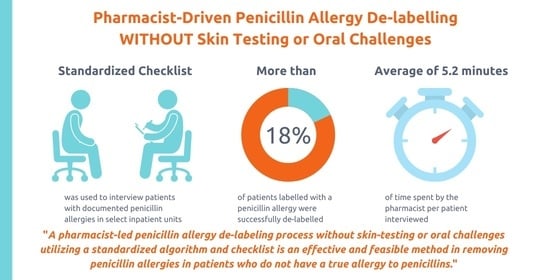
Effectiveness and Feasibility of Pharmacist-Driven Penicillin Allergy De-Labeling Pilot Program without Skin Testing or Oral Challenges
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shenoy, E.S.; Macy, E.; Rowe, T.; Blumenthal, K.G. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019, 321, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.A.; Bates, A.; Brigham, T.J.; Imam, J.S.; Burton, M.C. Clinical Outcomes Following Inpatient Penicillin Allergy Testing: A Systematic Review and Meta-Analysis. Allergy 2017, 72, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Lu, N.; Zhang, Y.; Li, Y.; Walensky, R.P.; Choi, H.K. Risk of Meticillin Resistant Staphylococcus Aureus and Clostridium Difficile in Patients with a Documented Penicillin Allergy: Population Based Matched Cohort Study. BMJ 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Ryan, E.E.; Li, Y.; Lee, H.; Kuhlen, J.L.; Shenoy, E.S. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin. Infect. Dis. 2018, 66, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Devchand, M.; Kirkpatrick, C.M.J.; Stevenson, W.; Garrett, K.; Perera, D.; Khumra, S.; Urbancic, K.; Grayson, M.L.; Trubiano, J.A. Evaluation of a Pharmacist-Led Penicillin Allergy de-Labelling Ward Round: A Novel Antimicrobial Stewardship Intervention. J. Antimicrob. Chemother. 2019, 74, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, A.J.; Pulver, L.S.; Korgenski, E.K.; Savitz, L.A.; Daly, J.A.; Byington, C.L. Clindamycin-Resistant Group B Streptococcus and Failure of Intrapartum Prophylaxis to Prevent Early-Onset Disease. J. Pediatr. 2010, 156, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Prevention of Perinatal Group B Streptococcal Disease. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm (accessed on 21 June 2020).
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns|ACOG. Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns (accessed on 21 June 2020).
- Ramsey, A.; Staicu, M.L. Use of a Penicillin Allergy Screening Algorithm and Penicillin Skin Testing for Transitioning Hospitalized Patients to First-Line Antibiotic Therapy. J. Allergy Clin. Immunol. Pract. 2018, 6, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.R.; Huebner, E.M.; Blumenthal, K.G. Acute Care Beta-Lactam Allergy Pathways: Approaches and Outcomes. Ann. Allergy Asthma Immunol. 2019, 123, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Li, Y.; Banerji, A.; Yun, B.J.; Long, A.A.; Walensky, R.P. The Cost of Penicillin Allergy Evaluation. J. Allergy Clin. Immunol. Pract. 2018, 6, 1019–1027.e2. [Google Scholar] [CrossRef] [PubMed]
- Evaluation and Diagnosis of Penicillin Allergy for Healthcare Professionals | Community | Antibiotic Use | CDC. Available online: https://www.cdc.gov/antibiotic-use/community/for-hcp/Penicillin-Allergy.html (accessed on 6 February 2020).
- Herbert, M.E.; Brewster, G.S.; Lanctot-Herbert, M. Ten Percent of Patients Who Are Allergic to Penicillin Will Have Serious Reactions If Exposed to Cephalosporins. West. J. Med. 2000, 172, 341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelkar, P.S.; Li, J.T.-C. Cephalosporin Allergy. N. Engl. J. Med. 2001, 345, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Campagna, J.D.; Bond, M.C.; Schabelman, E.; Hayes, B.D. The Use of Cephalosporins in Penicillin-Allergic Patients: A Literature Review. J. Emerg. Med. 2012, 42, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Zagursky, R.J.; Pichichero, M.E. Cross-Reactivity in β-Lactam Allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 72–81.e1. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters Drug Allergy: An Updated Practice Parameter. Ann. Allergy Asthma Immunol. 2010, 105, 78.
- Blumenthal, K.; Fu, X.; Zhang, Y.; Kuper, K.; Schulz, L.; Bhowmick, T.; Postelnick, M.; Lee, F.; Walensky, R. Association of Penicillin Allergy Documentation and Antibiotic Use: A National Inpatient Study. J. Allergy Clin. Immunol. 2020, 145, AB175. [Google Scholar] [CrossRef]
- Mattingly II, T.J.; Heil, E.L. The Economics of Penicillin Allergy Testing: Still Scratching the Value Surface. Clin. Infect. Dis. [CrossRef] [PubMed]
- Mattingly, T.J.; Fulton, A.; Lumish, R.A.; Williams, A.M.C.; Yoon, S.; Yuen, M.; Heil, E.L. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1649–1654.e4. [Google Scholar] [CrossRef] [PubMed]


| MB | Med/Surg | Total | |
|---|---|---|---|
| De-labeled | 4 | 8 | 12 |
| Intolerance | 1 | 3 | 4 |
| True allergy | 18 | 32 | 50 |
| Total | 23 | 43 | 66 |
| Time Spent (min) | |
|---|---|
| Mean | 5.2 |
| Median | 5 |
| Range | 12 |
| Minimum | 3 |
| Maximum | 15 |
| Prescribed Antibiotics after De-Labeling/or Re-Labeling as Intolerance | Tolerated a Beta-Lactam Agent after De-Labeling | Agents Tolerated | |
|---|---|---|---|
| De-labeled 7/12 (58.3%) | 7/7 (100%) | Amoxicillin/Clavulanate | 1 |
| Piperacillin/Tazobactam | 2 | ||
| Cephalexin | 5 | ||
| Cefazolin | 3 | ||
| Cefuroxime Axetil | 1 | ||
| Ceftriaxone | 3 | ||
| Cefepime | 2 | ||
| Intolerance 2/4 (50%) | 2/2 (100%) | Ampicillin/Sulbactam | 1 |
| Cefuroxime Axetil | 2 | ||
| Cefdinir | 1 | ||
| Total 9/16 (56.3%) | 9/9 (100%) | ||
| Patient 1: De-Labeling of an Amoxicillin Allergy Patient reported a one-time GI reaction and some tingling after taking eight tablets of amoxicillin on an empty stomach prior to a dental procedure. Patient repeatedly reported during multiple inpatient/outpatient visits that he did not have an allergy but the allergy was never removed from the chart. Pharmacy resident removed the amoxicillin allergy after the interview. However, upon reviewing this patient’s case, penicillin allergy was added to patients’ chart during a subsequent provider visit. The patient tolerated piperacillin/tazobactam, cephalexin, and cefepime after de-labeling. |
| Patient 2: Mislabeled Dicloxacillin Allergy Patient had a documented dicloxacillin allergy. When asked about this allergy, patient denied the allergy and reported being allergic to doxycycline. De-labeling was performed and doxycycline was added to patient’s allergy list. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-C.; Nelson, Z.J.; Wankum, M.A.; Gens, K.D. Effectiveness and Feasibility of Pharmacist-Driven Penicillin Allergy De-Labeling Pilot Program without Skin Testing or Oral Challenges. Pharmacy 2021, 9, 127. https://doi.org/10.3390/pharmacy9030127
Song Y-C, Nelson ZJ, Wankum MA, Gens KD. Effectiveness and Feasibility of Pharmacist-Driven Penicillin Allergy De-Labeling Pilot Program without Skin Testing or Oral Challenges. Pharmacy. 2021; 9(3):127. https://doi.org/10.3390/pharmacy9030127
Chicago/Turabian StyleSong, You-Chan, Zachary J. Nelson, Michael A. Wankum, and Krista D. Gens. 2021. "Effectiveness and Feasibility of Pharmacist-Driven Penicillin Allergy De-Labeling Pilot Program without Skin Testing or Oral Challenges" Pharmacy 9, no. 3: 127. https://doi.org/10.3390/pharmacy9030127
APA StyleSong, Y.-C., Nelson, Z. J., Wankum, M. A., & Gens, K. D. (2021). Effectiveness and Feasibility of Pharmacist-Driven Penicillin Allergy De-Labeling Pilot Program without Skin Testing or Oral Challenges. Pharmacy, 9(3), 127. https://doi.org/10.3390/pharmacy9030127