAfrican%20Americans%20and%20Hispanics,significant%20risk%20for%20getting%20HIV (accessed on 1 August 2020).
- Dugdale, C.; Zaller, N.; Bratberg, J.; Berk, W.; Flanigan, T. Missed Opportunities for HIV Screening in Pharmacies and Retail Clinics. J. Manag. Care Spec. Pharm. 2014, 20, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, D.V.; Young, S.; Phillips, L.; Clark, D. Patient attitudes regarding the role of the pharmacist and interest in expanded pharmacist services. Can. Pharm. J. (Ott.) 2014, 147, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurses Continue to Rate Highest in Honesty, Ethics. Available online: https://news.gallup.com/poll/274673/nurses-continue-rate-highest-honesty-ethics.aspx (accessed on 23 September 2020).
- HIV Testing in Retail Pharmacies. Available online: https://www.cdc.gov/hiv/effective-interventions/diagnose/hiv-testing-in-retail-pharmacies?Sort=Title%3A%3Aasc&Intervention%20Name=HIV%20Testing%20in%20Retail%20Pharmacies (accessed on 23 September 2020).
- Clinical Laboratory Improvement Amendments of 1988. Available online: https://www.gpo.gov/fdsys/pkg/STATUTE-102/pdf/STATUTE-102-Pg2903.pdf (accessed on 15 April 2021).
- Clinical Laboratory Improvement Amendments (CLIA). Available online: https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA (accessed on 23 September 2020).
- Darin, K.M.; Klepser, M.E.; Klepser, D.E.; Klepser, S.A.; Reeves, A.; Young, M.; Scarsi, K.K. Pharmacist-provided rapid HIV testing in two community pharmacies. J. Am. Pharm. Assoc. 2015, 55, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lecher, S.L.; Shrestha, R.K.; Botts, L.W.; Alvarez, J.; Moore, J.H., Jr.; Thomas, V.; Weidle, P.J. Cost analysis of a novel HIV testing strategy in community pharmacies and retail clinics. J. Am. Pharm. Assoc. 2015, 55, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, P.O.; Klepser, M.E.; Dering-Anderson, A.M.; Bauer, K.A.; Darin, K.M.; Klepser, S.; Matthias, K.R.; Scarsi, K. Point-of-care testing for infectious diseases: Opportunities, barriers, and considerations in community pharmacy. J. Am. Pharm. Assoc. 2014, 54, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Facility Search. Available online: https://fortress.wa.gov/doh/facilitysearch/ (accessed on 15 April 2020).
- Weidle, P.J.; Lecher, S.; Botts, L.W.; Jones, L.; Spach, D.H.; Alvarez, J.; Jones, R.; Thomas, V. HIV testing in community pharmacies and retail clinics: A model to expand access to screening for HIV infection. J. Am. Pharm. Assoc. 2014, 54, 486–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, N.D.; Dean, T.; Rivera, A.V.; Guffey, T.; Amesty, S.; Rudolph, A.; DeCuir, J.; Fuller, C.M. Pharmacy Intervention to Improve HIV Testing Uptake Using a Comprehensive Health Screening Approach. Public Health Rep. 2016, 131, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, A.C.; Andres, J.L.; Grover, A.B.; Megherea, O. Pharmacist Comfort and Awareness of HIV and HCV Point-of-Care Testing in Community Settings. Health Promot. Pract. 2019, 21, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ryder, P.T.; Meyerson, B.E.; Coy, K.C.; Hippel, C.D.V. Pharmacists’ perspectives on HIV testing in community pharmacies. J. Am. Pharm. Assoc. 2013, 53, 595–600. [Google Scholar] [CrossRef]
- Consolidated Framework for Implementation Research. Available online: https://cfirguide.org/ (accessed on 9 April 2021).
- Mattingly, A.N.; Mattingly, T.J., 2nd. Advancing the role of the pharmacy technician: A systematic review. J. Am. Pharm. Assoc. 2018, 58, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Senate Bill No. 159. Available online: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159 (accessed on 23 September 2020).
- Young, S.D.; Bendavid, E. The relationship between HIV testing, stigma, and health service usage. AIDS Care 2010, 22, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. Ann. Emerg. Med. 2007, 49, 575–777. [Google Scholar]
- Hill, H.; Cardosi, L.; Henson, L.; Fountain, M.; Desselle, S.; Hohmeier, K.C. Evaluating advanced pharmacy technician roles in the provision of point-of-care testing. J. Am. Pharm. Assoc. 2020, 60, E64–E69. [Google Scholar] [CrossRef] [PubMed]
- Siegel, K.; Meyer, I.H. Hope and resilience in suicide ideation and behavior of gay and bisexual men following notification of HIV infection. AIDS Educ. Prev. 1999, 11, 53–64. [Google Scholar] [PubMed]
- Stevens, P.E.; Hildebrandt, E. Life changing words: Women’s responses to being diagnosed with HIV infection. ANS Adv. Nurs. Sci. 2006, 29, 207–221. [Google Scholar] [CrossRef] [PubMed]
| Question | Very Likely n (%) | Likely n (%) | Neutral n (%) | Somewhat Likely n (%) | Not Likely n (%) | Omit n (%) |
|---|---|---|---|---|---|---|
| 4. If you were offered a 2 h training session on how to administer the point-of-care testing (POCT) and accurately interpret the results, how likely are you to implement human immunodeficiency virus (HIV) POCT in your community pharmacy? | 6 (23) | 13 (50) | 2 (8) | 2 (8) | 3 (11) | 0 (0) |
| 5. If you were offered a 4 h education session on HIV prevention and screening covering topics such as: Disease state overview, risk factors for transmission, special populations, and pre-exposure prophylaxis (PrEP), how likely are you to implement HIV POCT in your community pharmacy? | 7 (26) | 12 (46) | 3 (12) | 2 (8) | 2 (8) | 0 (0) |
| 6. If you were offered training on couples testing, post-test counseling and de-escalation techniques, how likely are you to implement HIV POCT in your community pharmacy? | 5 (19) | 10 (39) | 2 (8) | 4 (15) | 4 (15) | 1 (4) |
| 7. If you were offered semi-annual (every 6 months) continuing education (CE) training for HIV POCT, how likely are you to implement HIV POCT in your community pharmacy? | 4 (16) | 11 (42) | 5 (19) | 4 (16) | 2 (7) | 0 (0) |
| 8. Pharmacists are increasingly gaining authority to prescribe Pre-Exposure Prophylaxis (PrEP) for individuals who are at high risk for acquiring HIV through Collaborate Drug Therapy Agreements (CDTA). If you were offered training and education in identifying high-risk patients who may benefit from the use of PrEP, how likely are you to implement prescribing and dispensing of PrEP at your pharmacy? | 8 (31) | 13 (50) | 2 (8) | 1 (4) | 2 (7) | 0 (0) |
| 10. Pharmacists have historically reported a lack of staffing, pharmacist availability and physical space to provide clinical services in the community setting. If there was a pharmacist position solely dedicated to providing clinical services in your pharmacy (such as POCT, immunizations, etc.) how likely are you to implement HIV POCT in your community pharmacy? | 13 (50) | 8 (31) | 3 (12) | 0 (0) | 2 (7) | 0 (0) |
| 11. If Pharmacy Technicians, with the appropriate training and education, were providing the entirety of the HIV POCT service, how likely are you to implement these services in your pharmacy? | 3 (12) | 11 (42) | 3 (12) | 0 (0) | 9 (34) | 0 (0) |
| 12. If Pharmacy Technicians, with the appropriate training and education, were to administer the HIV POCT and refer to the pharmacist for interpretation, post-test counseling and referral, how likely are you to implement these services in your community pharmacy? | 9 (34) | 8 (31) | 2 (8) | 1 (4) | 6 (23) | 0 (0) |
| 13. HIV carries many stigma-related concerns and thus a private area to provide HIV POCT services is necessary. If your pharmacy had a private counseling room, how likely are you to implement HIV POCT in your community pharmacy? | 10 (38) | 13 (50) | 2 (8) | 0 (0) | 1 (4) | 0 (0) |
| 17. If there was a protocol in place for referral of patients who have a reactive HIV screening with specific local partners that offer confirmatory diagnostic testing, how likely are you to implement HIV POCT services in your community pharmacy? | 10 (38) | 11 (42) | 3 (12) | 0 (0) | 1 (4) | 1 (4) |
| 18. If there was a protocol in place for referral of patients who require comprehensive post-test counseling or referral for PrEP, how likely are you to implement HIV POCT services in your community pharmacy? | 8 (31) | 13 (50) | 4 (15) | 0 (0) | 1 (4) | 0 (0) |
| 19. If there was a standardized risk-determination questionnaire that would indicate whether the patient qualifies for PrEP, how likely are you to implement the prescribing/dispensing PrEP to high-risk patients? | 12 (46) | 8 (31) | 4 (15) | 1 (4) | 1 (4) | 0 (0) |
| 20. If there was a standardized script provided for post-test counseling on reactive and non-reactive HIV POCT, how likely are you to implement HIV POCT services in your community pharmacy? | 9 (35) | 12 (46) | 3 (11) | 0 (0) | 2 (8) | 0 (0) |
| Pharmacists’ Suggestions of Training that Would Help them Feel Comfortable Providing Human Immunodeficiency Virus (HIV) Point-of-Care Testing (POCT) (Question 9) |
| • Substantially more additional training about HIV in general (n = 7, 27%) |
| • Hands-on experience with testing (n = 5, 19%) |
| • Support from corporate leadership (n = 5, 19%) |
| • More staff/labor hours (n = 3, 12%) |
| • Assistance with development of a collaborative drug therapy agreement (CDTA) (n = 2, 8%) |
| • A detailed implementation plan (n = 2, 8%) |
| • Training on talking to couples about reactive results (n = 1, 4%) |
| • Training for de-escalation during emotional situations (n = 1, 4%) |
| • And a motivational interviewing refresher course (n = 1, 4%) |
| Pharmacists’ opinions of the amount of time needed to conduct the HIV POCT, discuss results, and provide education and counseling if the test itself takes 5 min (Question 14) |
| • 5 to 10 min (n = 1, 4%) |
| • 15 to 20 min (n = 4, 15%) |
| • 20 to 30 min (n = 14, 54%) |
| • 30 to 45 min (n = 4, 15%) |
| • More than 45 min (n = 2, 8%) |
| • Prefer not to answer (n = 1, 4%) |
| Challenges to implementing HIV POCT beyond staffing time and physical space identified by pharmacist participants (Question 16) |
| • Corporate support for implementation (n = 4, 15%) |
| • Adding HIV POCT into workflow (n = 3, 12%) |
| • Advertising the service to the community (n = 2, 8%) |
| • Cost of testing and potential for reimbursement (n = 1, 4%) |
| Concerns regarding referring patients with a reactive screening and collaborating with community partners identified by pharmacist participants (Question 21) |
| • Ensuring patients followed up with another provider and not “falling between the cracks” (n = 3, 12%) |
| • Cost of testing and insurance coverage for testing and pre-exposure prophylaxis (PrEP)/post-exposure prophylaxis (PEP) (n = 2, 8%) |
| • Obtaining liver and kidney function blood tests prior to beginning PreP/PEP (n = 1, 4%) |
| • Limitations of the pharmacy’s computer system to document encounters (n = 1, 4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKeirnan, K.; Kherghehpoush, S.; Gladchuk, A.; Patterson, S. Addressing Barriers to HIV Point-of-Care Testing in Community Pharmacies. Pharmacy 2021, 9, 84. https://doi.org/10.3390/pharmacy9020084
McKeirnan K, Kherghehpoush S, Gladchuk A, Patterson S. Addressing Barriers to HIV Point-of-Care Testing in Community Pharmacies. Pharmacy. 2021; 9(2):84. https://doi.org/10.3390/pharmacy9020084
Chicago/Turabian StyleMcKeirnan, Kimberly, Sorosh Kherghehpoush, Angie Gladchuk, and Shannon Patterson. 2021. "Addressing Barriers to HIV Point-of-Care Testing in Community Pharmacies" Pharmacy 9, no. 2: 84. https://doi.org/10.3390/pharmacy9020084
APA StyleMcKeirnan, K., Kherghehpoush, S., Gladchuk, A., & Patterson, S. (2021). Addressing Barriers to HIV Point-of-Care Testing in Community Pharmacies. Pharmacy, 9(2), 84. https://doi.org/10.3390/pharmacy9020084







