Integrated System of Reverse Osmosis and Forward Pressure-Assisted Osmosis from ZrO2 Base Polymer Membranes for Desalination Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
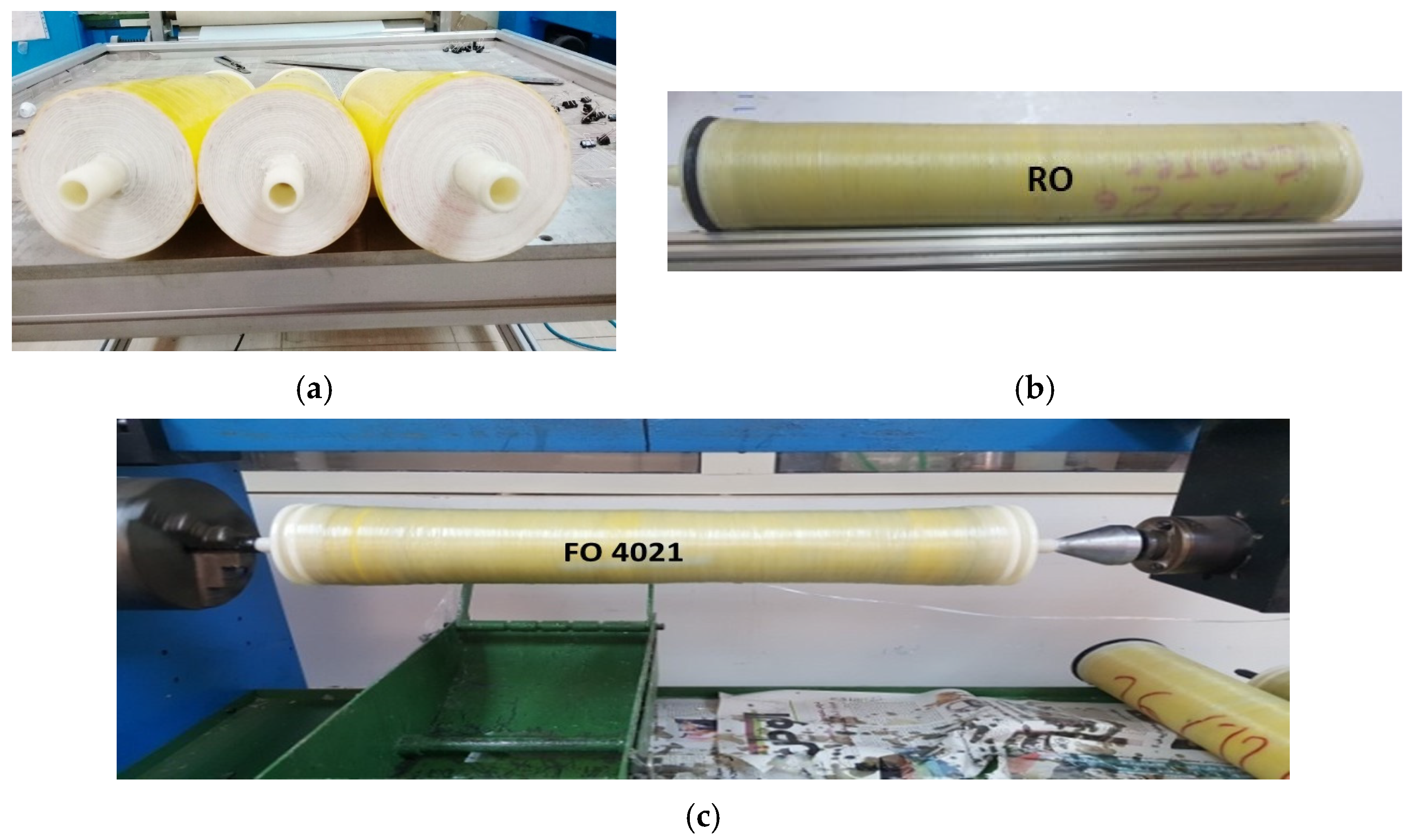
2.2. Preparation of Membranes and Module Fabrication
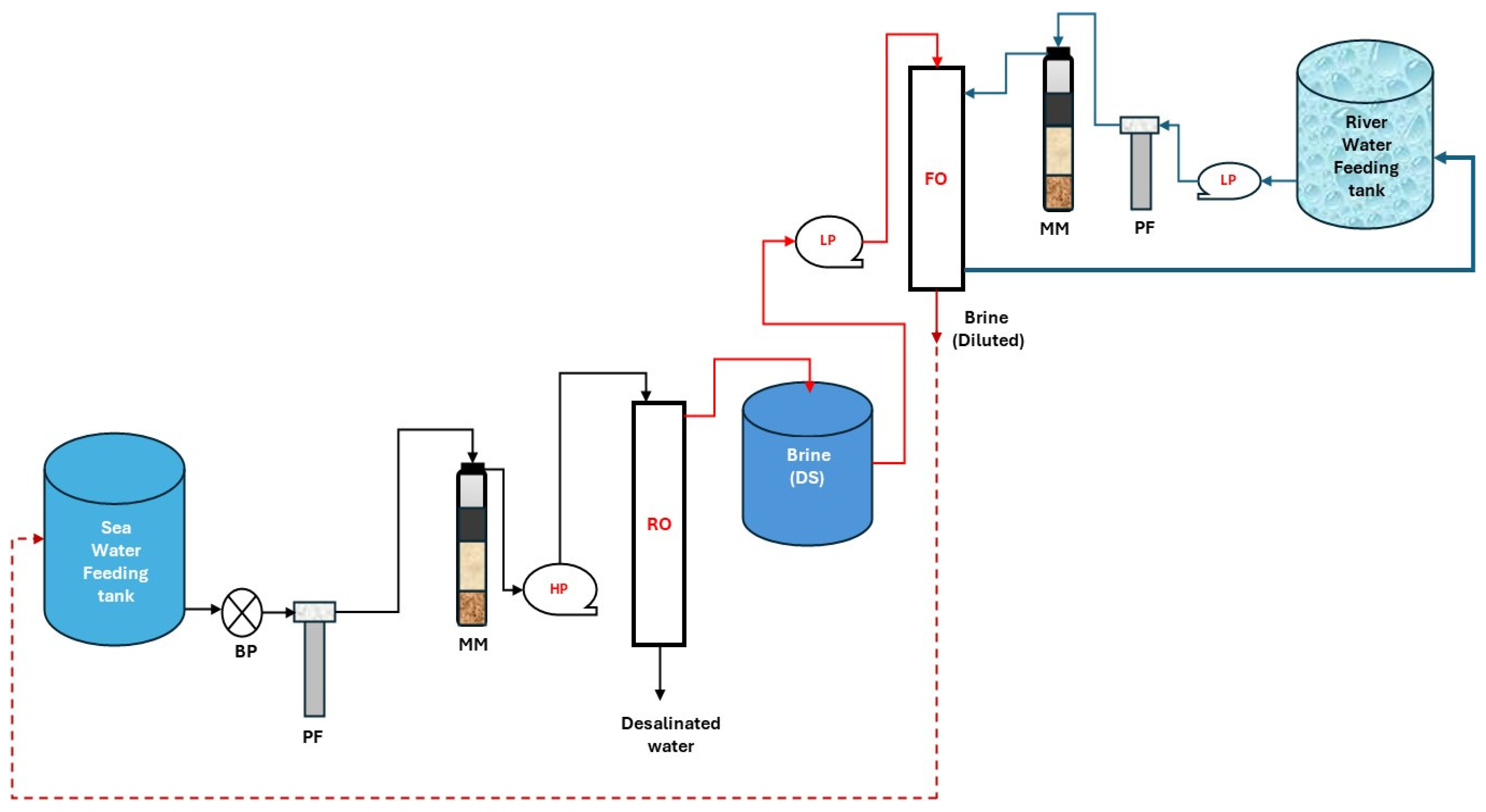
2.3. Pilot Systems for Membrane Module Testing
- TMP: transmembrane pressure (bar),
- Pf: feed pressure (bar),
- Pc: concentrate pressure (bar), and
- Pp: permeate pressure (bar).
- J = flux (L/m2·h)
- Qp = filtrate flow (L/h)
- Am = membrane surface area (m2)
- The recovery of a membrane was calculated using Equation (3):
- Qp = filtrate flow produced by the membrane unit (L/h)
- Qf = feed flow to the membrane unit (L/h)
- The salt rejection (R) was calculated as follows:
3. Results and Discussion
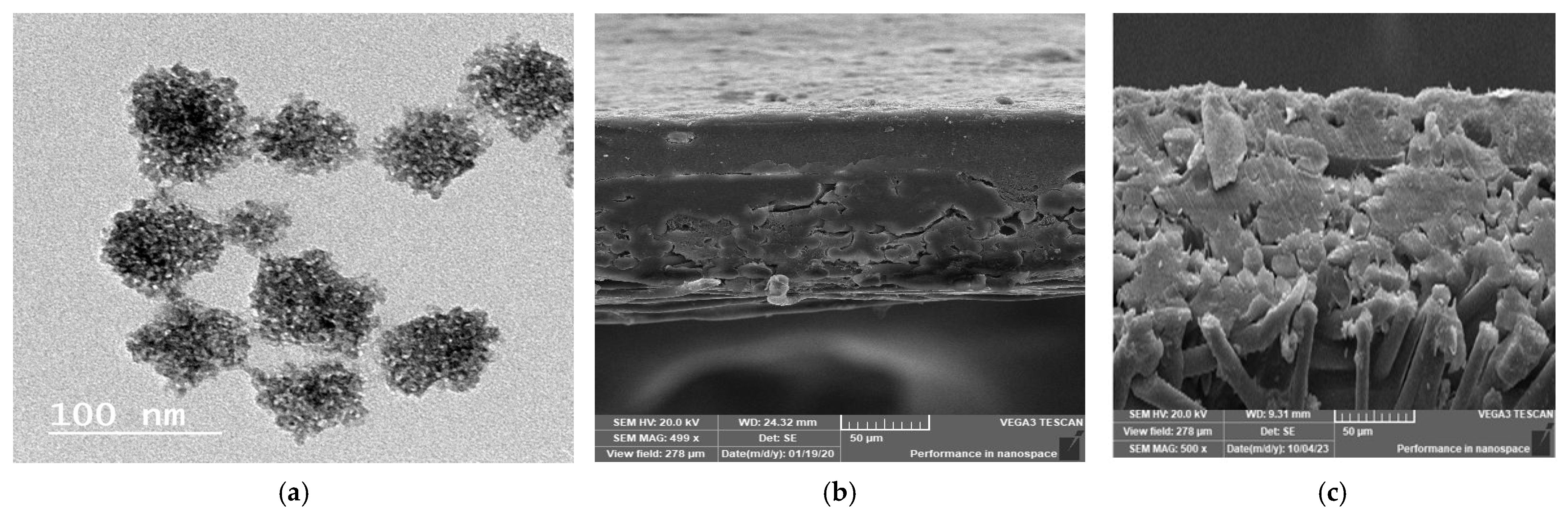
3.1. Prepared Nanoparticles and Membranes Characterization
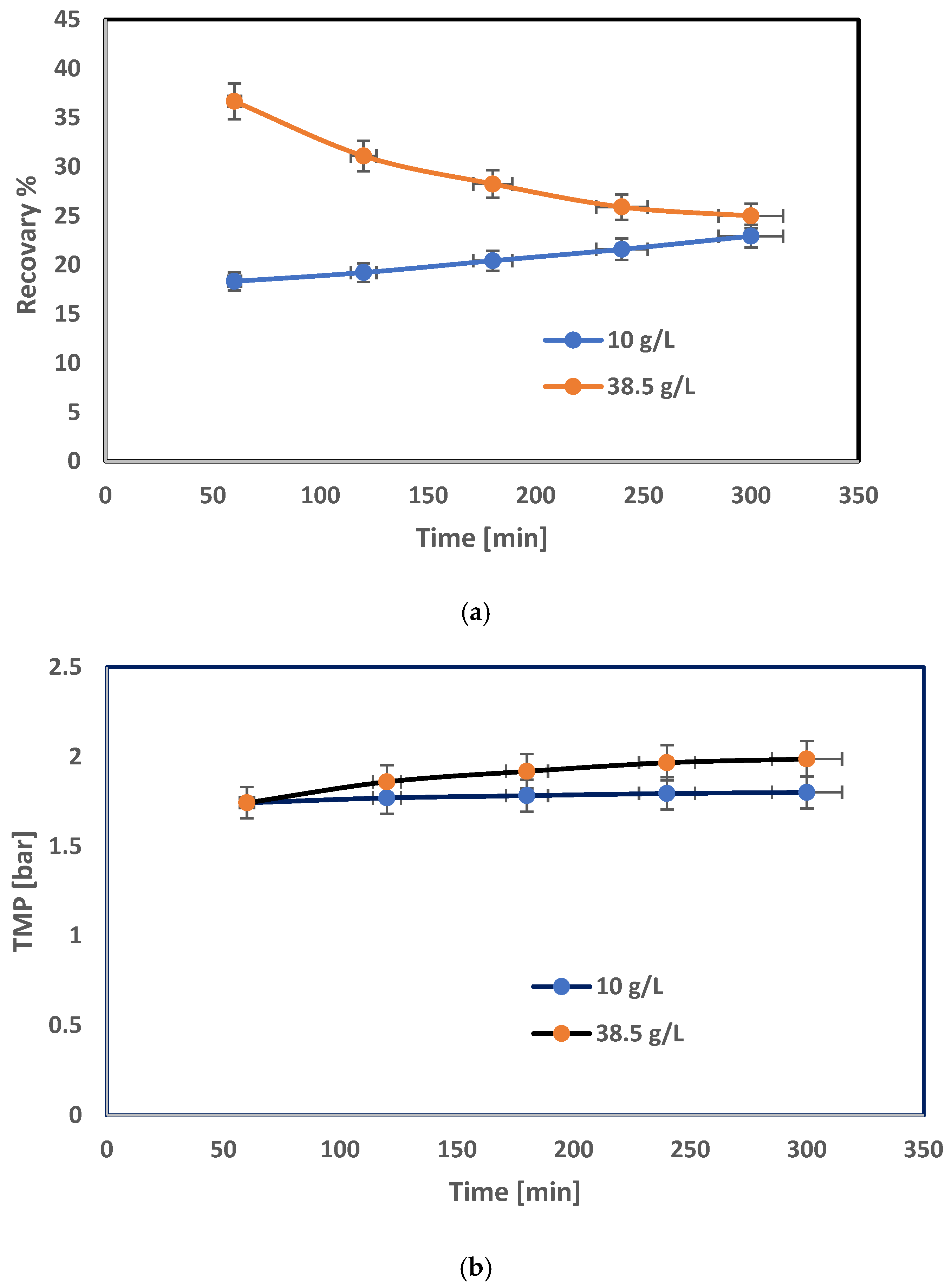
3.2. RO Spiral-Wound Membrane Module Results
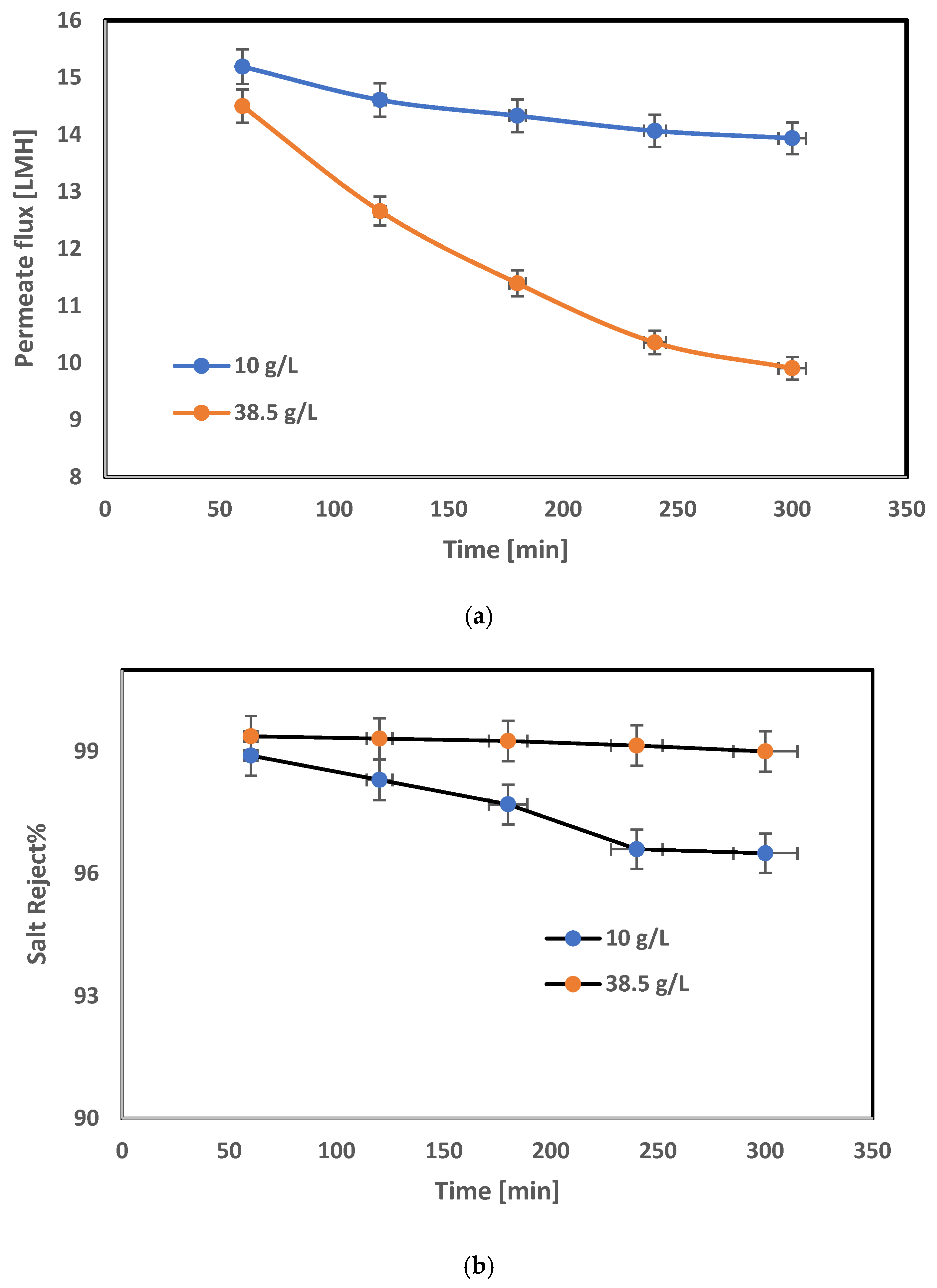
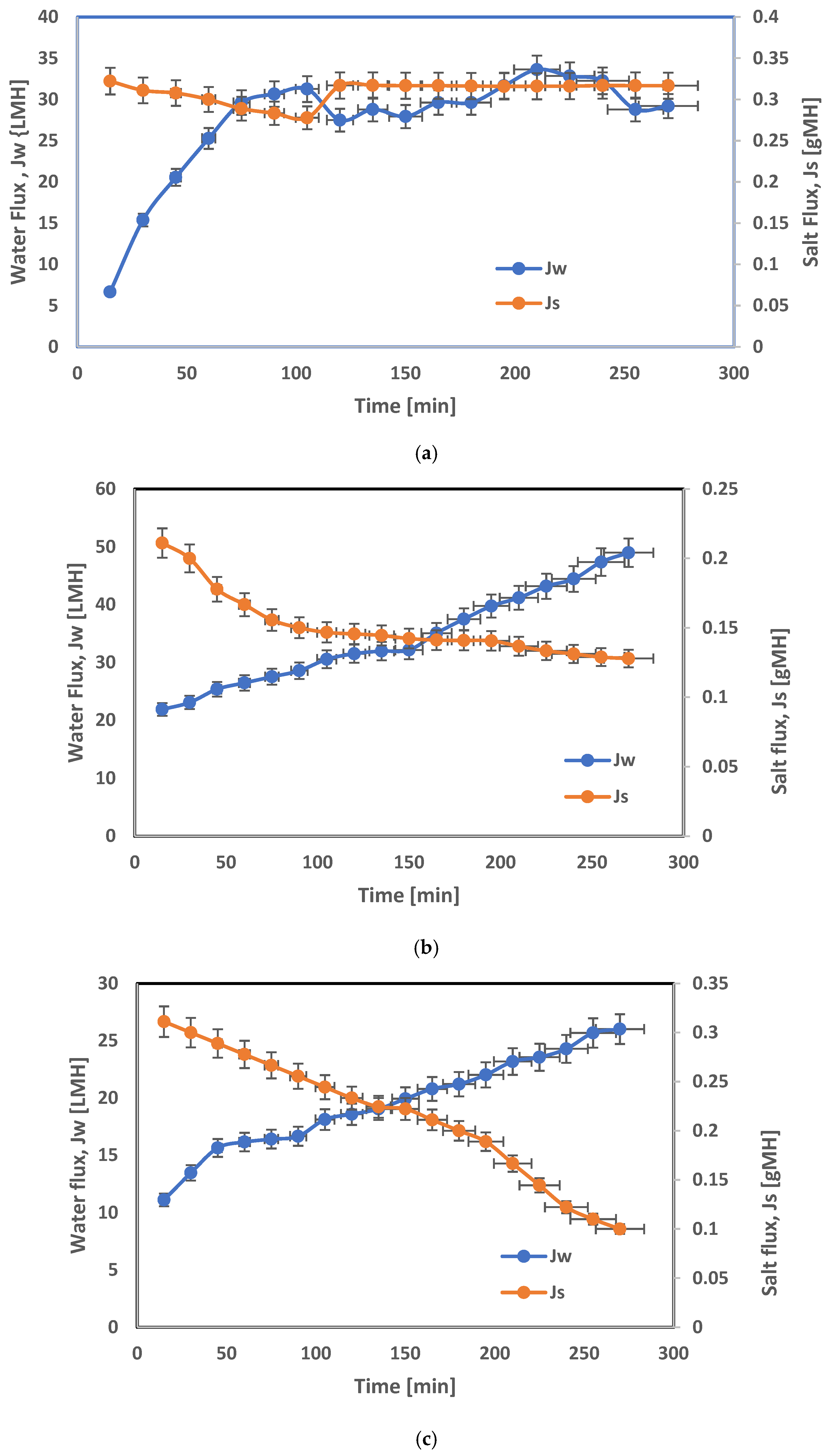
3.3. FO Spiral-Wound Membrane Module Results
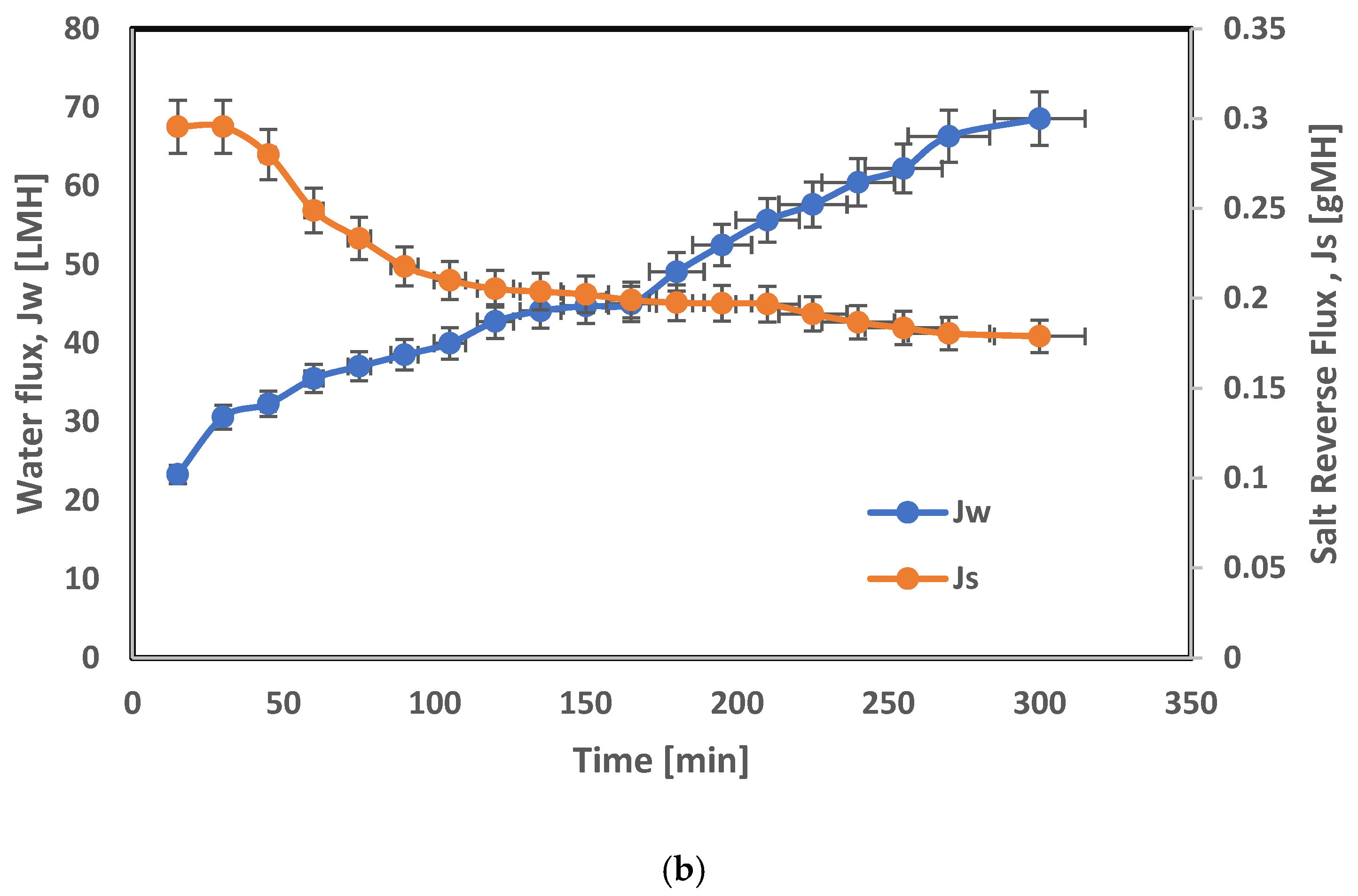
3.4. Integrated Membrane RO/PAO System Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.L.; Zhou, W.; Shen, L.; Li, B.; Sun, H.; Zeng, Q.; Tang, C.Y.; Lin, H.; Chung, T.S. New directions on membranes for removal and degradation of emerging pollutants in aqueous systems. Water Res. 2024, 251, 121111. [Google Scholar] [CrossRef] [PubMed]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Opia, A.C.; Ugwu, C.I. Membrane technology as viable means for water recovery: Challenges and future directions. J. Resour. Recovery 2024, 2, 1020. [Google Scholar] [CrossRef]
- Popova, A.; Boivin, S.; Shintani, T.; Fujioka, T. Development of high-integrity reverse osmosis membranes for enhanced removal of microorganisms. Desalination 2024, 572, 117155. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Ahmad, N.A.; Lee, W.J. Flux enhancement in reverse osmosis membranes induced by synergistic effect of incorporated palygorskite/chitin hybrid nanomaterial. J. Environ. Chem. Eng. 2021, 9, 105432. [Google Scholar] [CrossRef]
- Cui, L.; Wang, P.; Che, H.; Gao, X.; Chen, J.; Liu, B.; Ao, Y. Co nanoparticles modified N-doped carbon nanosheets array as a novel bifunctional photothermal membrane for simultaneous solar-driven interfacial water evaporation and persulfate mediating water purification. Appl. Catal. B Environ. 2023, 330, 122556. [Google Scholar] [CrossRef]
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane technology for sustainable water resources management: Challenges and future projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Yu, L.; Deana, K.; Lin, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Reddya, A.V.R.; Patelb, H.R. Chemically treated polyethersulfone/polyacrylonitrile blend ultrafiltration membranes for better fouling resistance. Desalination 2008, 221, 318–323. [Google Scholar] [CrossRef]
- Ehsan, S.; Toraj, M. Cellulose acetate (CA)/polyvinylpyrrolidone (PVP) blend asymmetric membranes: Preparation, morphology and performance. Desalination 2009, 249, 850–854. [Google Scholar]
- Mansor, E.S.; Abdallah, H.; Shaban, A.M. Development of TiO2/polyvinyl alcohol-cellulose acetate nanocomposite reverse osmosis membrane for groundwater-surface water interfaces purification. Mater. Sci. Eng. B 2023, 289, 116222. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Al-Najar, B.; Peters, C.D.; Albuflasa, H.; Hankins, N.P. Pressure and osmotically driven membrane processes: A review of the benefits and production of nano-enhanced membranes for desalination. Desalination 2020, 479, 114323. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A.; Zaragoza, G.; Ismail, A.F. Evaluation of FO-RO and PRO-RO designs for power generation and seawater desalination using impaired water feeds. Desalination 2015, 368, 27–35. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.; Le-Clech, P. Pressure enhanced fouling and adapted anti-fouling strategy in pressure assisted osmosis (PAO). J. Membr. Sci. 2015, 493, 557–567. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; Childress, A.E.; Le-Clech, P. Validation of assisted forward osmosis (AFO) process: Impact of hydraulic pressure. J. Membr. Sci. 2013, 447, 1–11. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on methodology for determining forward osmosis (FO) membrane characteristics: Water permeability (A), solute permeability (B), and structural parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Marghany, N.E.; Moustafa, M.M.; El-Sharkawy, A.M.; Ali, A.A. Zirconium oxide nanoparticles: Fabrication, study and application for removal of organic dye from aqueous media. Benha J. Appl. Sci. 2022, 7, 193–200. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shaban, A.M. Fabrication of high selectivity blend membranes based on polyvinyl alcohol for crystal violet dye removal. J. Environ. Chem. Eng. 2020, 8, 103706. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.B.; Zhang, X.S.; Zheng, G.D. Polyamide thin-film composite membranes prepared from 3,4,5-biphenyl triacyl chloride, 3,3,5,5-biphenyl tetraacyl chloride and m-phenylenediamine. J. Membr. Sci. 2007, 289, 258–267. [Google Scholar] [CrossRef]
- Liu, M.H.; Wu, D.H.; Yua, S.C.; Gao, C.J. Influence of the polyacyl chloride structure on the reverse osmosis performance, surface properties and chlorine stability of the thin-film composite polyamide membranes. J. Membr. Sci. 2009, 326, 205–214. [Google Scholar] [CrossRef]
- Kim, I.C.; Ka, Y.H.; Park, J.Y.; Lee, K.H. Preparation of fouling resistant nanofiltration and reverse osmosis membranes and their use for dyeing wastewater effluent. J. Ing. Eng. Chem. 2004, 10, 115–121. [Google Scholar]
- Huang, X.; Tian, F.; Chen, G.; Wang, F.; Weng, R.; Xi, B. Preparation and Characterization of Regenerated Cellulose Membrane Blended with ZrO2 Nanoparticles. Membranes 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Kilduff, J.E.; Belfort, G. Modes of Natural Organic Matter Fouling during Ultrafiltration. Environ. Sci. Technol. 2003, 37, 1676–1683. [Google Scholar] [CrossRef]
- Teixeira, M.; Rosa, M.; Nyström, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 160–166. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, S. Experimental Study of a 4040 Spiral-Wound Forward-Osmosis Membrane Module. Environ. Sci. Technol. 2011, 45, 7737–7745. [Google Scholar] [CrossRef] [PubMed]
- Abounahia, N.; Ibrar, I.; Kazwini, T.; Altaee, A.; Samal, A.K.; Zaidi, S.J.; Hawari, A.H. Desalination by the forward osmosis: Advancement and challenges. Sci. Total Environ. 2023, 886, 163901. [Google Scholar] [CrossRef]
- Coday, B.D.; Heil, D.M.; Xu, P.; Cath, T.Y. Effects of transmembrane hydraulic pressure on performance of forward osmosis membranes. Environ. Sci. Technol. 2013, 47, 2386–2393. [Google Scholar] [CrossRef]
- AlZainati, N.; Saleem, H.; Altaee, A.; Zaidi, S.J.; Mohsen, M.; Hawari, A.; Millar, G.J. Pressure retarded osmosis: Advancement, challenges and potential. J. Water Process Eng. 2021, 40, 101950. [Google Scholar] [CrossRef]
- Blandin, G.; Myat, D.T.; Verliefde, A.R.D.; Le-Clech, P. Pressure assisted osmosis using nanofiltration membranes (PAO-NF): Towards higher efficiency osmotic processes. J. Membr. Sci. 2017, 533, 250–260. [Google Scholar] [CrossRef]
- Ibraheem, B.M.; Al Aani, S.; Alsarayreh, A.A.; Alsalhy, Q.F.; Salih, I.K. Forward Osmosis Membrane: Review of Fabrication, Modification, Challenges and Potential. Membranes 2023, 13, 379. [Google Scholar] [CrossRef]
- Lee, S. Performance Comparison of Spiral-Wound and Plate-and-Frame Forward Osmosis Membrane Module. Membranes 2020, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Wang, X.; Cheng, C.; Zhang, K.; Yang, J.; Han, G.; Wang, Z.; Wang, X.; Wang, L. Desalination Characteristics of Cellulose Acetate FO Membrane Incorporated with ZIF-8 Nanoparticles. Membranes 2022, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.M.; Soliman, M.M.; Kandil, S.H.; Khalil, M.M.A. Emerging mixed matrix membranes based on zeolite nanoparticles and cellulose acetate for water desalination. Cellulose 2021, 28, 6417–6426. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Lu, J.; Shan, B.; Gao, C. Enhanced performance of cellulose triacetate membranes using binary mixed additives for forward osmosis desalination. Desalination 2016, 405, 68–75. [Google Scholar] [CrossRef]
- Ali, A.S.M.; Soliman, M.M.; Kandil, S.H.; Ebrahim, S.; Khalil, M. Tailoring nanocomposite membranes of cellulose of acetate Silica Nanoparticles for Desalination. J. Mater. 2022, 8, 1122–1130. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shalaby, M.S.; Shaban, A.M. Enhancement of reverse osmosis membranes for groundwater purification using cellulose acetate incorporated with ultrathin graphitic carbon nitride nanosheets. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100760. [Google Scholar] [CrossRef]
- Kim, T.; Choi, M.; Ahn, H.S.; Rho, J.; Jeong, H.M.; Kim, K. Fabrication and characterization of zeolitic imidazolate framework embedded cellulose acetate membranes for osmotically driven membrane process. Sci. Rep. 2019, 9, 5779. [Google Scholar] [CrossRef]
- El-Noss, M.; Isawi, H.; Shawky, H.A.; Gomaa, M.A.; Abdel-Mottaleb, M.S.A. Improvement of cellulose acetate forward osmosis membrane performance using zinc oxide nanoparticles. Desalin. Water Treat. 2020, 193, 19–33. [Google Scholar] [CrossRef]




| Parameter | Unit | Results |
|---|---|---|
| pH | - | 7.5 |
| TDS | mg/L | 38,528 |
| Conductivity | Ms/cm | 57.5 |
| Total Hardness | mg/L | 6500 |
| Calcium hardness | mg/L | 1800 |
| Magnesium hardness | mg/L | 5600 |
| Sodium | mg/L | 18,000 |
| Alkalinity | mg/L | 13,000 |
| Chloride | mg/L | 34,200 |
| Sulfate | mg/L | 1170 |
| Potassium | mg/L | 275 |
| Parameter | Unit | Results |
|---|---|---|
| pH | 8.14 | |
| Turbidity | NTU | 7.4 |
| TDS | mg/L | 282 |
| TSS | mg/L | 101 |
| COD | mg/L | 6.3 |
| BOD | mg/L | 3.8 |
| Alkalinity | mg/L | 127 |
| CO3 | mg/L | 8.9 |
| HCO3 | mg/L | 137 |
| Hardness | mg/L | 113 |
| NO3 | mg/L | 0.24 |
| Concentration [ppm] | Rir | Rr | Rm |
|---|---|---|---|
| 10,000 | 0.34 | 0.498 | 1.67 |
| 38,528 | 0.139 | 0.414 | 1.233 |
| Polymeric Material | Nano Particles | Process | Separation% | Permeate Flux LMH | Reference |
|---|---|---|---|---|---|
| Cellulose acetate | ZIF-8 | FO | 2.84 (Js; gLMH) | 50.14 | [32] |
| cellulose acetate | Zeolite | RO | 95.5 | 1.3 | [33] |
| Cellulose triacetate | ZnCl2-LA | FO | 98.3 | 11.5 | [34] |
| cellulose acetate | Silica | RO | 91 | 1.6 | [35] |
| Cellulose acetate/polyvinyl alcohol | Graphite carbon nitride nanosheets | RO | 95 | 10.5 | [36] |
| Cellulose acetate | ZIF-302/CA | FO | - | 16.8 | [37] |
| Cellulose acetate | ZnO | FO | 99.5% of Na+, 100% of Cl | 26.57 | [38] |
| Cellulose acetate/polyvinyl alcohol | ZrO2 | RO | 99 | 9.9 | This work |
| Cellulose triacetate | ZrO2 | FO | 93.2 Dilution% | 68.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaswad, S.O.; Abdallah, H.; Mansor, E.S. Integrated System of Reverse Osmosis and Forward Pressure-Assisted Osmosis from ZrO2 Base Polymer Membranes for Desalination Technology. Technologies 2024, 12, 253. https://doi.org/10.3390/technologies12120253
Alaswad SO, Abdallah H, Mansor ES. Integrated System of Reverse Osmosis and Forward Pressure-Assisted Osmosis from ZrO2 Base Polymer Membranes for Desalination Technology. Technologies. 2024; 12(12):253. https://doi.org/10.3390/technologies12120253
Chicago/Turabian StyleAlaswad, Saleh O., Heba Abdallah, and Eman S. Mansor. 2024. "Integrated System of Reverse Osmosis and Forward Pressure-Assisted Osmosis from ZrO2 Base Polymer Membranes for Desalination Technology" Technologies 12, no. 12: 253. https://doi.org/10.3390/technologies12120253
APA StyleAlaswad, S. O., Abdallah, H., & Mansor, E. S. (2024). Integrated System of Reverse Osmosis and Forward Pressure-Assisted Osmosis from ZrO2 Base Polymer Membranes for Desalination Technology. Technologies, 12(12), 253. https://doi.org/10.3390/technologies12120253






