Selected Techniques for Cutting SOx Emissions in Maritime Industry
Abstract
:1. Introduction
- Switch to fuel oils with lower sulfur content than HFO, such marine gas oil (MGO) or very low-sulfur fuel oils (VLFO);
- Switch to alternative fuels such as renewable diesel, LNG, methanol (CH3OH), ammonia (NH3) and hydrogen (H2);
- Consider a flue exhaust aftertreatment method that will reduce SOx emissions, enabling them to still use the cheapest fuel (HFO).
2. Industrial Flue Gas State-of-the-Art DeSOx Processes
- Wet desulfurization;
- Dry or semi-dry desulfurization;
- Bio-desulfurization.
- Limestone (CaCO3) or lime (CaO) sludge desulfurization process through gypsum (CaSO4 2H2O) production;
- Magnesium oxide (MgO) desulfurization through SO2 adsorption and production of MgSO4;
- Zinc oxide (ZnO) desulfurization process;
- Dual-alkali desulfurization process;
- Ammonia (NH3) desulfurization process.
- Sorbent injection method;
- Active carbon adsorption;
- Circulating fluidized bed desulfurization;
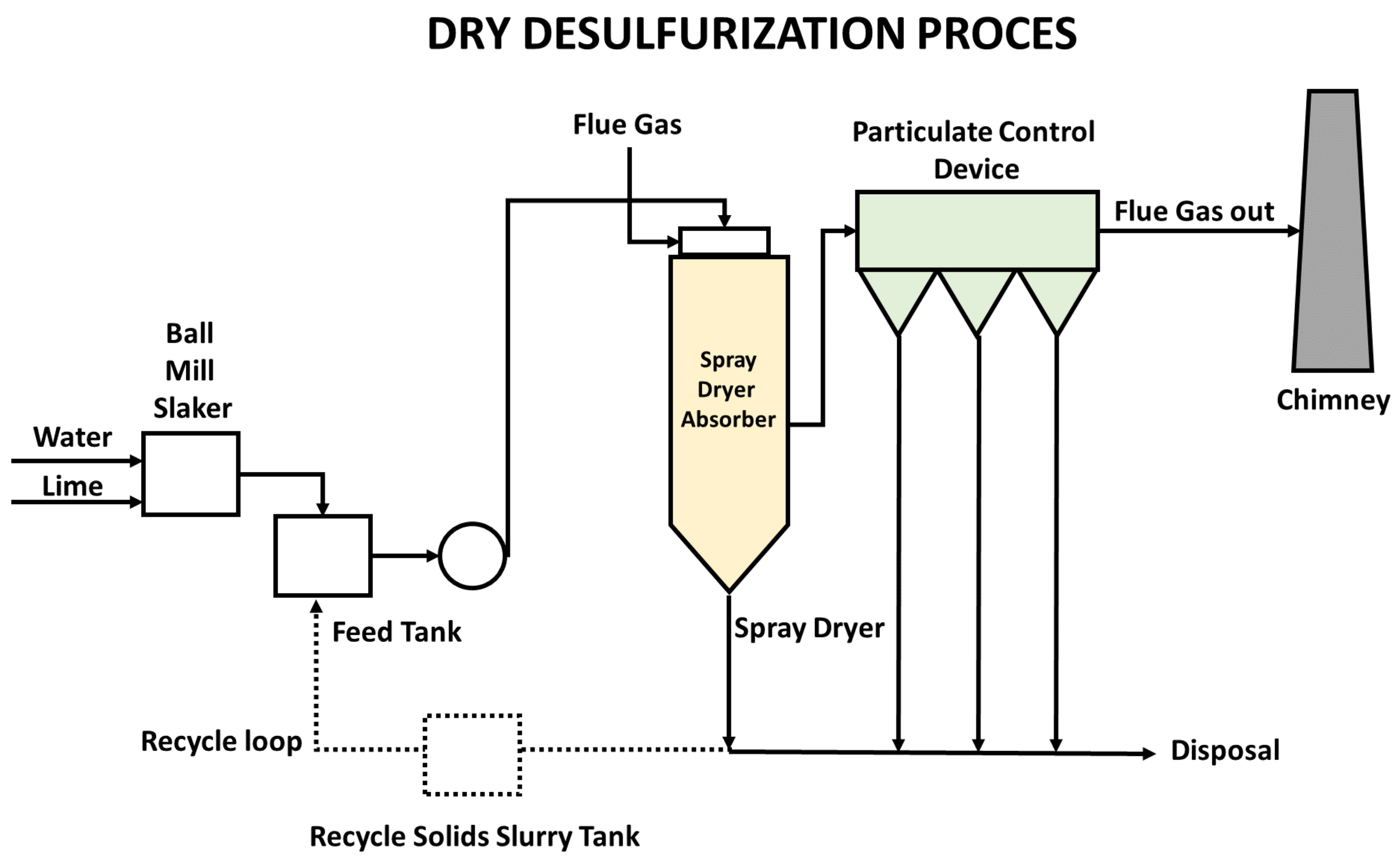
- Spray dry method.
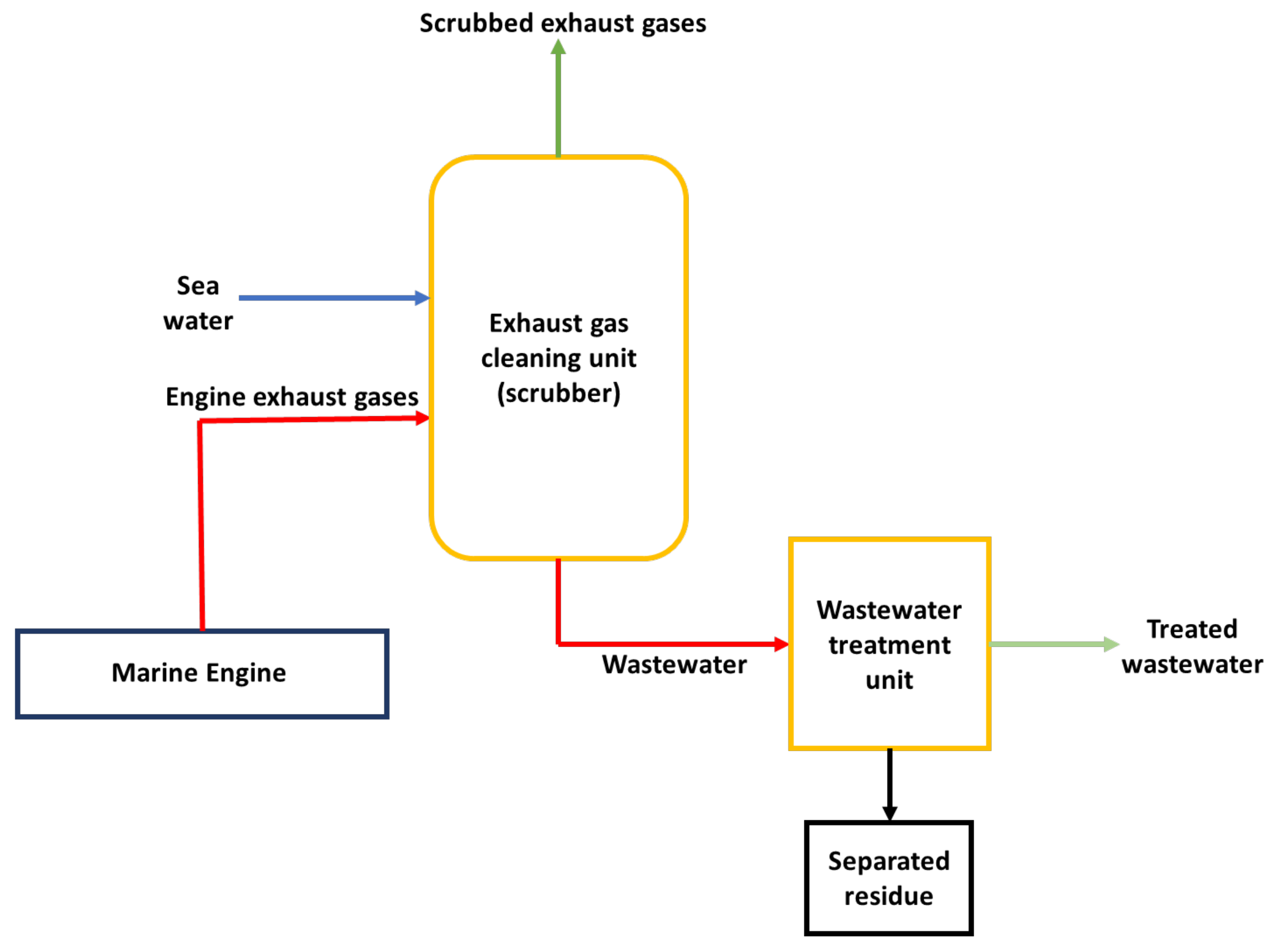
3. State-of-the-Art DeSOx Processes for Marine Diesel Engines
- Open-loop (81%);
- Closed-loop (2%);
- Hybrid (17%).
4. Beyond the State of the Art
- High cost, which varies between EUR 2–6 million [60], depending on the power of the engine and the type of scrubber used;
- Even more countries issuing regulations that disband the use of scrubbers or scrubber discharges at their ports or coast;
- The environmental problem shifting to the oceans, even though the released emissions comply with regulations.
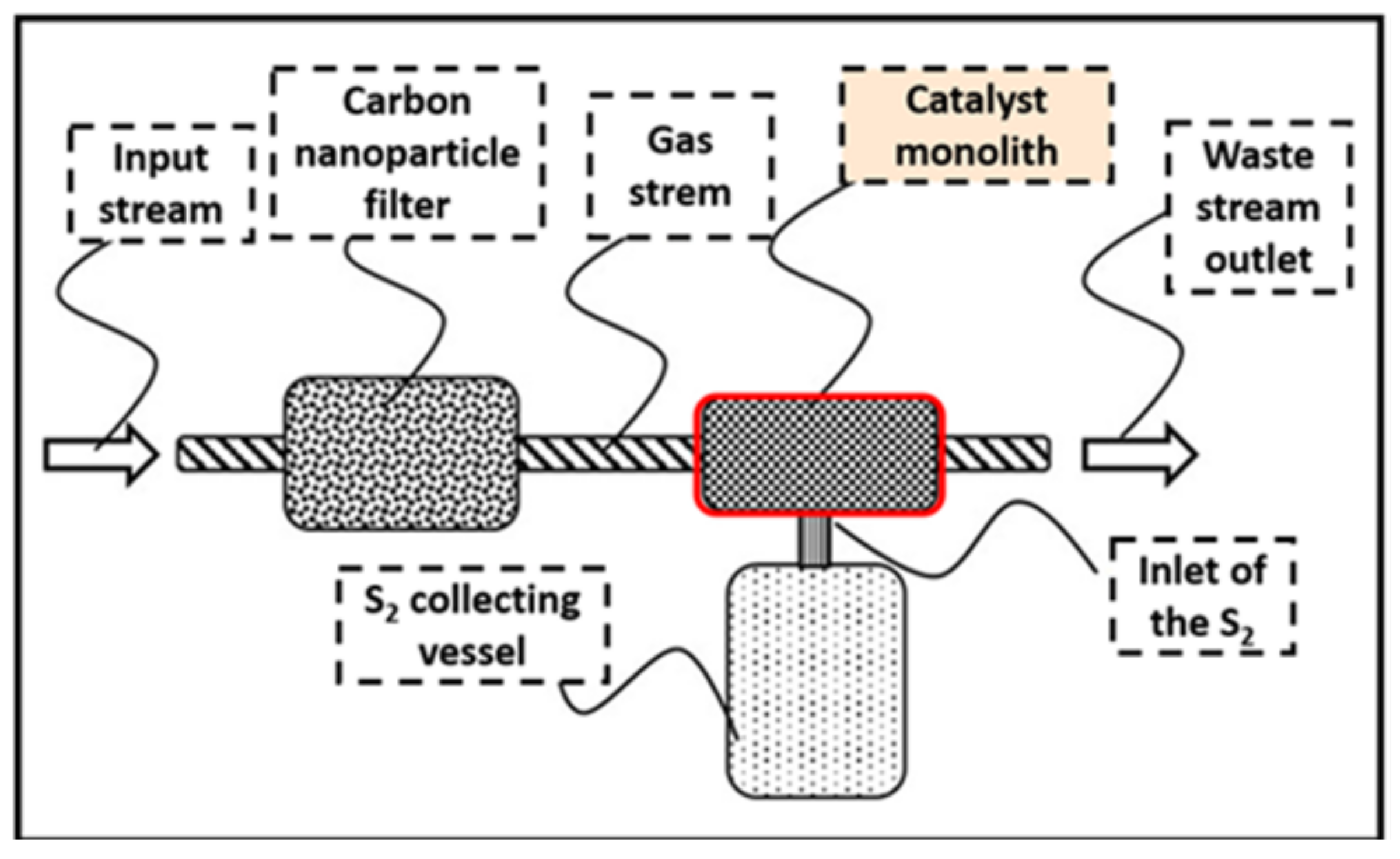
5. Metal Oxide Catalysts for the Reduction of SO2 to Elemental Sulfur
6. Conclusions and Suggestions for the Future
- Results obtained in this paper: It seems from the above that highly active and selective catalysts have been developed for the selective reduction of SO2 towards elemental sulfur. Most authors emphasize on the role of ceria and γ-Al2O3, attributing the high catalytic activity that these supports exhibit to their oxygen defective fluorite structure and to the high concentration of acidic sites, respectively. Impregnating these supports with transition metals such as Fe, Cu, Ni and Co greatly promote their catalytic activity, selectivity and stability. Most of the studies that emphasized screening a wide range of active metals for this reaction proved that Fe might be the most active compared to the others.
- Limitations on the conducted review: On the other hand, there are very few studies focusing on noble metals, possibly because of their susceptibility to sulfur poisoning. The literature lacks studies that emphasize on the role of oxygen in the gas feed stream, which is an important topic to consider if these catalysts are developed for commercial DeSOx solutions. Monolithos Catalysts & Recycling Ltd. has proposed a very promising DeSOx solution that can be easily applied both for land based and marine applications. Compared to the other solutions proposed in the literature, a SOx selective catalytic reduction system can overcome problems that current state-of-the-art solutions exhibit, such as secondary environmental pollution, high operational and capital cost, low DeSOx efficiency and/or waste management.
- Prospects for future research: The research in the near future should focus mainly on supported catalytic systems to treat SOx emissions, simultaneously to NOx and hydrocarbon emissions, taking advantage of the compositions and the concentration of the flue gas streams that are formed from the HFO fuel, which is used on marine sector. Additionally, significant improvement steps should be performed on the particulate matter treatment, assisted with catalytic supported phases in order to enhance the catalytic efficiency and meet the strict environmental criteria of the IMO MARPOL regulations. Byproducts of the flue gas treatment should be taken under serious consideration to avoid side pollution by the desulfurization catalytic system, in order to obtain an integrated, environmental and economically feasible solution requiring the least maintenance and expertise. Finally, a prediction method for probable recovery of partially missing or completely lost data based on the improvement of the combined gas treatment technologies should also be considered and should take place to enhance the evolution, development and demonstration of these systems.
7. Patents
Author Contributions
Funding
Conflicts of Interest
References
- European Parliament. Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021 establishing the framework for achieving climate neutrality and amending Regulations (EC) No 401/2009 and (EU) 2018/1999 (‘European Climate Law’). in PE/27/2021/REV/1. 9.7.2021. Off. J. Eur. Union 2021, 50, 1–17. [Google Scholar]
- United Nations-Paris Agreement. 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 6 June 2022).
- Cruz, T.T.D.; Perrella Balestieri, J.A.; de Toledo Silva, J.M.; Vilanova, M.R.N.; Oliveira, O.J.; Ávila, I. Life cycle assessment of carbon capture and storage/utilization: From current state to future research directions and opportunities. Int. J. Greenh. Gas Control 2021, 108, 103309. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, C.; Zhang, X.; Li, J. The effect of SiO2 on a novel CeO2–WO3/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B 2013, 140, 276–282. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Zipper, C.E.; Gilroy, L. Sulfur Dioxide Emissions and Market Effects under the Clean Air Act Acid Rain Program. J. Air Waste Manag. Assoc. 1998, 48, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, W.; Wong, C.-M.; Wang, Z.; Quoc Thach, T.; Chen, B.; Kan, H. Short-term exposure to sulfur dioxide and daily mortality in 17 Chinese cities: The China air pollution and health effects study (CAPES). Environ. Res. 2012, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.Y.; Yuan, T.H.; Shie, R.H.; Chen, C.F.; Chan, C.C. Increased incidence of allergic rhinitis, bronchitis and asthma, in children living near a petrochemical complex with SO2 pollution. Environ. Int. 2016, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- IMO 2020—Cutting Sulphur Oxide Emissions. Available online: https://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx (accessed on 6 June 2022).
- Zhong, Q.; Shen, H.; Yun, X.; Chen, Y.; Ren, Y.; Xu, H.; Shen, G.; Du, W.; Meng, J.; Li, W.; et al. Global Sulfur Dioxide Emissions and the Driving Forces. Environ. Sci. Technol. 2020, 54, 6508–6517. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; de Vos, P.; Stapersma, D.; Visser, K.; Ding, Y. Fuel Consumption and Emissions of Ocean-Going Cargo Ship with Hybrid Propulsion and Different Fuels over Voyage. J. Mar. Sci. Eng. 2020, 8, 588. [Google Scholar] [CrossRef]
- International Maritime Organisation-Maritime Facts and Figures. Available online: https://www.imo.org/en/KnowledgeCentre/Pages/MaritimeFactsFigures-Default.aspx (accessed on 6 June 2022).
- Sirimanne, S.N.; Hoffman, J.; Juan, W.; Asariotis, R.; Assaf, M.; Ayala, G.; Benamara, H.; Chantrel, D.; Hoffmann, J.; Premti, A.; et al. Review of Maritime Transport 2019; United Nations: Geneva, Switzerland, 2020. [Google Scholar]
- Crippa, M.; Janssens-Maenhout, G.; Dentener, F.; Guizzardi, D.; Sindelarova, K.; Muntean, M.; Van Dingenen, R.; Granier, C. Forty years of improvements in European air quality: Regional policy-industry interactions with global impacts. Atmos. Chem. Phys. 2016, 16, 3825–3841. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Streets, D.G.; Zhang, Q.; Wang, S.; Carmichael, G.R.; Cheng, Y.F.; Wei, C.; Chin, M.; Diehl, T.; Tan, Q. Sulfur dioxide emissions in China and sulfur trends in East Asia since 2000. Atmos. Chem. Phys. 2010, 10, 6311–6331. [Google Scholar] [CrossRef]
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Dahiya, S.; Anhäuser, A.; Farrow, A.; Thieriot, H.; Kumar, A.; Myllyvirta, L. Ranking the World’s Sulfur Dioxide (SO2) Hotspots: 2019–2020; Delhi Center for Research on Energy and Clean Air-Greenpeace India: Chennai, India, 2020; p. 48. [Google Scholar]
- Li, C.; McLinden, C.; Fioletov, V.; Krotkov, N.; Carn, S.; Joiner, J.; Streets, D.; He, H.; Ren, X.; Li, Z.; et al. India Is Overtaking China as the World’s Largest Emitter of Anthropogenic Sulfur Dioxide. Sci. Rep. 2017, 7, 14304. [Google Scholar] [CrossRef]
- Agency, E.E. Sulphur dioxide (SO2) emissions. (5 November 2021). Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea-32-sulphur-dioxide-so2-emissions-1/assessment-3 (accessed on 6 June 2022).
- Faber, J.; Hanayama, S.; Zhang, S.; Pereda, P.; Comer, B.; Hauerhof, E.; van der Loeff, W.S.; Smith, T.; Zhang, Y.; Kosaka, H.; et al. Fourth IMO GHG Study 2020 Executive Summary; 4 Albert Embankment; SE1 7SR; International Maritime Organisation: London, UK, 2021. [Google Scholar]
- Dos Santos, V.A.; Pereira da Silva, P.; Serrano, L.M.V. The Maritime Sector and Its Problematic Decarbonization: A Systematic Review of the Contribution of Alternative Fuels. Energies 2022, 15, 3571. [Google Scholar] [CrossRef]
- World Bunker Prices. Available online: https://shipandbunker.com/prices (accessed on 6 June 2022).
- Deniz, C.; Zincir, B. Environmental and economical assessment of alternative marine fuels. J. Clean. Prod. 2016, 113, 438–449. [Google Scholar] [CrossRef]
- Rehmatulla, N.; Smith, T. Barriers to energy efficient and low carbon shipping. Ocean Eng. 2015, 110, 102–112. [Google Scholar] [CrossRef]
- Gilbert, P.; Walsh, C.; Traut, M.; Kesieme, U.; Pazouki, K.; Murphy, A. Assessment of full life-cycle air emissions of alternative shipping fuels. J. Clean. Prod. 2018, 172, 855–866. [Google Scholar] [CrossRef]
- Gilbert, P.; Bows-Larkin, A.; Mander, S.; Walsh, C. Technologies for the high seas: Meeting the climate challenge. Carbon Manag. 2014, 5, 447–461. [Google Scholar] [CrossRef]
- Mishchuk, O.; Tkachenko, R.; Izonin, I. Missing Data Imputation Through SGTM Neural-Like Structure for Environmental Monitoring Tasks. In Advances in Intelligent Systems and Computing (AISC); Springer: Berlin, Germany, 2019; Volume 938. [Google Scholar]
- Sethi, S. A Guide To Scrubber System On Ship. 2021. Available online: https://www.marineinsight.com/tech/scrubber-system-on-ship/ (accessed on 6 June 2022).
- Teuchies, J.; Cox, T.J.S.; Van Itterbeeck, K.; Meysman, F.J.R.; Blust, R. The impact of scrubber discharge on the water quality in estuaries and ports. Environ. Sci. Eur. 2020, 32, 103. [Google Scholar] [CrossRef]
- Davin, S. The trouble with scrubbers: Shipping’s emissions “solution” creates new pollution. 2020. Available online: https://wwf.ca/stories/scrubbers-creates-new-pollution/ (accessed on 6 June 2022).
- Georgeff, E.; Mao, X.; Comer, B. A Whale of A Problem? Heavy Fuel Oil, Exhaust Gas Cleaning Systems, and British Columbia’s Resident Killer Whales; International Council on Clean Transportation: 1500 K Street NW Suite 650, Washington, DC, USA, 2019. [Google Scholar]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Efstathiou, A.M. NOx Control via H2-Selective Catalytic Reduction (H2-SCR) Technology for Stationary and Mobile Applications. Recent Pat. Mater. Sci. 2012, 5, 87–104. [Google Scholar] [CrossRef]
- Costa, C.N.; Savva, P.G.; Fierro, J.L.G.; Efstathiou, A.M. Industrial H2-SCR of NO on a Novel Pt/MgO-CeO2 Catalyst. Appl. Catal. B: Environ. 2007, 75, 147–156. [Google Scholar] [CrossRef]
- Savva, Z.; Petallidou, K.C.; Damaskinos, C.M.; Olympiou, G.G.; Stathopoulos, V.N.; Efstathiou, A.M. H2-SCR of NOx on low-SSA CeO2-supported Pd: The effect of Pd particle size. Appl. Catal. A Gen. 2021, 615, 118062. [Google Scholar] [CrossRef]
- Akiho, H.; Ito, S.; Matsuda, H. Effect of oxidizing agents on selenate formation in a wet FGD. Fuel 2010, 89, 2490–2495. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jozewicz, W.; Singer, C. SO2 Scrubbing Technologies: A Review. Environ. Prog. 2001, 20, 219–228. [Google Scholar] [CrossRef]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Poullikkas, A. Review of Design, Operating, and Financial Considerations in Flue Gas Desulfurization Systems. Energy Technol. Policy 2015, 2, 92–103. [Google Scholar] [CrossRef]
- Dou, B.; Pan, W.; Jin, Q.; Wang, W.; Li, Y. Prediction of SO2 removal efficiency for wet Flue Gas Desulfurization. Energy Convers. Manag. 2009, 50, 2547–2553. [Google Scholar] [CrossRef]
- Karatepe, N. A Comparison of Flue Gas Desulfurization Processes. Energy Sources 2000, 22, 197–206. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T.-A. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Guo, R.-T.; Pan, W.-G.; Zhang, X.-B.; Xu, H.-J.; Ren, J.-X. Dissolution rate of magnesium hydrate for wet flue gas desulfurization. Fuel 2011, 90, 7–10. [Google Scholar] [CrossRef]
- Jia, Y.; Yin, L.; Xu, Y.; Chen, Y.; Ding, X. Simulation of the absorption of SO2 by ammonia in a spray scrubber. Chem. Eng. Process. 2017, 116, 60–67. [Google Scholar] [CrossRef]
- Hashemi, S.M.H.; Mehrabani-Zeinabad, A.; Zare, M.H.; Shirazian, S. SO2 Removal from Gas Streams by Ammonia Scrubbing: Process Optimization by Response Surface Methodology. Chem. Eng. Technol. 2019, 42, 45–52. [Google Scholar] [CrossRef]
- Rokni, E.; Hsein Chi, H.; Levendis, Y.A. In-Furnace Sulfur Capture by Cofiring Coal With Alkali-Based Sorbents. J. Energy Resour. Technol. 2017, 139(4), 042204. [Google Scholar] [CrossRef]
- Yue, X.; Yang, H.R.; Lu, J.F.; Zhang, H. Latest development of CFB boilers in China. In 20th International Conference on Fluidized Bed Combustion, 20th International Conference on Fluidized Bed Combustion, January 2010; Springer: Berlin, Germany, 2010; pp. 3–12. [Google Scholar] [CrossRef]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Hassellöv, I.-M.; Koski, M.; Broeg, K.; Marin-Enriquez, O.; Tronczynski, J.; Dulière, V.; Murray, C.; Bailey, S.A.; Redfern, J.; de Jong, K.; et al. Ices Viewpoint Background Document: Impact from Exhaust Gas Cleaning Systems (Scrubbers) on the Marine Environment (Ad Hoc); ICES Scientific Reports: Copenhagen, Denmark, 2022; Volume 2, Issue 86. [Google Scholar]
- Osipova, L.; Georgeff, E.; Comer, B. Global Scrubber Washwater Discharges under IMO’s 2020 Fuel Sulfur Limit; International Council on Clean Transportation (ICCT): Washington, DC, USA, 2021. [Google Scholar]
- Safety4Sea. Update: Scrubber Discharges Bans in Ports. 2021. Available online: https://safety4sea.com/update-scrubber-discharges-bans-in-ports/ (accessed on 7 June 2022).
- Port Information Guide, V.F.P. Authority, Editor. 2022: Port of Vancouver. Available online: https://www.portvancouver.com/marine-operations/port-information-guide (accessed on 8 June 2022).
- Comments on the Vancouver Fraser Port Authority Notice of Amendment to the Port Information Guide to Introduce Exhaust gas Cleaning Systems (EGCS) Wash Water Discharge Requirements. Issued 24 November 2021. Available online: https://theicct.org/comments-vancouver-fraser-port-scrubbers-dec21/ (accessed on 7 July 2022).
- Comer, B. Vancouver’s New Scrubber Restrictions Mean Cleaner Waters; The International Council on Clean Transportation: 2022. Available online: https://theicct.org/vancouver-can-scrubbers-marine-mar22/ (accessed on 6 June 2022).
- Dulière, V.; Baetens, K.; Lacroix, G. Potential impact of wash water effluents from scrubbers on water acidification in the southern North Sea. Final. Proj. Rep. 2020, 31. Rue Vautier 29, 1000 Brussels, Belgium. [Google Scholar]
- Zannis, T.C.; Katsanis, J.S.; Christopoulos, G.P.; Yfantis, E.A.; Papagiannakis, R.G.; Pariotis, E.G.; Rakopoulos, D.C.; Rakopoulos, C.D.; Vallis, A.G. Marine Exhaust Gas Treatment Systems for Compliance with the IMO 2020 Global Sulfur Cap and Tier III NOx Limits: A Review. Energies 2022, 15, 3638. [Google Scholar] [CrossRef]
- Kim, A.-R.; Seo, Y.-J. The reduction of SOx emissions in the shipping industry: The case of Korean companies. Mar. Policy 2018, 100, 98–106. [Google Scholar] [CrossRef]
- Lehtoranta, K.; Aakko-Saksa, P.; Murtonen, T.; Vesala, H.; Ntziachristos, L.; Rönkkö, T.; Karjalainen, P.; Kuittinen, N.; Timonen, H. Particulate Mass and Nonvolatile Particle Number Emissions from Marine Engines Using Low-Sulfur Fuels, Natural Gas, or Scrubbers. Environ. Sci. Technol. 2019, 53, 3315–3322. [Google Scholar] [CrossRef]
- Yakoumis, I.; Sakkas, K.M.; Moschovi, A.M. Method Device Process Abatement SO2 Emissions Internal Combustion Engines. EP 3 939 690 A1, 19-01-2022.
- Zhu, Y.; Zhou, W.; Xia, C.; Hou, Q. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere 2022, 13, 731. [Google Scholar] [CrossRef]
- Sung, Y.; Choi, M.; Park, T.; Choi, C.; Park, Y.; Choi, G. Synergistic effect of mixer and mixing chamber on flow mixing and NOx reduction in a marine urea-SCR system. Chem. Eng. Process. 2020, 150, 107888. [Google Scholar] [CrossRef]
- Happel, J.; Hnatow, M.A.; Bajars, L.; Kundrath, M. Lanthanum Titanate Catalyst-Sulfur Dioxide Reduction. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 154–158. [Google Scholar] [CrossRef]
- Happel, J.; Leon, A.L.; Hnatow, M.A.; Bajars, L. Catalysts Composition Optimization for the Reduction of Sulfur Dioxide by Carbon Monoxide. Ind. Eng. Chem. Prod. Res. Dev. 1977, 16, 150–154. [Google Scholar] [CrossRef]
- Liu, W.; Sarofim, A.F.; Flytzani-Stephanopoulos, M. Reduction of sulfur dioxide by carbon monoxide to elemental sulfur over composite oxide catalysts. Appl. Catal. B 1994, 4, 167–186. [Google Scholar] [CrossRef]
- Liu, W.; Wadia, C.; Flytzani-Stephanopoulos, M. Transition metal/fluorite-type oxides as active catalysts for reduction of sulfur dioxide to elemental sulfur by carbon monoxide. Catal. Today 1996, 28, 391–403. [Google Scholar] [CrossRef]
- Zhu, T.; Dreher, A.; Flytzani-Stephanopoulos, M. Direct reduction of SO2 to elemental sulfur by methane over ceria-based catalysts. Appl. Catal. B 1999, 21, 103–120. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M.; Zhu, T.; Li, Y. Ceria-based catalysts for the recovery of elemental sulfur from SO2-laden gas streams. Catal. Today 2000, 62, 145–158. [Google Scholar] [CrossRef]
- Yu, J.-J.; Yu, Q.; Jin, Y.; Chang, S.-G. Reduction of Sulfur Dioxide by Methane to Elemental Sulfur over Supported Cobalt Catalysts. Ind. Eng. Chem. Res. 1997, 36, 2128–2133. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; White, M.G. Adsorption and Reaction of Sulfur Dioxide on Alumina and Sodium-Impregnated Alumina. J. Phys. Chem. 1996, 100, 7550–7557. [Google Scholar] [CrossRef]
- Ma, J.; Fang, M.; Lau, N.T. Activation of La2O3for the Catalytic Reduction of SO2by CO. J. Catal. 1996, 163, 271–278. [Google Scholar] [CrossRef]
- Ge, T.; Zuo, C.; Wei, L.; Li, C. Sulfur production from smelter off-gas using CO–H2 gas mixture as the reducing agent over modified Fe/γ-Al2O3 catalysts. Chin. J. Chem. Eng. 2018, 26, 1920–1927. [Google Scholar] [CrossRef]
- Ge, T.; Zuo, C.; Chen, H.; Muhammad, Y.; Wei, L.; Li, C. Catalytic Activity and Molecular Behavior of Lanthanum Modified CoSx/γ-Al2O3 Catalysts for the Reduction of SO2 to Sulfur in Smelter Off-Gas Using CO-H2 Mixture as Reductant. Ind. Eng. Chem. Res. 2019, 58, 3595–3605. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; He, J.; Peng, C.; Wu, T. Recovery of elemental sulphur via selective catalytic reduction of SO2 over sulphided CoMo/γ-Al2O3 catalysts. Fuel 2015, 147, 67–75. [Google Scholar] [CrossRef]
- Paik, S.C.; Chung, J.S. Selective hydrogenation of SO2 to elemental sulfur over transition metal sulfides supported on Al2O3. Appl. Catal. B 1996, 8, 267–279. [Google Scholar] [CrossRef]
- Paik, S.C.; Kim, H.; Chung, J.S. The catalytic reduction of SO2 to elemental sulfur with H2 or CO. Catal. Today 1997, 38, 193–198. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lin, S.-S.; Hwang, W.-U.; Weng, H.-S. Supported Transition-Metal Oxide Catalysts for Catalytic Reduction of SO2 with CO as a Reducing Agent. Ind. Eng. Chem. Res. 2002, 41, 666–671. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Formation and Decomposition of Sulfite and Sulfate Species on Pt/Pd Catalysts: An SO2 Oxidation and Sulfur Exposure Study. ACS Catal. 2019, 9, 640–648. [Google Scholar] [CrossRef]
- Sharma, H.N.; Sharma, V.; Mhadeshwar, A.B.; Ramprasad, R. Why Pt Survives but Pd Suffers From SOx Poisoning? J. Phys. Chem. Lett. 2015, 6, 1140–1148. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Jirsak, T.; Chaturvedi, S.; Hrbek, J. Surface Chemistry of SO2 on Sn and Sn/Pt(111) Alloys: Effects of Metal−Metal Bonding on Reactivity toward Sulfur. J. Am. Chem. Soc. 1998, 120, 11149–11157. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lin, S.-S.; Sung, P.-C.; Weng, H.-S. Catalytic reduction of SO2 over supported transition-metal oxide catalysts with C2H4 as a reducing agent. Appl. Catal. B 2003, 40, 331–345. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Li, N.; Wang, X.; Liu, Z.; Zhang, T. Catalytic Reduction of SO2 with CO over Supported Iron Catalysts. Ind. Eng. Chem. Res. 2006, 45, 4582–4588. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Pahlavanzadeh, H.; Khani, M.; Ale ebrahim, H.; Mozaffari, A. Catalytic reduction of SO2 with CH4 to elemental sulfur: A comparative analysis of alumina, copper-alumina and nickel-alumina catalysts. Iran. J. Chem. Chem. Eng. 2018, 15, 94–107. [Google Scholar]
- Han, G.B.; Park, N.-K.; Yoon, S.H.; Lee, T.J. Investigation of Catalytic Reduction of Sulfur Dioxide with Carbon Monoxide over Zirconium Dioxide Catalyst for Selective Sulfur Recovery. Ind. Eng. Chem. Res. 2008, 47, 1427–1434. [Google Scholar] [CrossRef]
- Han, G.B.; Park, N.K.; Yoon, S.H.; Lee, T.J. Catalytic reduction of sulfur dioxide with carbon monoxide over tin dioxide for direct sulfur recovery process. Chemosphere 2008, 72, 1744–1750. [Google Scholar] [CrossRef]
- Ngwenya, T.; Nongwe, I.; Jewell, L.L. Reduction of sulphur dioxide using carbon monoxide over gold supported catalysts. Gold Bull. 2018, 51, 153–162. [Google Scholar] [CrossRef]
- Park, N.-K.; Lee, T.H.; Lee, T.J.; Baek, J.-I.; Lee, J.B. Catalytic reduction of SO2 under the regeneration of off-gas containing oxygen over Cu-Sn-Zr-based oxides for the hot coal gas desulfurization process. Catal. Today 2016, 265, 131–137. [Google Scholar] [CrossRef]
- Ebrahim Mousavi, S.; Pahlavanzadeh, H.; Ale Ebrahim, H. Preparation, Characterization and Optimization of High Surface Area Ce-La-Cu Ternary Oxide Nanoparticles. E-J. Surf. Sci. Nanotechnol. 2017, 15, 87–92. [Google Scholar] [CrossRef]
- Hossein Khangah, A.; Javad Sarraf, M.; Ale Ebrahim, H.; Tabatabaee, M. Preparing and Optimization of Cerium-Lanthanum-Cobalt Ternary Mixed Oxide as Catalyst for SO2 Reduction to Sulfur. E-J. Surf. Sci. Nanotechnol. 2019, 17, 16–26. [Google Scholar] [CrossRef] [Green Version]

| Method/Sorbent | Main Product | Main Advantage | Main Disadvantage |
|---|---|---|---|
| Wet limestone (CaCO3) | Gypsum (CaSO4·2H2O) | High efficiency (>95%) | High cost |
| Lime (CaO) | Gypsum (CaSO4·2H2O) | High efficiency (>95%) | High cost |
| Magnesium oxide (MgO) | Mg(SO4) | Cheaper | Efficiency varies greatly |
| Zinc oxide (ZnO) | Zn(SO3) | Suitable for zinc smelting plants | Limited applications |
| Dual alkali (NaOH, Na2CO3) | CaSO3, CaSO4 | High efficiency up to 98% | Not mature and applicable at large scale |
| Ammonia (NH3) | (NH4)2SO4 | Very high efficiency | NH3 is toxic |
| Method | Main Advantage | Main Disadvantage |
|---|---|---|
| Sorbent injection | Low cost, no wastewater | Low efficiency (20–50%) |
| Active carbon | Easy to apply | High cost, low efficiency |
| Dry circulating fluidized bed | High efficiency | Particulate matter production |
| Spray dry | 90% efficiency | Difficult to apply at large-size boilers |
| Type | Advantages | Disadvantages |
|---|---|---|
| Open-Loop |
|
|
| Closed-Loop |
|
|
| Hybrid |
|
|
| Country | Prohibited Scrubber Use |
|---|---|
| Bahrain | at port or at anchor |
| Belgium | within 3 nautical miles of the coast |
| Brazil | territorial seas |
| China | in domestic emission control areas |
| Egypt | in all ports and Suez canals |
| Gibraltar | in Gibraltar waters |
| Ireland | in Dublin and Waterford ports |
| Malaysia | in territorial seas (12 nautical miles) |
| Norway | in the World Heritage Fjords Sea areas of Geirangerfjord and Nærøyfjord |
| Pakistan | in the ports of Karachi and Bin Qasim |
| Panama | in the Panama Canal |
| Portugal | in any port or at berth |
| Singapore | in any port |
| Spain | in the ports of Algeciras, Cartagena and Huelva |
| United States | California, Connecticut and Hawaii ports or waters |
| United Arab Emirates | in the port of Fujairah |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, C.; Kourtelesis, M.; Moschovi, A.M.; Sakkas, K.M.; Yakoumis, I. Selected Techniques for Cutting SOx Emissions in Maritime Industry. Technologies 2022, 10, 99. https://doi.org/10.3390/technologies10050099
Papadopoulos C, Kourtelesis M, Moschovi AM, Sakkas KM, Yakoumis I. Selected Techniques for Cutting SOx Emissions in Maritime Industry. Technologies. 2022; 10(5):99. https://doi.org/10.3390/technologies10050099
Chicago/Turabian StylePapadopoulos, Christos, Marios Kourtelesis, Anastasia Maria Moschovi, Konstantinos Miltiadis Sakkas, and Iakovos Yakoumis. 2022. "Selected Techniques for Cutting SOx Emissions in Maritime Industry" Technologies 10, no. 5: 99. https://doi.org/10.3390/technologies10050099
APA StylePapadopoulos, C., Kourtelesis, M., Moschovi, A. M., Sakkas, K. M., & Yakoumis, I. (2022). Selected Techniques for Cutting SOx Emissions in Maritime Industry. Technologies, 10(5), 99. https://doi.org/10.3390/technologies10050099







