Examination of Polymer Blends by AFM Phase Images
Abstract
:1. Introduction
2. Materials and Methods
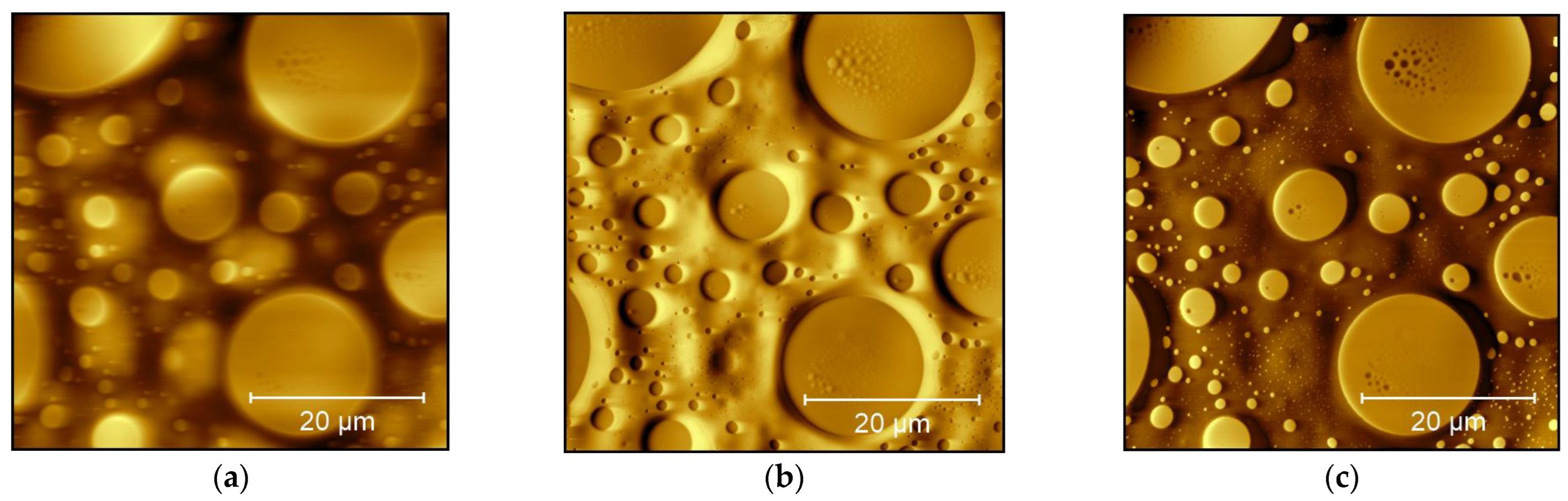
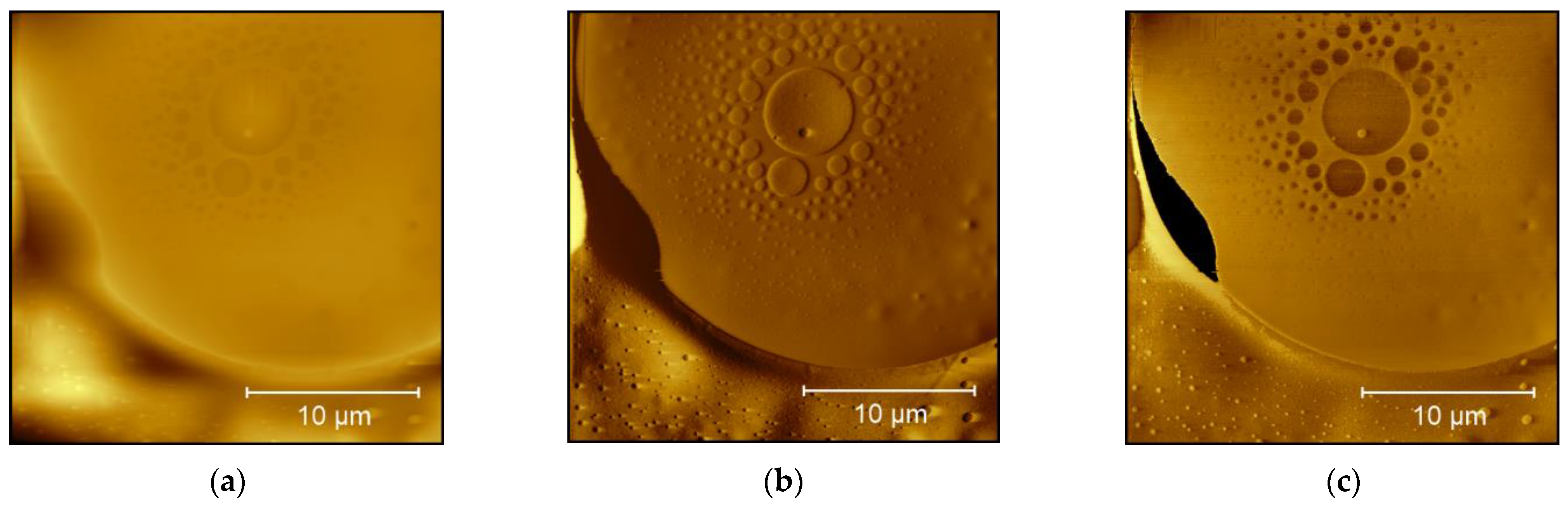
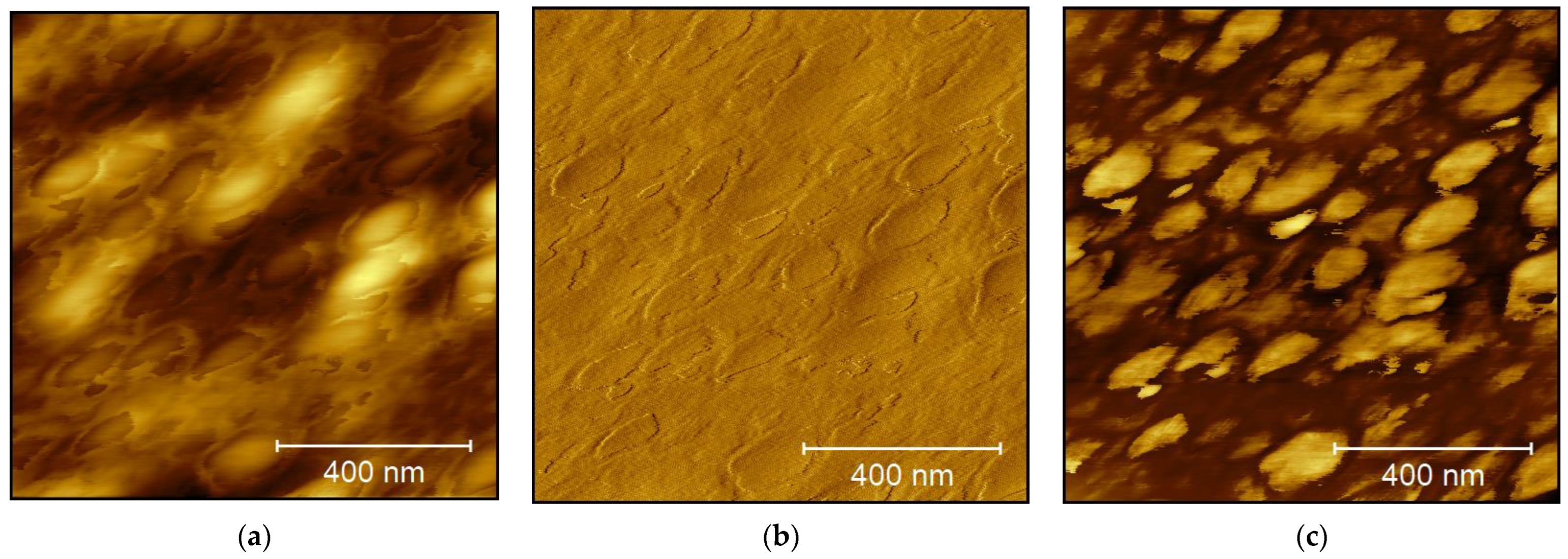
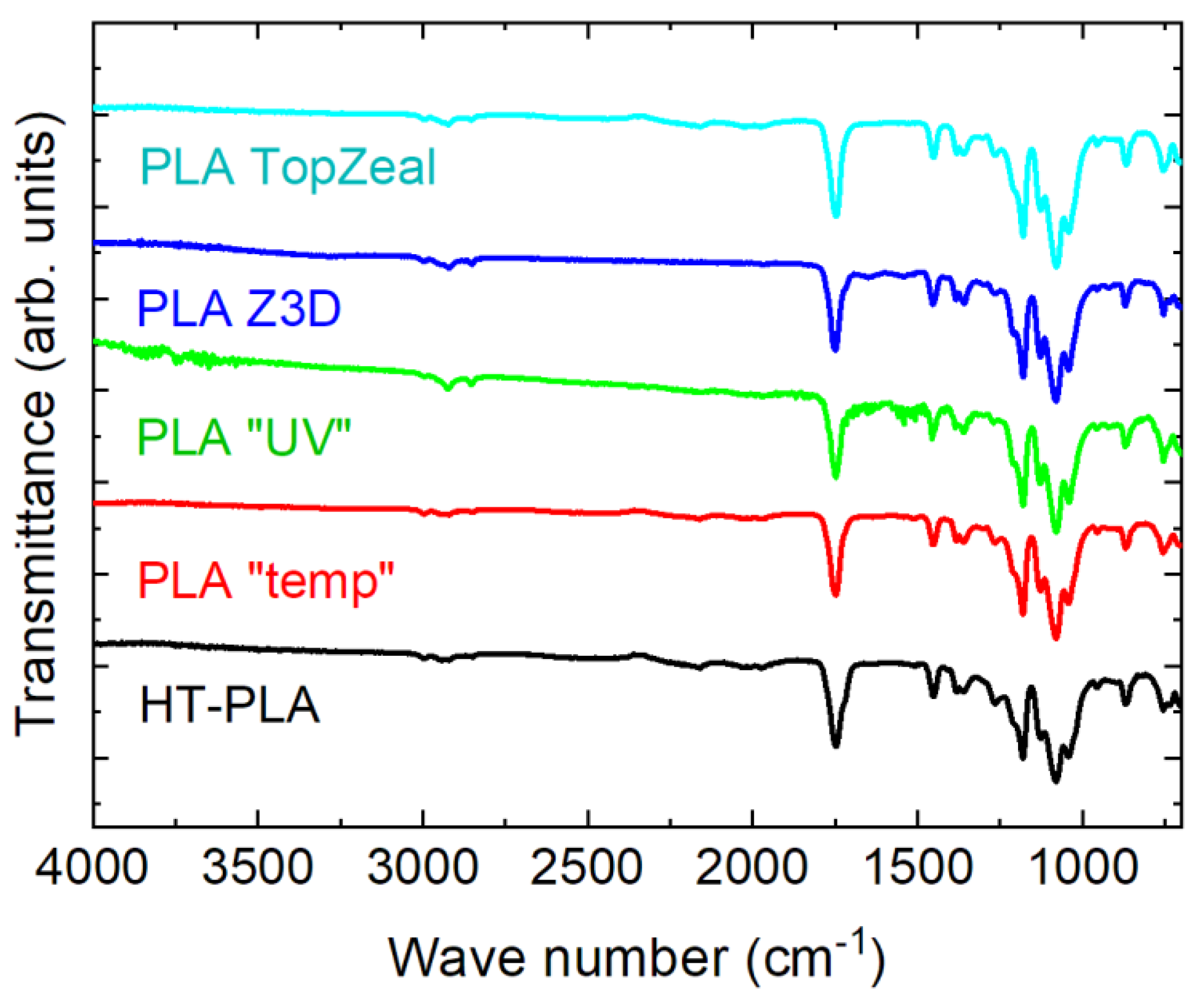
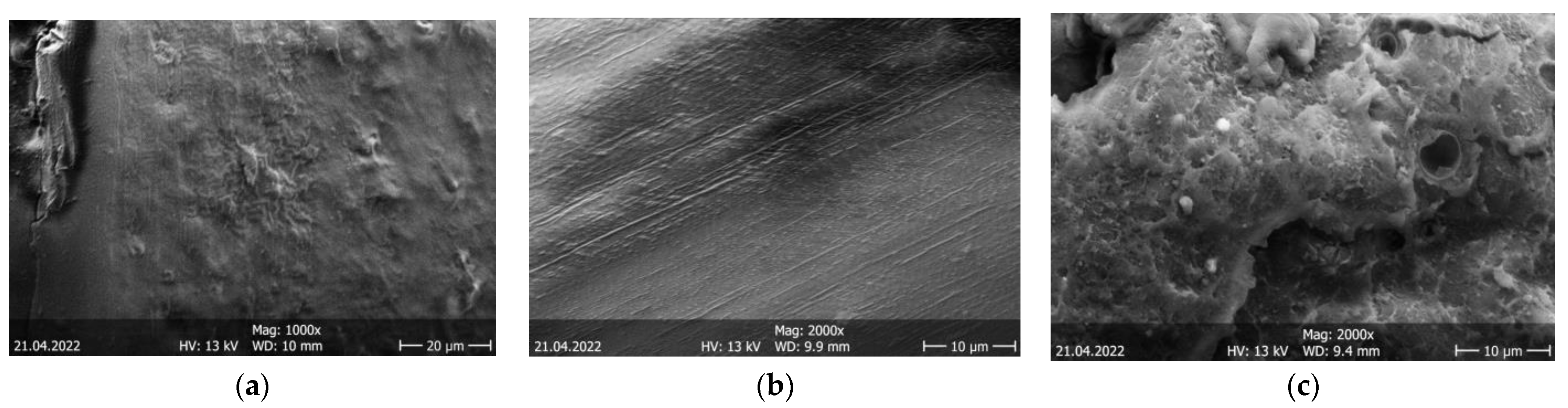
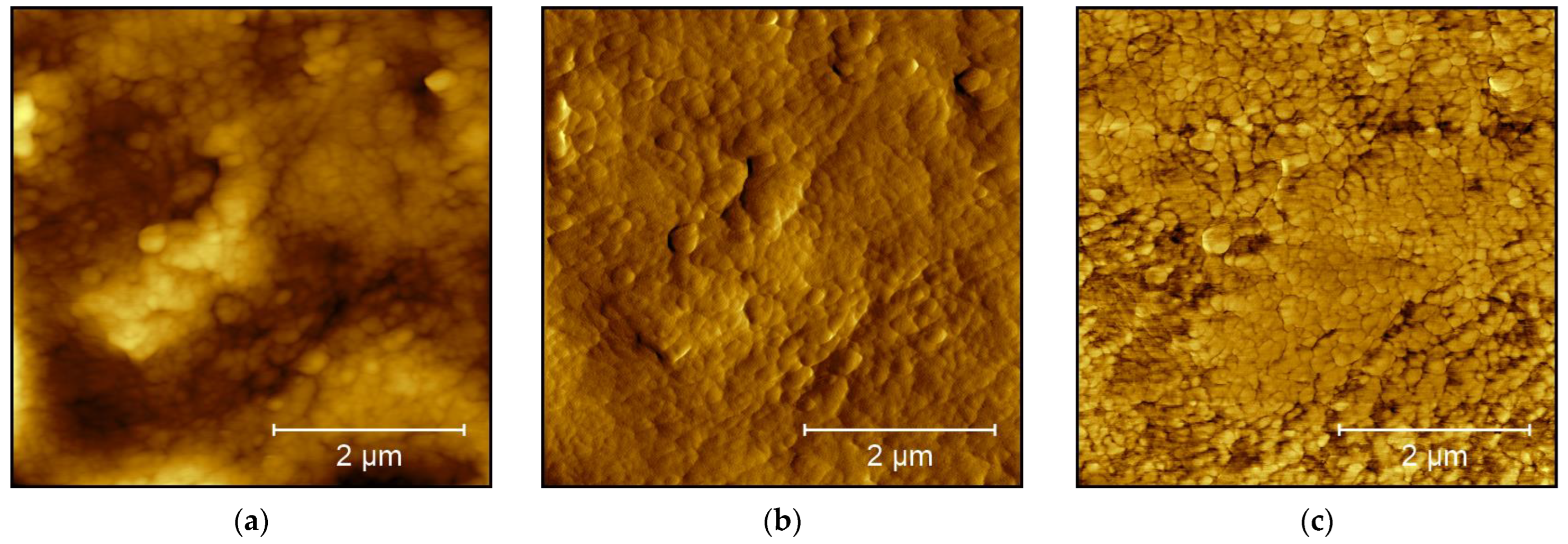
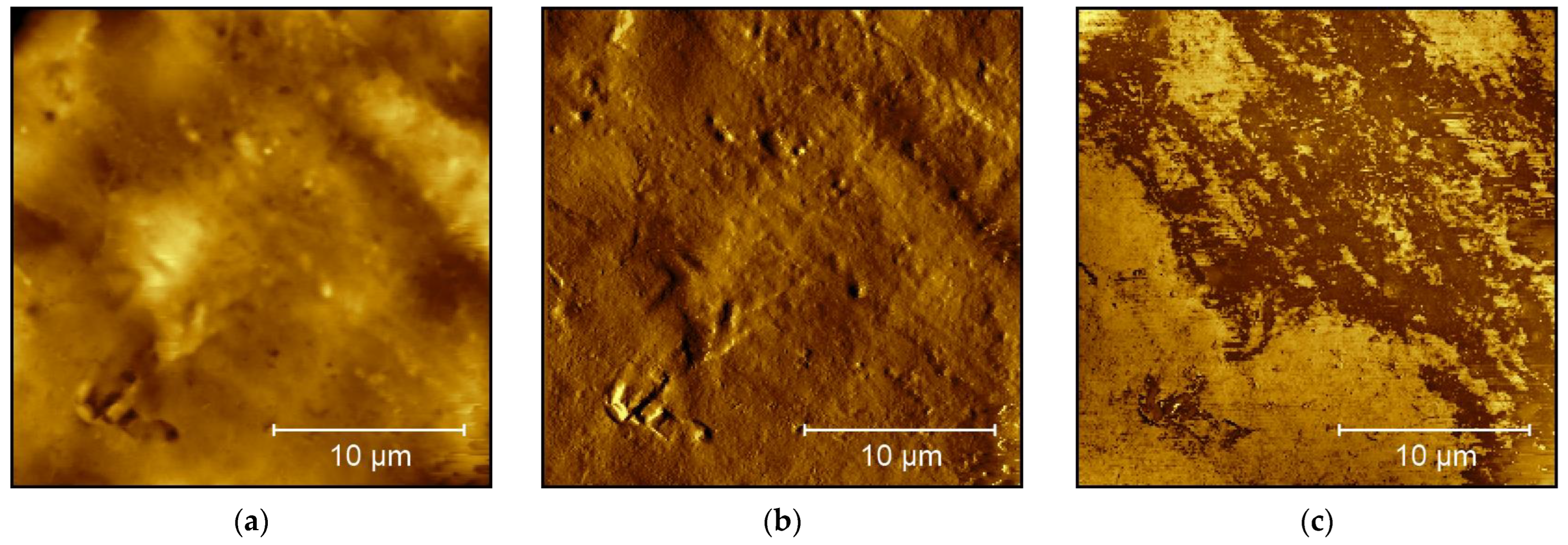
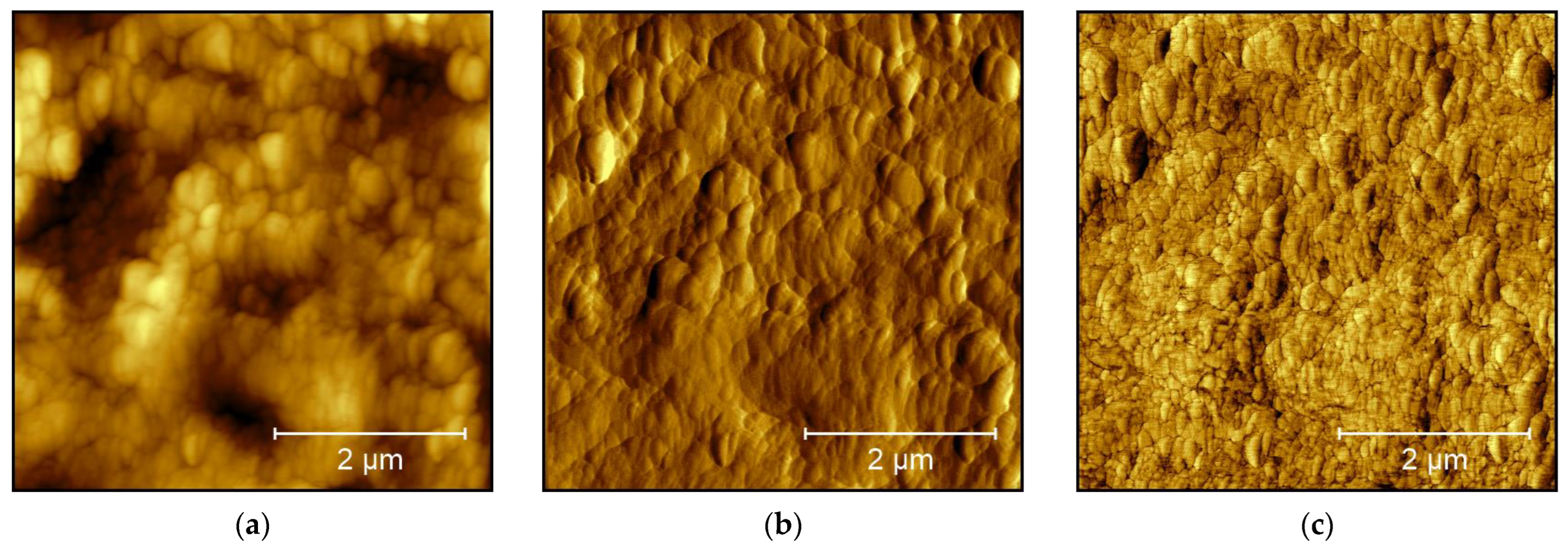
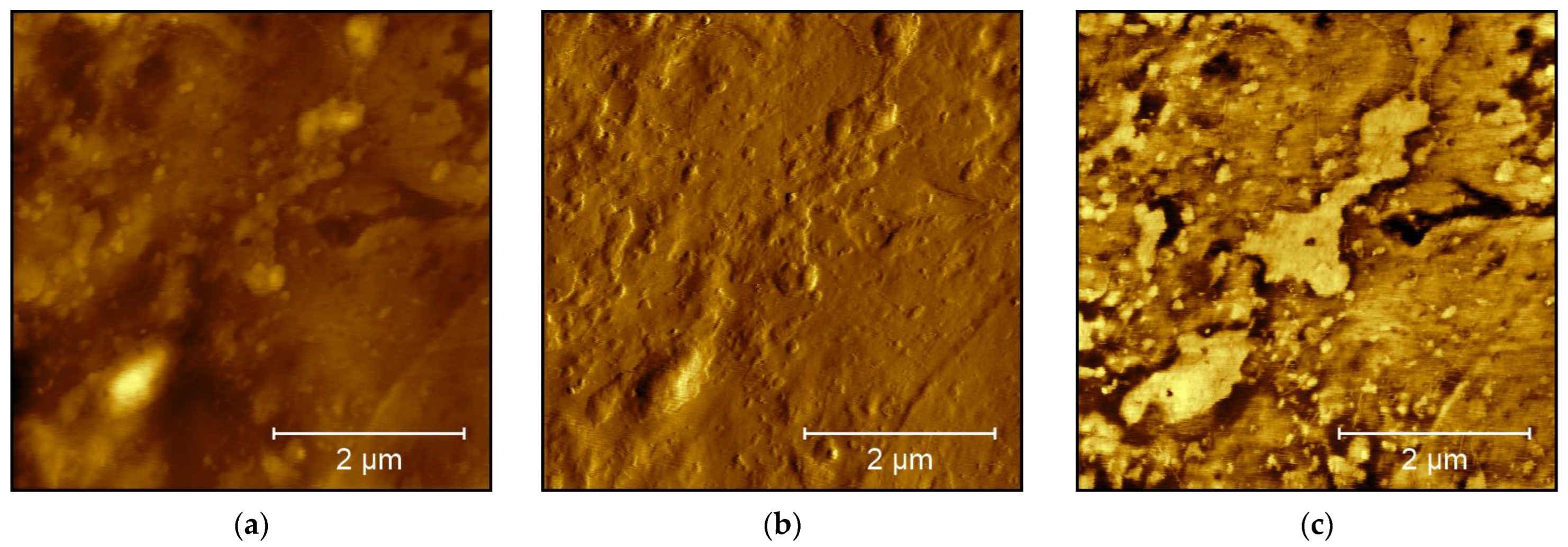
3. Results and Discussion
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Parot, P.; Dufrene, Y.F.; Hinterdorfer, P.; Le Grimellec, C.; Navajas, D.; Pellequer, J.-L.; Scheuring, S. Past, present and future of atomic force microscopy in life sciences and medicine. J. Mol. Recognit. 2007, 20, 418–431. [Google Scholar] [CrossRef]
- Hoogenboom, B.W. Stretching the resolution limit of atomic force microscopy. Nat. Struct. Mol. Biol. 2021, 28, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D. Atomic force microscopy of biomaterials surfaces and interfaces. Surf. Sci. 2001, 491, 303–332. [Google Scholar] [CrossRef]
- García, R.; San Paulo, A. Amplitude curves and operating regimes in dynamic atomic force microscopy. Ultramicroscopy 2000, 82, 79–83. [Google Scholar] [CrossRef]
- Haugstad, G.; Jones, R.R. Mechanisms of dynamic force microscopy on polyvinyl alcohol: Region-specific non-contact and intermittent contact regimes. Ultramicroscopy 1999, 76, 77–86. [Google Scholar] [CrossRef]
- San Paulo, A.; García, R. High-resolution imaging of antibodies by tapping-mode atomic force microscopy: Attractive and repulsive tip-sample interaction regimes. Biophys. J. 2000, 78, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Round, A.N.; Miles, M.J. Exploring the consequences of attractive and repulsive interaction regimes in tapping mode atomic force microscopy of DNA. Nanotechnology 2004, 15, S176–S183. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic force microscopy (AFM) on biopolymers and hydrogels for biotechnological applications—Possibilities and limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Raghavan, D.; Gu, X.; Nguyen, T.; Van Landingham, M.; Karim, A. Mapping polymer heterogeneity using atomic force microscopy phase imaging and nanoscale indentation. Macromolecules 2000, 33, 2573–2583. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B.; Hu, Q.C.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM–IR: Combining Atomic Force Microscopy and Infrared Spectroscopy for Nanoscale Chemical Characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.-P.; Caprarescu, S.; Iurciuc (Tincu), C.E.; Riess, G. Micellization of pH-sensitive poly(butadiene)-block-poly(2 vinylpyridine)-block-poly(ethylene oxide) triblock copolymers: Complex formation with anionic surfactants. J. Appl. Polym. Sci. 2017, 134, 45313. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Jalal, V.J.; Tahir, D.A.; Mohamad, A.H.; Abdullah, O.G. Effect of PEG as a plasticizer on the electrical and optical properties of polymer blend electrolyte MC-CH-LiBF4 based films. Results Phys. 2019, 15, 102735. [Google Scholar] [CrossRef]
- Caprarescu, S.; Radu, A.-L.; Purcar, V.; Sarbu, A.; Vaireanu, D.-I.; Ianchis, R.; Ghiurea, M. Removal of Copper Ions from Simulated Wastewaters Using Different Bicomponent Polymer Membranes. Water Air Soil Pollut. 2014, 225, 2079. [Google Scholar] [CrossRef]
- Cole, D.P.; Riddick, J.C.; Iftekhar Jaim, H.M.; Strawhecker, K.E.; Zander, N.E. Interfacial mechanical behavior of 3D printed ABS. J. Appl. Polym. Sci. 2016, 133, 43671. [Google Scholar] [CrossRef]
- León, A.S.; Domínguez-Calvo, A.; Molina, S.I. Materials with enhanced adhesive properties based on acrylonitrile-butadiene-styrene (ABS)/thermoplastic polyurethane (TPU) blends for fused filament fabrication (FFF). Mater. Des. 2019, 182, 108044. [Google Scholar] [CrossRef]
- Peng, B.G.; Yang, Y.C.; Ju, T.X.; Cavicchi, K.A. Fused Filament Fabrication 4D Printing of a Highly Extensible, Self-Healing, Shape Memory Elastomer Based on Thermoplastic Polymer Blends. ACS Appl. Mater. Interfaces 2021, 13, 12777–12788. [Google Scholar] [CrossRef]
- Moore, J.D. Acrylonitrile-butadiene-styrene (ABS)—A review. Composites 1973, 4, 118–130. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Kadum, K.M. Morphology studies and mechanical properties for PS/SBS blends. Int. J. Eng. Technol. 2012, 12, 19–27. [Google Scholar]
- Sauer, B.B.; McLean, R.S.; Thomas, R.R. Tapping Mode AFM Studies of Nano-Phases on Fluorine-Containing Polyester Coatings and Octadecyltrichlorosilane Monolayers. Langmuir 1998, 14, 3045. [Google Scholar] [CrossRef]
- Adhikari, R. Tapping mode atomic force microscopy (TMAFM) of some multi-component polymers. J. Nepal Chem. Soc. 2012, 29, 96–103. [Google Scholar] [CrossRef]
- Haviland, D.B.; van Eysden, C.A.; Forchheimer, D.; Platz, D.; Kassa, H.G.; Leclère, P. Probing viscoelastic response of soft material surfaces at the nanoscale. Soft Matter. 2016, 12, 619–624. [Google Scholar] [CrossRef]
- Leclère, P.; Lazzaroni, R.; Bredas, J.L.; Yu, J.M.; Dubois, P.; Jerome, R. Microdomain Morphology Analysis of Block Copolymers by Atomic Force Microscopy with Phase Detection Imaging. Langmuir 1996, 12, 4317. [Google Scholar] [CrossRef]
- McLean, R.S.; Sauer, B.B. Tapping-Mode AFM Studies Using Phase Detection for Resolution of Nanophases in Segmented Polyurethanes and Other Block Copolymers. Macromolecules 1997, 30, 8314. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT-Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Franca, D.C.; Almeida, T.G.; Abels, G.; Canedo, E.L.; Carvalho, L.H.; Wellen, R.M.R.; Haag, K.; Koschek, K. Tailoring PBAT/PLA/Babassu films for suitability of agriculture mulch application. J. Nat. Fibers 2019, 16, 933–943. [Google Scholar] [CrossRef]
- Siyamak, S.; Ibrahim, N.A.; Abdolmohammadi, S.; Yunus, W.M.Z.W.; Rahman, M.Z.A.B. Effect of fiber esterification on fundamental properties of oil palm empty fruit bunch fiber/poly(butylene adipate-co-terephthalate) biocomposites. Int. J. Mol. Sci. 2012, 13, 1327–1346. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.Q.; Wu, Q.S.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly(lactic acid) blends. J. Nanomater. 2010, 2010, 287082. [Google Scholar] [CrossRef]
- Storck, J.L.; Ehrmann, G.; Güth, U.; Uthoff, J.; Homburg, S.V.; Blachowicz, T.; Ehrmann, A. Investigation of Low-Cost FDM-Printed Polymers for Elevated-Temperature Applications. Polymers 2022, 14, 2826. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamaguchi, K.; Miyazaki, H. Fabrication of thermochromic composite using monodispersed VO2 coated SiO2 nanoparticles prepared by modified chemical solution deposition. Compos. Sci. Technol. 2007, 67, 3487–3490. [Google Scholar] [CrossRef]
- Maaza, M.; Nemraoui, O.; Sella, C.; Beye, A.C.; Baruch-Barak, B. Thermal induced tunability of surface plasmon resonance in Au–VO2 nano-photonics. Opt. Commun. 2005, 254, 188–195. [Google Scholar] [CrossRef]
- Zhou, H.J.; Cao, X.; Jiang, M.; Bao, S.H.; Jin, P. Surface plasmon resonance tunability in VO2/Au/VO2 thermochromic structure. Laser Photonics Rev. 2014, 8, 617–625. [Google Scholar] [CrossRef]
- Faucheu, J.; Bourgeat-Lami, E.; Prevot, V. A Review of Vanadium Dioxide as an Actor of Nanothermochromism: Challenges and Perspectives for Polymer Nanocomposites. Adv. Eng. Mater. 2019, 21, 1800438. [Google Scholar] [CrossRef]
- Garshasbi, S.; Santamouris, M. Using advanced thermochromic technologies in the built environment: Recent development and potential to decrease the energy consumption and fight urban overheating. Sol. Energy Mater. Sol. Cells 2019, 191, 21–32. [Google Scholar] [CrossRef]
- DeJournett, T.J.; Spicer, J.B. The influence of oxygen on the microstructural, optical and photochromic properties of polymer-matrix, tungsten-oxide nanocomposite films. Sol. Energy Mater. Sol. Cells 2014, 120, 102–108. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, B.; Li, H.H.; Guo, J.J.; Zhao, S.Q.; Wu, G.H. Preparation and properties of photochromic regenerated silk fibroin/Tungsten trioxide nanoparticles hybrid fibers. Compos. Commun. 2021, 27, 100810. [Google Scholar] [CrossRef]
- Schmidt, U.; Hild, S.; Ibach, W.; Hollricher, O. Characterization of Thin Polymer Films on the Nanometer Scale with Confocal Raman AFM. Macromol. Symp. 2005, 230, 133–143. [Google Scholar] [CrossRef]
- Artyushkova, K.; Farrar, J.O.; Fulghum, J.E. Data fusion of XPS and AFM images for chemical phase identification in polymer blends. Surf. Interface Anal. 2009, 41, 119–126. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Peng, L.; Zhang, N.; Su, J.X.; Ye, N.B.; Luo, Z.F.; Han, C.C.; Huang, X.B.; Su, Z.H. Miscibility of isotactic polypropylene with random and block ethylene-octene copolymers studied by atomic force microscopy-infrared. Polymer 2022, 259, 125354. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, E.; Güth, U.; Brockhagen, B.; Döpke, C.; Ehrmann, A. Examination of Polymer Blends by AFM Phase Images. Technologies 2023, 11, 56. https://doi.org/10.3390/technologies11020056
Werner E, Güth U, Brockhagen B, Döpke C, Ehrmann A. Examination of Polymer Blends by AFM Phase Images. Technologies. 2023; 11(2):56. https://doi.org/10.3390/technologies11020056
Chicago/Turabian StyleWerner, Enrico, Uwe Güth, Bennet Brockhagen, Christoph Döpke, and Andrea Ehrmann. 2023. "Examination of Polymer Blends by AFM Phase Images" Technologies 11, no. 2: 56. https://doi.org/10.3390/technologies11020056
APA StyleWerner, E., Güth, U., Brockhagen, B., Döpke, C., & Ehrmann, A. (2023). Examination of Polymer Blends by AFM Phase Images. Technologies, 11(2), 56. https://doi.org/10.3390/technologies11020056












