Synthetic Micro/Nanomotors for Drug Delivery
Abstract
:1. Introduction
2. Designing Micro/Nanomotors
2.1. Tubular and Rod Motors
2.2. Janus Motors
2.3. Roughness
3. Environmental Factors Affecting the Motor Motion
3.1. Viscosity
3.2. Temperature
4. Powering the Motion of Micro/Nanomotors
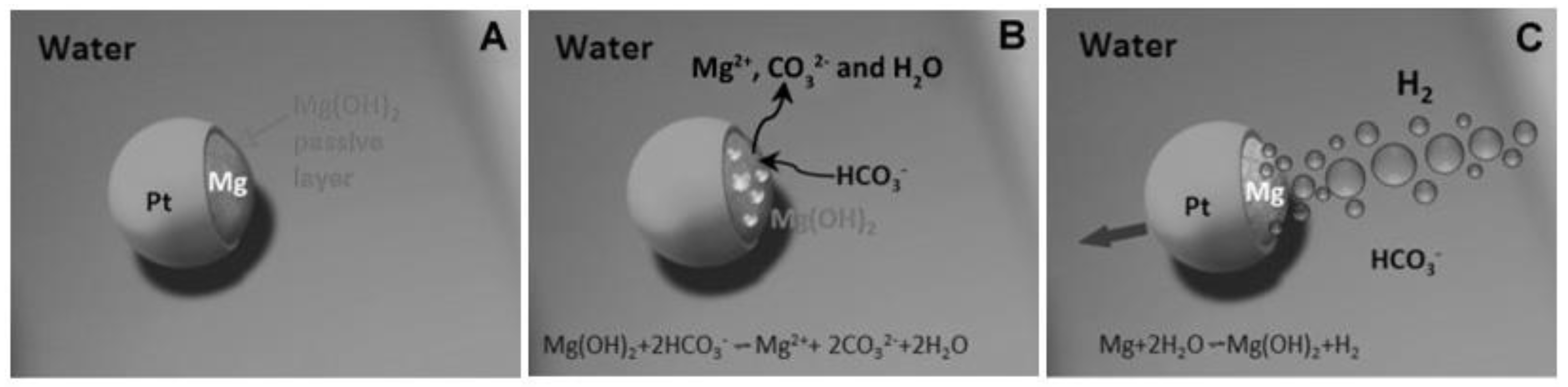
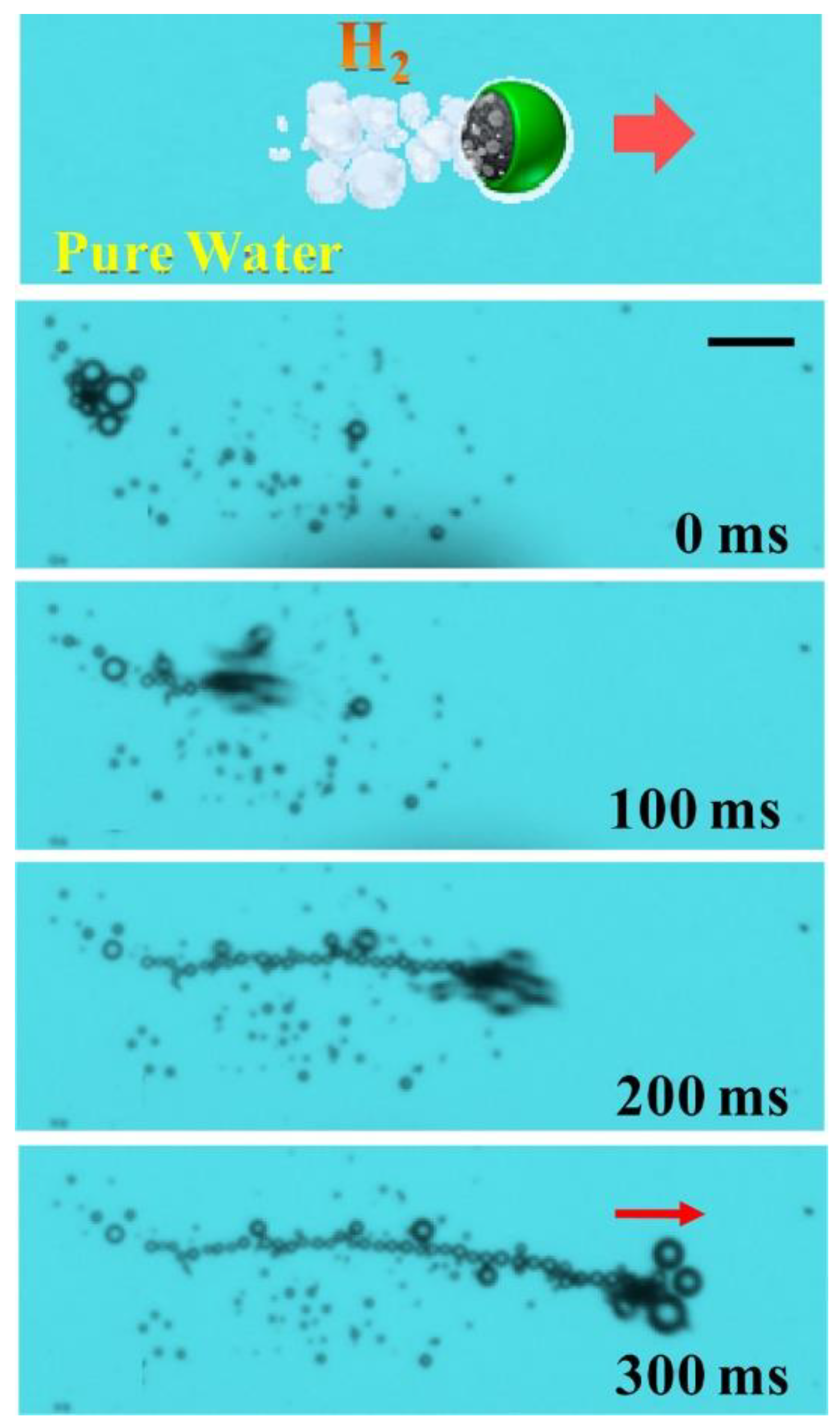
4.1. Endogenous Powered Micro/Nanomotors
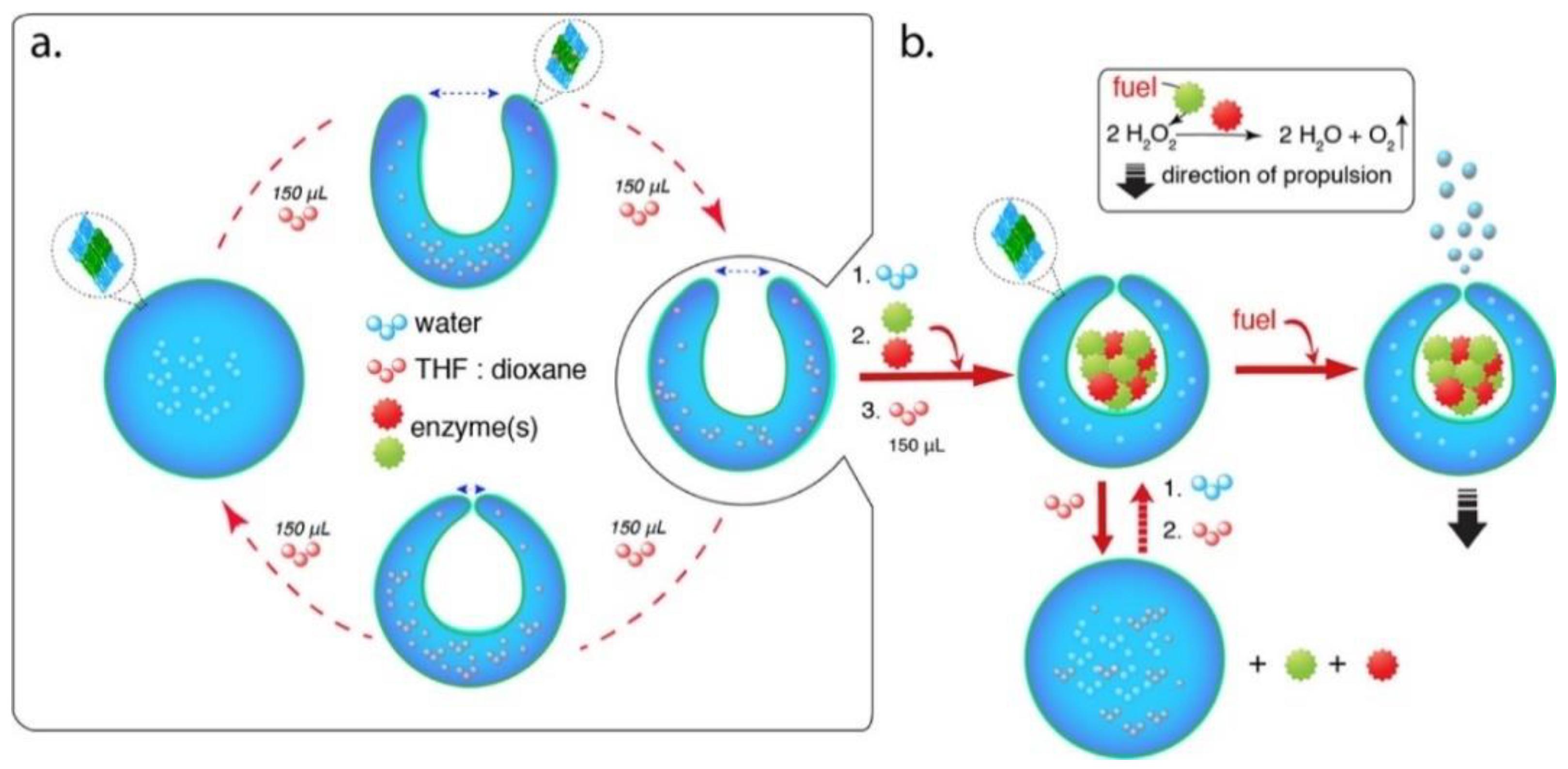
Enzyme-Actuated Micro/Nanomotors
4.2. Externally Actuated Micro/Nanomotors
4.2.1. Magnetically Guided Micro/Nanomotors
4.2.2. Electric Propulsion of Micro/Nanomotors
4.2.3. Light-Actuated Micro/Nanomotors
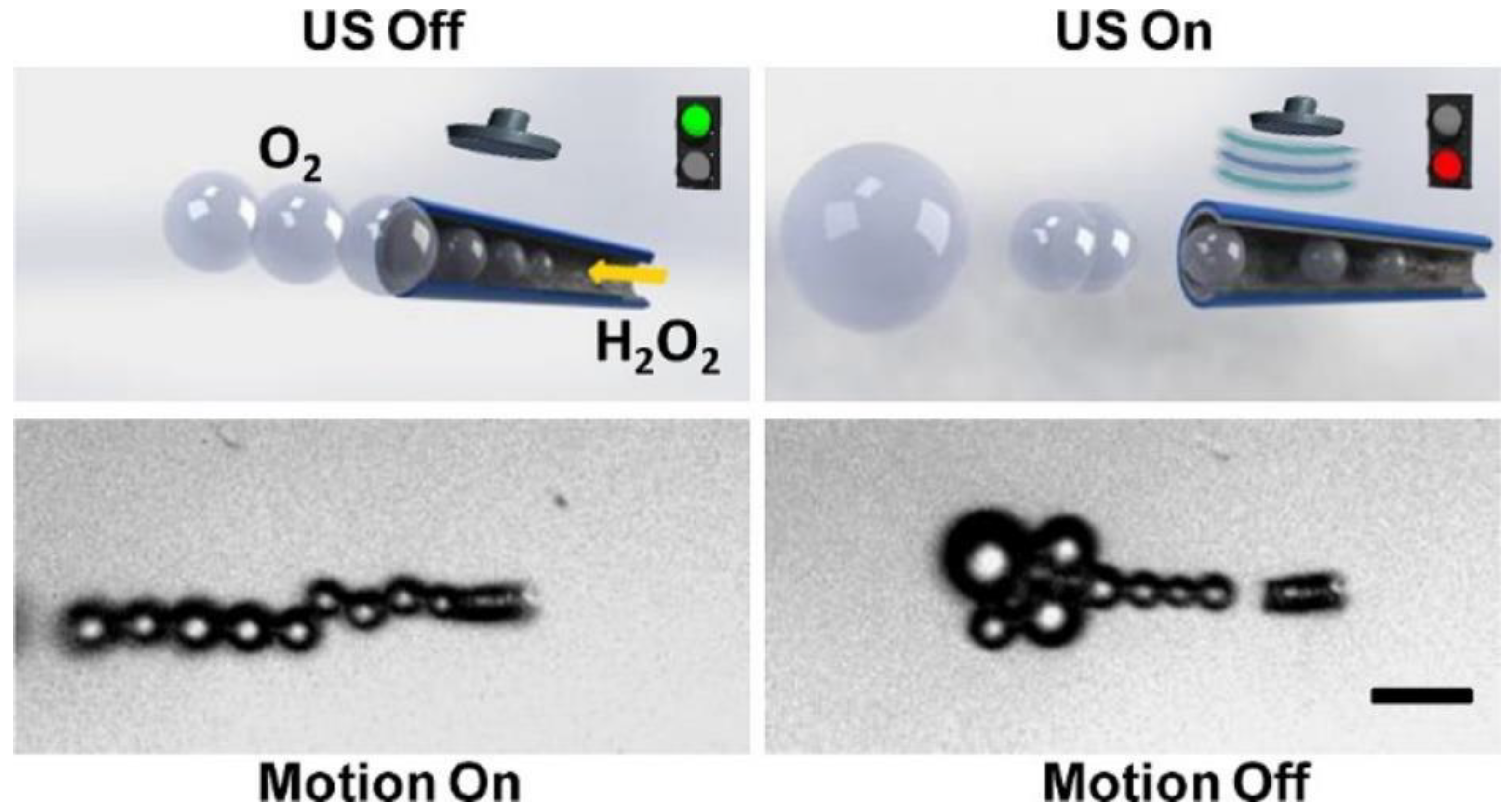
4.2.4. Ultrasound-Actuated Micro/Nanomotors
5. Towards the Biocompatibility of Micro/Nanomotors
6. Micro-/Nano-Motors in Drug Delivery
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez, S.; Soler, L.; Katuri, J. Chemically Powered Micro-and Nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Self-propelling micro-/nano-motors: Mechanisms, applications, and challenges in drug delivery. Int. J. Pharm. 2021, 596, 120275. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Khan, A.; Rahim, M.A.; Naeem, A.; Fahad, M.; Badshah, S.F.; Jabar, A.; Janakiraman, A.K. Micro and nanorobot-based drug delivery: An overview. J. Drug Target. 2022, 30, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Ma, X.; Katuri, J.; Simmchen, J.; Stanton, M.M.; Trichet-Paredes, C.; Soler, L.; Sanchez, S. Nano and micro architectures for self-propelled motors. Sci. Technol. Adv. Mater. 2015, 16, 014802. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Xu, H.; Schmidt, O.G. Micro-and nano-motors: The new generation of drug carriers. Ther. Deliv. 2018, 1, 303–316. [Google Scholar] [CrossRef]
- Abdelmohsen, L.K.E.A.; Peng, F.; Tu, Y.; Wilson, D.A. Micro-and nano-motors for biomedical applications. J. Mater. Chem. B 2014, 2, 2395–2408. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Li, T.; Song, W.; Zhang, G. A unified model of drag force for bubble-propelled catalytic micro/nano-motors with different geometries in low Reynolds number flows. J. Appl. Phys. 2015, 117, 104308. [Google Scholar] [CrossRef]
- Li, J.; Ávila, B.E.-F.d.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Solovev, A.A.; Xi, W.; Gracias, D.H.; Harazim, S.M.; Deneke, C.; Sanchez, S.; SchmidT, O.G. Self-Propelled Nanotools. ACS Nano 2012, 6, 1751–1756. [Google Scholar] [CrossRef]
- Campuzano, S.; Ávila, B.E.-F.d.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Wang, J. Nano/micro-vehicles for efficient delivery and (bio)sensing at cellular level. Chem. Sci. 2017, 8, 6750–6763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, R.; Wu, H.; Cai, Y.; Ren, B. A Review on Artificial Micro/Nanomotors for Cancer-Targeted Delivery, Diagnosis, and Therapy. Nano-Micro Lett. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cui, H.; Wang, Y.; Du, X. Microfluidic platforms toward rational material fabrication for biomedical applications. Small 2019, 16, 1903798. [Google Scholar] [CrossRef] [PubMed]
- Karshalev, E.; Ávila, B.E.-F.D.; Beltrán-Gastélum, M.; Angsantikul, P.; Tang, S.; Mundaca-Uribe, R.; Zhang, F.; Zhao, J.; Wang, L.Z. Micromotor Pills as a Dynamic Oral Delivery Platform. ACS Nano 2018, 12, 8397–8405. [Google Scholar] [CrossRef]
- Fu, D.; Wang, Z.; Tu, Y.; Peng, F. Interactions between Biomedical Micro-/Nano-Motors and the Immune Molecules, Immune Cells, and the Immune System: Challenges and Opportunities. Adv. Healthc. Mater. 2021, 7, 2001788. [Google Scholar] [CrossRef]
- Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.-E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics 2020, 12, 665. [Google Scholar] [CrossRef]
- Hato, T.; Dagher, P.C. How the Innate Immune System Senses Trouble and Causes Trouble. Clin. J. Am. Soc. Nephrol. 2015, 10, 1459–1469. [Google Scholar] [CrossRef]
- Ou, J.; Liu, K.; Jiang, J.; Wilson, D.A.; Liu, L.; Wang, F.; Wang, S.; Tu, Y.; Peng, F. Micro-/Nanomotors toward Biomedical Applications: The Recent Progress in Biocompatibility. Small 2020, 16, 1906184. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef]
- Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines 2017, 8, 267. [Google Scholar] [CrossRef]
- Huang, W.; Manjare, M.; Zhao, Y. Catalytic Nanoshell Micromotors. J. Phys. Chem. C 2013, 117, 21590–21596. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, Q.; Liu, L.; Liu, Q.; Bai, T.; Wang, Q. A Viscosity-Based Model for Bubble-Propelled Catalytic Micromotors. Micromachines 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Mei, Y.; Ureña, E.B.; Huang, G.; Schmidt, O.G. Catalytic Microtubular Jet Engines Self-Propelled by Accumulated Gas Bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Shklyaev, S. Janus droplet as a catalytic micromotor. Eur. Phys. Lett. 2015, 110, 54002. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, B.; Zhao, Y.-P. Bubble Driven Quasioscillatory Translational Motion of Catalytic Micromotors. Phys. Rev. Lett. 2012, 109, 128305. [Google Scholar] [CrossRef]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Sattayasamitsathit, S.; Gao, W.; Santos, L.; Fedorak, Y.; Singh, V.V.; Orozco, J.; Galarnyk, M.; Wang, J. Self-Propelled Activated Carbon Janus Micromotors for Efficient Water Purification. Small 2015, 11, 499–506. [Google Scholar] [CrossRef]
- Maria-Hormigos, R.; Jurado-Sanchez, B.; Vazquez, L.; Escarpa, A. Carbon Allotrope Nanomaterials Based Catalytic Micromotors. Chem. Mater. 2016, 28, 8962–8970. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Li, L.; Wang, J.; Song, W.; Zhang, G. Microrocket Based Viscometer. ECS J. Solid State Sci. Technol. 2015, 4, S3020–S3023. [Google Scholar] [CrossRef]
- Zhao, G.; Nguyen, N.-T.; Pumera, M. Reynolds numbers influence the directionality of self-propelled microjet engines in the 10−4 regime. Nanoscale 2013, 5, 7277–7283. [Google Scholar] [CrossRef]
- Sanchez, S.; Ananth, A.N.; Fomin, V.M.; Marlitt, V.; Schmidt, O.G. Superfast Motion of Catalytic Microjet Engines at Physiological Temperature. J. Am. Chem. Soc. 2011, 133, 14860–14863. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, I.L.; Cherkasov, V.R.; Tregubov, A.A.; Buiucli, S.R.; Nikitin, M.P. Smart materials on the way to theranostic nanorobots: Molecular machines and nanomotors, advanced biosensors, and intelligent vehicles for drug delivery. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/Nanorobots at Work in Active Drug Delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Y.; Peng, F. The Energy Conversion behind Micro-and Nanomotors. Micromachines 2021, 12, 222. [Google Scholar] [CrossRef]
- Kagan, D.; Laocharoensuk, R.; Zimmerman, M.; Clawson, C.; Balasubramanian, S.; Kang, D.; Bishop, D.; Sattayasamitsathit, S.; Zhang, L.; Wang, J. Rapid Delivery of Drug Carriers Propelled and Navigated by Catalytic Nanoshuttles. Small 2010, 6, 2741–2747. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Wu, Z.; Möhwald, H.; He, Q. Self-Propelled Polymer Multilayer Janus Capsules for Effective Drug Delivery and Light-Triggered Release. ACS Appl. Mater. Interfaces 2014, 6, 10476–10481. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; André, A.A.M.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable Hybrid Stomatocyte Nanomotors for Drug Delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef]
- Jang, B.; Wang, W.; Wiget, S.; Petruska, A.J.; Chen, X.; Hu, C.; Hong, A.; Folio, D.; Ferreira, A.; Pané, S.; et al. Catalytic Locomotion of Core–Shell Nanowire Motors. ACS Nano 2016, 10, 9983–9991. [Google Scholar] [CrossRef]
- Popescu, M.; Uspal, W.; Dietrich, S. Self-diffusiophoresis of chemically active colloids. Eur. Phys. J. Spec. Top. 2016, 225, 2189–2206. [Google Scholar] [CrossRef]
- Velegol, D.; Garg, A.; Guh, R.; Kar, A.; Kumara, M. Origins of concentration gradients for diffusiophoresis. Soft Matter 2016, 12, 4686–4703. [Google Scholar] [CrossRef]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling Behavior of Light-Powered Autonomous Micromotors in Water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Wang, Z.; Tian, F.; You, J.A.; Xu, S. A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme. Micromachines 2018, 9, 537. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Orozco, J.; Wang, J. Highly Efficient Catalytic Microengines: Template Electrosynthesis of Polyaniline/Platinum Microtubes. J. Am. Chem. Soc. 2011, 133, 11862–11864. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Solovev, A.A.; Mei, Y.; Schmidt, O.G. Dynamics of Biocatalytic Microengines Mediated by Variable Friction Control. J. Am. Chem. Soc. 2010, 32, 13144–13145. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-Propelled Micromotors Driven by the Magnesium-Water Reaction and Their Hemolytic Properties. Angew. Chem. Int. Ed. 2013, 52, 7208–7212. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2015, 46, 7787–7812. [Google Scholar] [CrossRef]
- Ouyang, L.; Ma, M.; Huang, M.; Duan, R.; Wang, H.; Sun, L.; Zhu, M. Enhanced hydrogen generation properties of MgH2-based hydrides by breaking the magnesium hydroxide passivation layer. Energies 2015, 8, 4237–4252. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Wang, J. Water-Driven Micromotors. ACS Nano 2012, 6, 8432–8438. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; de Ávila, B.E.F.; Li, T.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Water-Powered Cell-Mimicking Janus Micromotor. Adv. Funct. Mater. 2015, 15, 7497–7501. [Google Scholar] [CrossRef]
- Ma, X.; Sánchez, S. Bio-catalytic mesoporous Janus nano-motors powered by catalase enzyme. Tetrahedron 2017, 73, 4883–4886. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Wang, L.; Ma, X. Fundamentals and applications of enzyme powered micro/nano-motors. Bioact. Mater. 2021, 6, 1727–1749. [Google Scholar] [CrossRef] [PubMed]
- Hortelão, A.C.; Patiño, T.; Perez-Jiménez, A.; Blanco, À.; Sánchez, S. Enzyme-Powered Nanobots Enhance Anticancer Drug Delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Schattling, P.; Thingholm, B.; Städler, B. Enhanced Diffusion of Glucose-Fueled Janus Particles. Chem. Mater. 2015, 27, 7412–7418. [Google Scholar] [CrossRef]
- Simmchen, J.; Baeza, A.; Ruiz, D.; Esplandiu, M.J.; Vallet-Regí, M. Asymmetric Hybrid Silica Nanomotors for Capture and Cargo Transport: Towards a Novel Motion-Based DNA Sensor. Small 2012, 8, 2053–2059. [Google Scholar] [CrossRef]
- Abdelmohsen, L.K.E.A.; Nijemeisland, M.; Pawar, G.M.; Janssen, G.-J.A.; Nolte, R.J.M.; Hest, J.C.M.v.; Wilson, D.A. Dynamic Loading and Unloading of Proteins in Polymeric Stomatocytes: Formation of an Enzyme-Loaded Supramolecular Nanomotor. ACS Nano 2016, 10, 2652–2660. [Google Scholar] [CrossRef]
- Wilson, D.A.; Nolte, R.J.M.; Hest, J.C.M.v. Autonomous movement of platinum-loaded stomatocytes. Nat. Chem. 2012, 4, 268–274. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pané, S. Recent developments in magnetically driven micro-and nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Fabrication of Micro/Nanoscale Motors. Chem. Rev. 2015, 115, 8704–8735. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Dreyfus, R.; Baudry, J.; Roper, M.L.; Fermigier, M.; Stone, H.A.; Bibette, J. Microscopic artificial swimmers. Nature 2005, 437, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Schamel, D.; Mark, A.G.; Gibbs, J.G.; Miksch, C.; Morozov, K.I.; Leshansky, A.M.; Fischer, P. Nanopropellers and Their Actuation in Complex Viscoelastic Media. ACS Nano 2014, 8, 8794–8801. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Mhanna, R.; Zhang, L.; Ding, Y.; Fujita, S.; Nelson, B.J. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release. Sens. Actuators B Chem. 2014, 195, 676–681. [Google Scholar] [CrossRef]
- Qiu, F.; Fujita, S.; Mhanna, R.; Zhang, L.; Simona, B.R.; Nelson, B.J. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv. Funct. Mater. 2015, 25, 1666–1671. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular Cargo Delivery: Toward Assisted Fertilization by Sperm-Carrying Micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef]
- Schwarz, L.; Karnaushenko, D.D.; Hebenstreit, F.; Naumann, R.; Schmidt, O.G.; Medina-Sánchez, M. A Rotating Spiral Micromotor for Noninvasive Zygote Transfer. Sci. Adv. 2020, 7, 2000843. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K.M.; Weihs, D.; Wang, J. Magnetically Powered Flexible Metal Nanowire Motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef]
- Jang, B.; Gutman, E.; Stucki, N.; Seitz, B.F.; Wendel-García, P.D.; Newton, T.; Pokki, J.; Ergeneman, O.; Pané, S.; Or, Y.; et al. Undulatory Locomotion of Magnetic Multilink Nanoswimmers. Nano Lett. 2015, 15, 4829–4833. [Google Scholar] [CrossRef]
- Chang, S.T.; Paunov, V.N.; Petsev, D.N.; Velev, O.D. Remotely powered self-propelling particles and micropumps based on miniature diodes. Nat. Mater 2007, 6, 235–240. [Google Scholar] [CrossRef]
- Ni, S.; Marini, E.; Buttinoni, I.; Wolf, H.; Isa, L. Hybrid colloidal microswimmers through sequential capillary assembly. Soft Matter 2017, 13, 4252–4259. [Google Scholar] [CrossRef]
- Calvo-Marzal, P.; Manesh, K.M.; Kagan, D.; Balasubramanian, S.; Cardona, M.; Flechsig, G.-U.; Posner, J.; Wang, J. Electrochemically-triggered motion of catalytic nanomotors. Chem. Commun. 2009, 2009, 4509–4511. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yin, Z.; Cheong, R.; Zhu, F.Q.; Cammarata, R.C.; Chien, C.L.; Levchenko, A. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires. Nat. Nanotechnol. 2010, 5, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Chowdhury, M.M.; Alam, K. Rotating-Electric-Field-Induced Carbon-Nanotube-Based Nanomotor in Water: A Molecular Dynamics Study. Small 2017, 13, 1603978. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gallegos, J.J.; Tom, A.R.; Fa, D. Electric-Field-Guided Precision Manipulation of Catalytic Nanomotors for Cargo Delivery and Powering Nanoelectromechanical Devices. ACS Nano 2018, 12, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bian, Q.; Wang, R.; Gao, J. Micro/nanorobots for precise drug delivery via targeted transport and triggered release: A review. Int. J. Pharm. 2022, 616, 121551. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Choudhury, U.; Fischer, P.; Mark, A.G. Non-Equilibrium Assembly of Light-Activated Colloidal Mixtures. Adv. Mater. 2017, 29, 1701328. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Tang, X.; Tang, S.-Y.; Sivan, V.; Zhang, W.; Mitchell, A.; Kalantar-zadeha, K.; Khoshmanesha, K. Photochemically induced motion of liquid metal marbles. Appl. Phys. Lett. 2013, 103, 174104. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Qin, H.; Zhao, Z.; Liu, H. Light-Driven and Light-Guided Microswimmers. Adv. Funct. Mater. 2016, 26, 3164–3171. [Google Scholar] [CrossRef]
- Ryazantsev, Y.S.; Velarde, M.G.; Rubio, R.G.; Guzmán, E.; Ortega, F.; López, P. Thermo-and soluto-capillarity: Passive and active drops. Adv. Colloid Interface Sci. 2017, 247, 52–80. [Google Scholar] [CrossRef]
- Ryazantsev, Y.S.; Velarde, M.G.; Guzmán, E.; Rubio, R.G.; Ortega, F.; Montoya, J.J. On the Autonomous Motion of Active Drops or Bubbles. J. Colloid Interface Sci. 2018, 527, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lia, C.; Huang, X. Actuators based on liquid crystalline elastomer materials. Nanoscale 2013, 5, 5225–5240. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mamiya, J.-i.; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zheng, J.; Zhao, Y.; Zhu, B.; Cheng, R.; Wang, J.; Liu, J.; Tang, J.; Tang, J. From Strong Dichroic Nanomotor to Polarotactic Microswimmer. Adv. Funct. Mater. 2019, 31, 1903329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, R.; Wang, C.; Xu, S.; Chen, D.; Liang, Y.; Ren, B.; Gao, W.; Cai, Y. Glucose-Fueled Micromotors with Highly Efficient Visible-Light Photocatalytic Propulsion. ACS Appl. Mater. Interfaces 2019, 11, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhou, M.; Du, X.; Li, X.; Li, J.; Xu, T.; Zhang, X. Hollow mesoporous carbon@Pt Janus nanomotors with dual response of H2O2 and near-infrared light for active cargo delivery. Appl. Mater. Today 2019, 17, 85–91. [Google Scholar] [CrossRef]
- Xuan, M.; Wu, Z.; Shao, J.; Dai, L.; Si, T.; He, Q. Near Infrared Light-Powered Janus Mesoporous Silica Nanoparticle Motors. J. Am. Chem. Soc. 2016, 138, 6492–6497. [Google Scholar] [CrossRef]
- He, W.; Frueh, J.; Hu, N.; Liu, L.; Gai, M.; He, Q. Guidable Thermophoretic Janus Micromotors Containing Gold Nanocolorifiers for Infrared Laser Assisted Tissue Welding. Adv. Sci. 2016, 3, 1600206. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Clergeaud, G.; Andresen, T.L.; Boisen, A. Micromotors for drug delivery in vivo: The road ahead. Adv. Drug Deliv. Rev. 2019, 138, 41–55. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation forces: Classical theory and recent advances. In Recent Research Developments in Acoustics; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2003; Volume 1, pp. 39–67. [Google Scholar]
- Wang, W.; Castro, L.A.; Hoyos, M.; Mallouk, T.E. Autonomous Motion of Metallic Microrods Propelled by Ultrasound. ACS Nano 2012, 6, 6122–6132. [Google Scholar] [CrossRef]
- Kagan, D.; Benchimol, M.J.; Claussen, J.C.; Chuluun-Erdene, E.; Esener, S.; Wang, J. Acoustic Droplet Vaporization and Propulsion of Perfluorocarbon-Loaded Microbullets for Targeted Tissue Penetration and Deformation. Angew. Chem. Int. Ed. 2012, 51, 7519–7522. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized Ultrasound-Propelled Magnetically Guided Nanomotors: Toward Practical Biomedical Applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Soto, F.; Gao, W.; Garcia-Gradilla, V.; Li, J.; Zhang, X.; Wang, J. Ultrasound-Modulated Bubble Propulsion of Chemically Powered Microengines. J. Am. Chem. Soc. 2014, 136, 8552–8555. [Google Scholar] [CrossRef]
- Wan, M.; Li, T.; Chen, H.; Mao, C.; Shen, J. Biosafety, Functionalities, and Applications of Biomedical Micro/nanomotors. Angew. Chem. Int. Ed. 2021, 60, 13158–13176. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-Guided Hybrid Neutrophil Micromotors for Targeted Drug Transport. Angew. Chem. Int. Ed. 2017, 56, 12935–12939. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef]
- Alapan, Y.; Yasa, O.; Schauer, O.; Giltinan, J.; Tabak, A.F.; Sourjik, V.; Sitti, M. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci. Robot. 2018, 3, eaar4423. [Google Scholar] [CrossRef]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Gong, H.; Wei, F.; Zhuang, J.; Karshalev, E.; Ávila, B.E.F.d.; Huang, C.; Zhou, Z.; Li, Z.; et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 2020, 5, eaba6137. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.W.; Miguel-Lopez, A.; Ma, X.; Sitti, M.; Sánchez, S. Biohybrid Microtube Swimmers Driven by Single Captured Bacteria. Small 2017, 13, 1603679. [Google Scholar] [CrossRef]
- Ma, X.; Jannasch, A.; Albrecht, U.R.; Hahn, K.; Miguel-Lopez, A.; Schaffer, E.; Sánchez, S. Enzyme-Powered Hollow Mesoporous Janus Nanomotors. Nano Lett. 2015, 15, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Z.; Zheng, J.; Zhan, X.; Tang, J. Light-Driven Micro/Nanomotor for Promising Biomedical Tools: Principle, Challenge, and Prospect. Acc. Chem. Res. 2018, 51, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Nijemeisland, M.; Abdelmohsen, L.; Huck, W.; Wilson, D.A.; Van Hest, J.C. A Compartmentalized Out-of-Equilibrium Enzymatic Reaction Network for Sustained Autonomous Movement. ACS Cent. Sci. 2016, 2, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Grifantini, K. The State of Nanorobotics in Medicine. IEEE Pulsa 2019, 10, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Timur, S.S.; Kuralay, F.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Current Status of Micro/Nanomotors in Drug Delivery. J. Drug Target. 2021, 29, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Reinisova, L.; Hermanova, S.; Pumera, M. Micro/nanomachines: What is needed for them to become a real force in cancer therapy? Nanoscale 2019, 11, 6519–6532. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Angsantikul, P.; Li, J.; Lopez-Ramirez, M.A.; Ramírez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 272. [Google Scholar] [CrossRef]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial Micromotors in the Mouse’s Stomach: A Step toward in Vivo Use of Synthetic Motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef]
- Baylis, J.R.; Yeon, J.H.; Thomson, M.H.; Kazerooni, A.; Wang, X.; John, A.E.S.; Lim, E.B.; Chien, D.; Lee, A.; Zhang, J.Q.; et al. Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci. Adv. 2015, 1, e1500379. [Google Scholar] [CrossRef]
- Kim, D.-I.; Lee, H.; Kwon, S.-H.; Sung, Y.J.; Song, W.K.; Park, S. Bilayer Hydrogel Sheet-Type Intraocular Microrobot for Drug Delivery and Magnetic Nanoparticles Retrieval. Adv. Healthc. Mater. 2020, 9, 2000118. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [CrossRef]
- Villa, K.; Krejčová, L.; Novotný, F.; Heger, Z.; Sofer, Z.; Pumera, M. Cooperative Multifunctional Self-Propelled Paramagnetic Microrobots with Chemical Handles for Cell Manipulation and Drug Delivery. Adv. Funct. Mater. 2018, 28, 1804343. [Google Scholar] [CrossRef]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2011, 8, 460–467. [Google Scholar] [CrossRef]
- Feng, X.; Wang, l.; Chen, J.; Zeng, W.; Liu, R.; Lin, X.-J.; Ma, Y.; Jiahui, W. Self-propelled Manganese Oxide-Based Catalytic Micromotors for Drug Delivery. RSC Adv. 2016, 6, 65624–65630. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Y.; He, W.; Lin, X.; Sun, J.; He, Q. Self-Propelled Polymer-Based Multilayer Nanorockets for Transportation and Drug Release. Angew. Chem. Int. Ed. 2013, 52, 7000–7003. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Lin, X.; Dai, L.; He, Q. Self-Propelled Janus Mesoporous Silica Nanomotors with Sub-100 nm Diameters for Drug Encapsulation and Delivery. ChemPhysChem 2014, 15, 2255–2260. [Google Scholar] [CrossRef]
- Khezri, B.; Mohsen, S.; Mousavi, B.; Krejčová, L.; Heger, Z.; Sofer, Z.; Pumera, M. Ultrafast Electrochemical Trigger Drug Delivery Mechanism for Nanographene Micromachines. Adv. Funct. Mater. 2019, 29, 1806696. [Google Scholar] [CrossRef]





| Type | Energy | Penetration | Motion Ability | Persistence | Safety |
|---|---|---|---|---|---|
| Endogenous powered motors | Chemical | Not applicable | Requires external force for positioning | Not as good, chemical energy can be depleted when it decreases gradually, limiting the motion of the engines | Depends on the fuel: hydrogen peroxide is a toxic fuel, whereas glucose and urea are safe |
| Exogenous powered motors (Externally triggered) | Magnetic | Good, weak magnetic fields can be enough | Precise 3D navigation in fluids under the action of rotating magnetic fields | Good, engines can keep moving under the guidance of the external field | Used magnetic fields are generally safe, metallic components can present toxicity upon long-term exposure |
| Electric | Weak, strong electric fields are needed | Requires the combination of electric fields and additional fields for ensuring the directional motion | Strong electric field can affect human body, metallic components can present toxicity upon long-term exposure | ||
| Light | Depends on the type of light, different penetration | Normally exploited for triggering other reactions. However, it can provide directional motion | Depend on the type of light, ultraviolet light may be harmful, whereas other lights are commonly safe | ||
| Ultrasound | Good | Commonly combined with magnetic fields, provides directional motion | Ultrasound irradiation can cause oxidative stress in cells, metallic components can present toxicity upon long-term exposure |
| Type of MNM | Power Source | Disease | Drug | Reference |
|---|---|---|---|---|
| Mg-based motors | Chemical (catalytic motors powered by gastric acids) | gastrointestinal bacteria | clarithromycin | Esteban-Fernández de Ávila et al. [108] |
| Zn-based motors | Gao et al. [109] | |||
| Carbonate and tranexamic acid particles | Chemical | hemorrhages | thrombin | Baylis et al. [110] |
| Hydrogel/magnetic particle hybrid | Magnetic fields | eye diseases | doxorubicin | Kim et al. [111] |
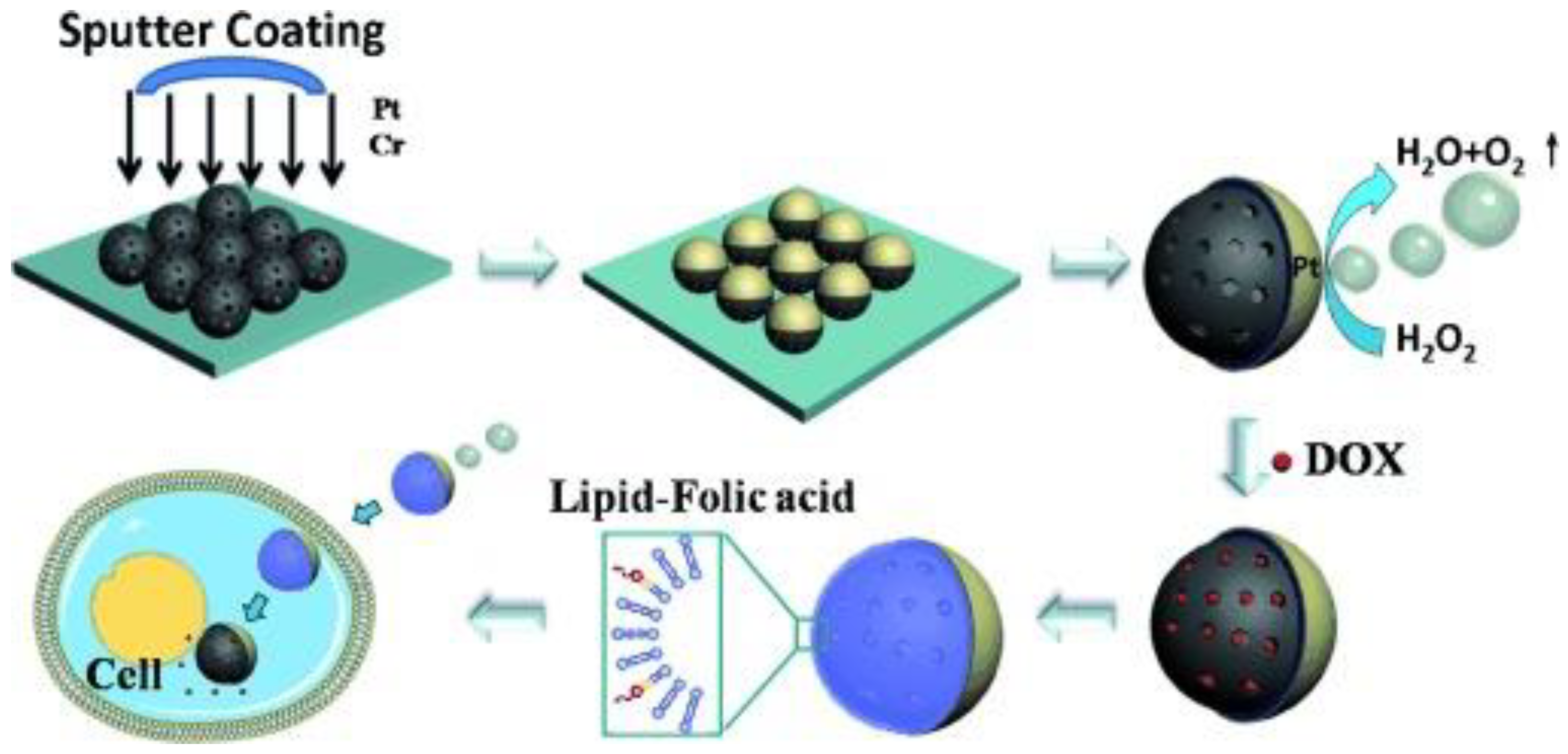
| Iron oxide particles with one hemisphere coated by Pt and decorated with tosylated groups | Chemical (catalytic motors powered by decomposition of hydrogen peroxide) | cancer | doxorubicin | Villa et al. [113] |
| Ni/(Au50/Ag50)/Ni/Pt) nanowires and Fe3O4 particles | Chemical (catalytic motors powered by decomposition of hydrogen peroxide) combined with magnetic field (directionality control) | Kagan et al. [35] | ||
| Pt nanorockets coated by a Layer-by-Layer film of chitosan and alginate (tubular motors) | Chemical (catalytic motors powered by decomposition of hydrogen peroxide) | Wu et al. [116] | ||
| Janus nanomotors with caps of chromium and platinum | Xuan et al. [117] | |||
| Silica-based nanoparticles decorated with urease | Chemical (enzymatic degradation of urea by urease) | Hortelao et al. [53] | ||
| Flexible nickel-silver swimmers | Magnetic field | Gao et al. [114] | ||
| Carbon-platinum tubular Janus motors | Chemical (catalytic motors powered by decomposition of hydrogen peroxide) and light (near infrared radiation) | Xing et al. [86] | ||
| Tubular motors of platinum and reduced graphene oxide | Electric field | Kehzri et al. [118] | ||
| Tubular (PEDOT/MnO2) micromotors | Chemical (catalytic motors powered by decomposition of hydrogen peroxide) | camptothecin | Feng et al. [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.; Maestro, A. Synthetic Micro/Nanomotors for Drug Delivery. Technologies 2022, 10, 96. https://doi.org/10.3390/technologies10040096
Guzmán E, Maestro A. Synthetic Micro/Nanomotors for Drug Delivery. Technologies. 2022; 10(4):96. https://doi.org/10.3390/technologies10040096
Chicago/Turabian StyleGuzmán, Eduardo, and Armando Maestro. 2022. "Synthetic Micro/Nanomotors for Drug Delivery" Technologies 10, no. 4: 96. https://doi.org/10.3390/technologies10040096
APA StyleGuzmán, E., & Maestro, A. (2022). Synthetic Micro/Nanomotors for Drug Delivery. Technologies, 10(4), 96. https://doi.org/10.3390/technologies10040096







