Characterization of Commercial and Custom-Made Printing Filament Materials for Computed Tomography Imaging of Radiological Phantoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filament Fabrication
2.2. Cubes and Cylindrical Samples
2.3. Computed Tomography (CT) Scans of Twenty-Nine 3D Printed and Melted Samples
2.4. Hounsfield Units Measurements
3. Results
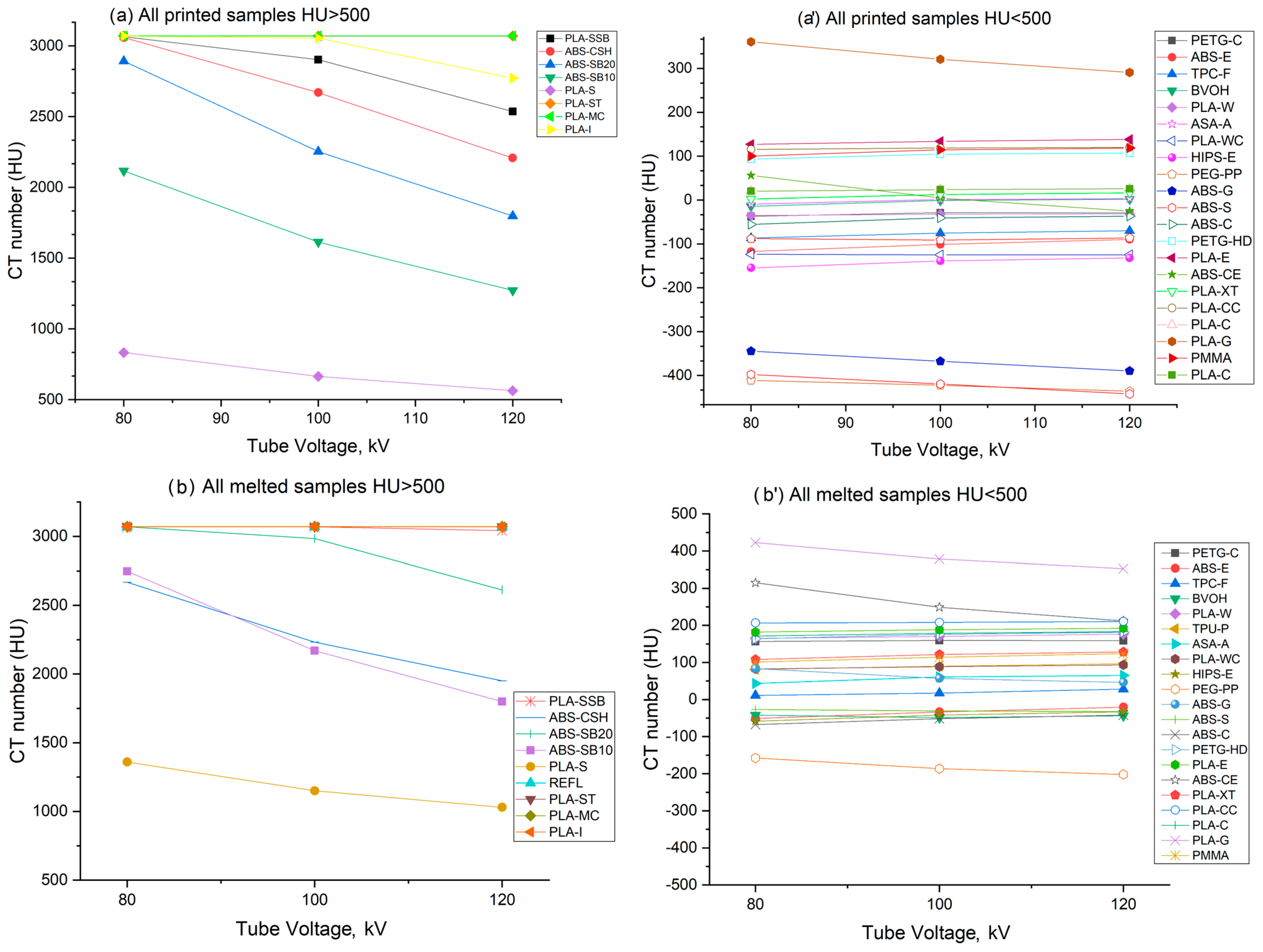
3.1. Measured Hounsfield Units
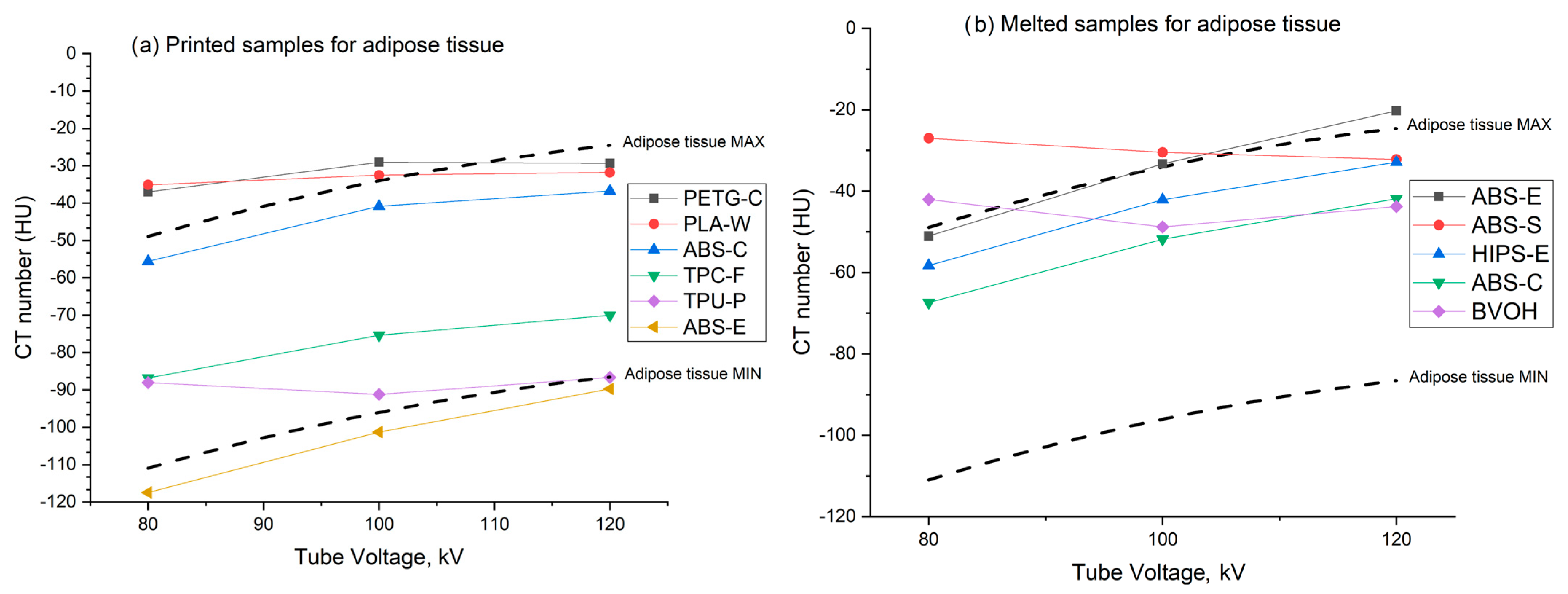
3.2. Adipose Tissue Mimicking Materials
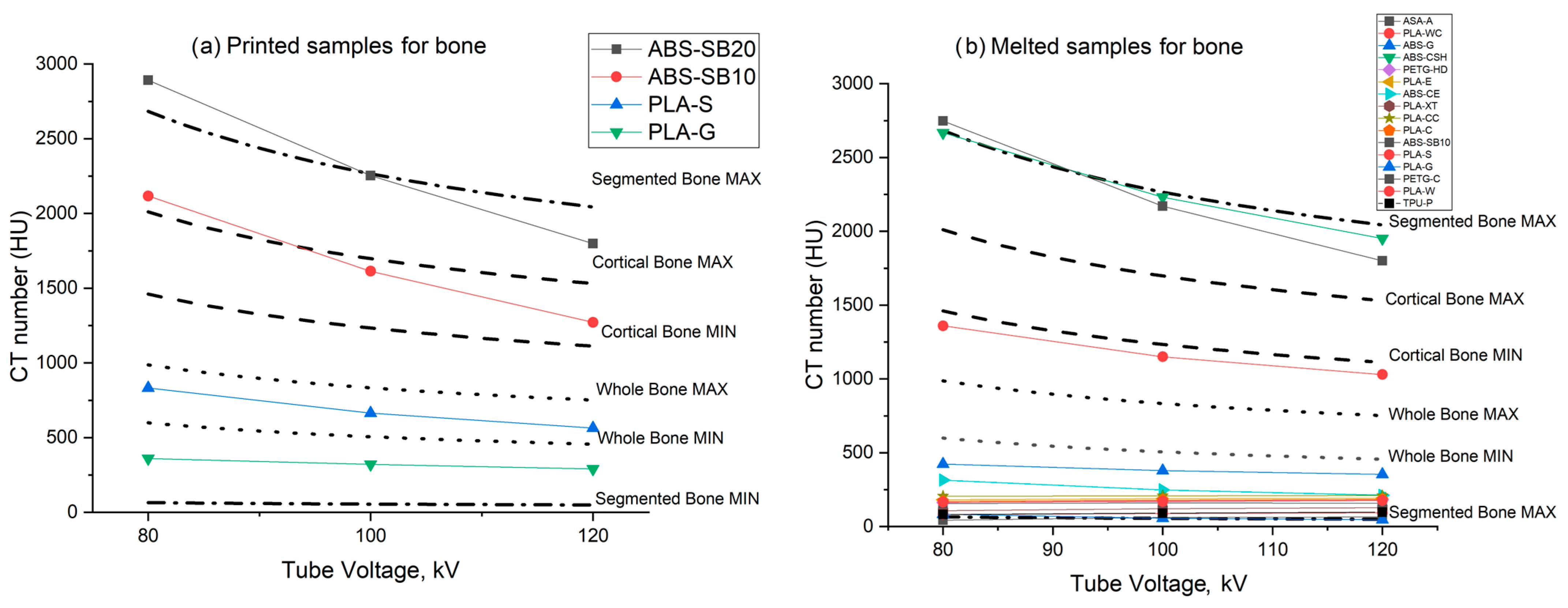
3.3. Bone Tissue Mimicking Materials
3.4. Soft Tissue Mimicking Materials
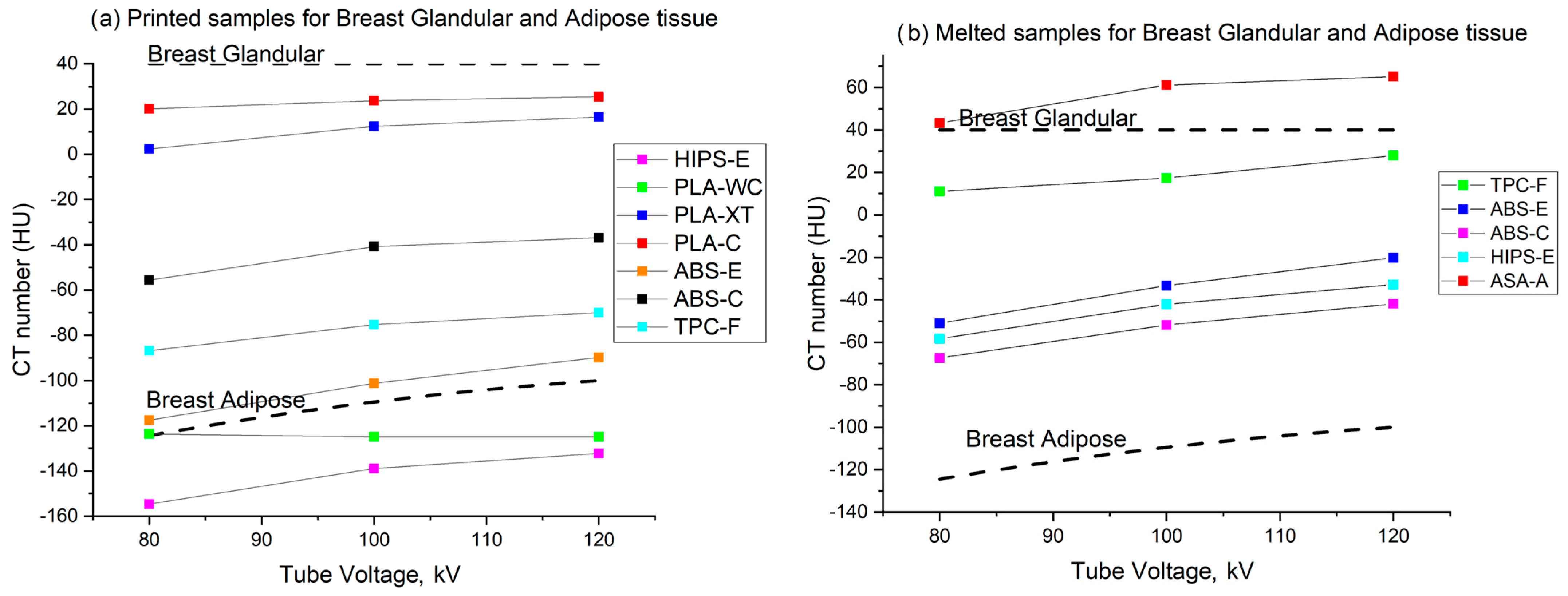
3.5. Breast Tissue Mimicking Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| PETG-HD | PETG-C | PLA-E | PLA-CC | |||||
| kV | printed | melted | printed | melted | printed | melted | printed | melted |
| 80 | +93 ± 3 | +163 ± 3 | −37 ± 3 | +156 ± 39 | +127 ± 2 | +181 ± 3 | +115 ± 3 | +206 ± 2 |
| 100 | +104 ± 2 | +175 ± 2 | −29 ± 4 | +159 ± 31 | +133 ± 1 | +188 ± 4 | +118 ± 4 | +207 ± 2 |
| 120 | +106 ± 2 | +181 ± 3 | −29 ± 3 | +158 ± 2 | +138 ± 1 | +192 ± 2 | +119 ± 4 | +210 ± 2 |
| PLA-C | PLA-W | PLA-WC | PLA-G | |||||
| kV | printed | melted | printed | melted | printed | melted | printed | melted |
| 80 | +20 ± 3 | +171 ± 2 | −35 ± 1 | +165 ± 3 | −123 ± 10 | +82 ± 5 | +360 ± 1 | +422 ± 5 |
| 100 | +23 ± 2 | +178 ± 1 | −32 ± 1 | +170 ± 3 | −124 ± 13 | +88 ± 6 | +320 ± 2 | +378 ± 5 |
| 120 | +25 ± 1 | +183 ± 2 | −31 ± 1 | +176 ± 6 | −124 ± 12 | +93 ± 5 | +290 ± 2 | +353 ± 4 |
| PLA-XT | ABS-CE | ABS-E | ABS-G | |||||
| kV | printed | melted | printed | melted | printed | melted | printed | melted |
| 80 | +2 ± 4 | +108 ± 5 | +55 ± 3 | +315 ± 3 | −117 ± 2 | −51 ± 4 | −344 ± 6 | +83 ± 6 |
| 100 | +12 ± 5 | +121 ± 4 | +4 ± 3 | +248 ± 3 | −101 ± 1 | −33 ± 3 | −367 ± 5 | +57 ± 7 |
| 120 | +16 ± 6 | +128 ± 5 | -25 ± 3 | +212 ± 3 | −89 ± 2 | −20 ± 4 | −389 ± 8 | +46 ± 6 |
| ABS-S | ABS-C | TPC-F | BVOH | |||||
| kV | printed | melted | Printed | melted | printed | melted | printed | melted |
| 80 | −397 ± 37 | −27 ± 3 | −55 ± 2 | −67 ± 12 | −86 ± 2 | 11 ± 30 | −14 ± 6 | −42 ± 24 |
| 100 | −419 ± 35 | −30 ± 3 | −40 ± 4 | −51 ± 12 | −75 ± 3 | 17 ± 32 | −1 ± 6 | −48 ± 25 |
| 120 | −442 ± 38 | −32 ± 2 | −36 ± 4 | −41 ± 12 | −70 ± 4 | 28 ± 31 | 1 ± 5 | −43 ± 24 |
| TPU-P | ASA-A | HIPS-E | PEG-PP | |||||
| kV | printed | melted | Printed | melted | printed | melted | printed | melted |
| 80 | −88 ± 8 | +81 ± 9 | −9 ± 1 | +43 ± 8 | −154 ± 4 | −58 ± 1 | −410 ± 4 | −157 ± 6 |
| 100 | −91 ± 7 | +90 ± 9 | 0 ± 2 | +61 ± 7 | −138 ± 3 | −42 ± 2 | −422 ± 4 | −186 ± 6 |
| 120 | −86 ± 7 | +96 ± 10 | +3 ± 1 | +65 ± 8 | −132 ± 2 | −32 ± 2 | −435 ± 4 | −201 ± 7 |
| PLA-SSB | ABS-CSH | ABS-SB10 | ABS-SB20 | |||||
| kV | printed | melted | printed | melted | printed | melted | printed | melted |
| 80 | +3065 ± 4 | +3071 ± 0.1 | +3059 ± 6 | +2668 ± 52 | +2117 ± 10 | +2748 ± 38 | +2893 ± 5 | +3070 ± 1 |
| 100 | +2902 ± 34 | +3069 ± 4 | +2670 ± 28 | +2232 ± 57 | +1614 ± 6 | +2171 ± 30 | +2253 ± 5 | +2985 ± 16 |
| 120 | +2536 ± 8 | +3042 ± 15 | +2208 ± 46 | +1951 ± 52 | +1272 ± 5 | +1800 ± 28 | +1798 ± 8 | +2612 ± 30 |
| PLA-S | ||||||||
| kV | printed | melted | ||||||
| 80 | +832 ± 13 | +1360 ± 14 | ||||||
| 100 | +664 ± 12 | +1151 ± 12 | ||||||
| 120 | +563 ± 11 | +1030 ± 2 | ||||||
References
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Durfee, W.K.; Iaizzo, P.A. Chapter 21—Medical Applications of 3D Printing. In Engineering in Medicine; Iaizzo, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 527–543. [Google Scholar]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Manero, A.; Smith, P.; Sparkman, J.; Dombrowski, M.; Courbin, D.; Kester, A.; Womack, I.; Chi, A. Implementation of 3D Printing Technology in the Field of Prosthetics: Past, Present, and Future. Int. J. Environ. Res. Public Health 2019, 16, 1641. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.K.; Rampant, P.; Smith, W.; Waterhouse, D.; Rowshanfarzad, P.; Ebert, M.A. Investigation of the effects of spinal surgical implants on radiotherapy dosimetry: A study of 3D printed phantoms. Med. Phys. 2021, 48, 4586–4597. [Google Scholar] [CrossRef]
- Okkalidis, N. 3D printing methods for radiological anthropomorphic phantoms. Phys. Med. Biol. 2022, 67, 15TR04. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701161. [Google Scholar] [CrossRef]
- Tümer, E.H.; ErbilTümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Tariq, A.; Arif, Z.U.; Khalid, M.Y.; Hossain, M.; Rasool, P.I.; Umer, R.; RamakrishnaTariq, A.; Arif, Z.U.; Khalid, M.Y.; Hossain, M.; et al. Recent Advances in the Additive Manufacturing of Stimuli-Responsive Soft Polymers. Adv. Eng. Mater. 2023, 25, 2301074. [Google Scholar] [CrossRef]
- Robinson, A.P.; Tipping, J.; Cullen, D.M.; Hamilton, D.; Brown, R.; Flynn, A.; Oldfield, C.; Page, E.; Price, E.; Smith Robinson, A.P.; et al. Organ-specific SPECT activity calibration using 3D printed phantoms for molecular radiotherapy dosimetry. EJNMMI Phys. 2016, 3, 12. [Google Scholar] [CrossRef]
- Frandon, J.; Akessoul, P.; Hamard, A.; Bezandry, E.; Loffroy, R.; Addala, T.; Bertrand, M.M.; Beregi, J.-P.; GreffierFrandon, J.; Akessoul, P.; et al. Comparison of acquisition and iterative reconstruction parameters in abdominal computed tomography-guided procedures: A phantom study. Quant. Imaging Med. Surg. 2021, 12, 281–291. [Google Scholar] [CrossRef]
- Kettenbach, J.; Kara, L.; Toporek, G.; Fuerst, M.; Kronreif, G. A robotic needle-positioning and guidance system for CT-guided puncture: Ex vivo results. Minim. Invasive Ther. Allied Technol. 2014, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bliznakova, K. The advent of anthropomorphic three-dimensional breast phantoms for X-ray imaging. Phys. Medica 2020, 79, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.B.; Negus, I.S.; Wiltshire, S.J.; Thorne, G.C.; Young, P.; Alzheimer’s Disease Neuroimaging Initiative. Creation of an anthropomorphic CT head phantom for verification of image segmentation. Med. Phys. 2020, 47, 2380–2391. [Google Scholar] [CrossRef]
- Gear, J.I.; Cummings, C.; Craig, A.J.; Divoli, A.; Long, C.D.C.; Tapner, M.; Flux, G.D. Abdo-Man: A 3D-printed anthropomorphic phantom for validating quantitative SIRT. EJNMMI Phys. 2016, 3, 17. [Google Scholar] [CrossRef]
- Madamesila, J.; McGeachy, P.; Barajas, J.E.V.; Khan, R. Characterizing 3D printing in the fabrication of variable density phantoms for quality assurance of radiotherapy. Phys. Medica 2016, 32, 242–247. [Google Scholar] [CrossRef]
- Filippou, V.; Tsoumpas, C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound. Med. Phys. 2018, 45, E740–E760. [Google Scholar] [CrossRef]
- Ivanov, D.; Bliznakova, K.; Buliev, I.; Popov, P.; Mettivier, G.; Russo, P.; Di Lillo, F.; Sarno, A.; Vignero, J.; Bosmans, H. Suitability of low density materials for 3D printing of physical breast phantoms. Phys. Med. Biol. 2018, 63, 175020. [Google Scholar] [CrossRef] [PubMed]
- Kunert, P.; Trinkl, S.; Giussani, A.; Reichert, D.; Brix, G. Tissue equivalence of 3D printing materials with respect to attenuation and absorption of X-rays used for diagnostic and interventional imaging. Med. Phys. 2022, 49, 7766–7778. [Google Scholar] [CrossRef]
- Ma, X.; Figl, M.; Unger, E.; Buschmann, M.; Homolka, P. X-ray attenuation of bone, soft and adipose tissue in CT from 70 to 140 kV and comparison with 3D printable additive manufacturing materials. Sci. Rep. 2022, 12, 14580. [Google Scholar] [CrossRef]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef]
- Mettivier, G.; Sarno, A.; Varallo, A.; Russo, P. Attenuation coefficient in the energy range 14–36 keV of 3D printing materials for physical breast phantoms. Phys. Med. Biol. 2022, 67, 175012. [Google Scholar] [CrossRef]
- Savi, M.; Villani, D.; Andrade, M.; Rodrigues, O.; Potiens, M. Study on attenuation of 3D printing commercial filaments on standard X-ray beams for dosimetry and tissue equivalence. Radiat. Phys. Chem. 2021, 182, 109365. [Google Scholar] [CrossRef]
- Alssabbagh, M.; Tajuddin, A.A.; Abdulmanap, M.; Zainon, R. Evaluation of 3D printing materials for fabrication of a novel multi-functional 3D thyroid phantom for medical dosimetry and image quality. Radiat. Phys. Chem. 2017, 135, 106–112. [Google Scholar] [CrossRef]
- Hatamikia, S.; Kronreif, G.; Unger, A.; Oberoi, G.; Jaksa, L.; Unger, E.; Koschitz, S.; Gulyas, I.; Irnstorfer, N.; Buschmann, M.; et al. 3D printed patient-specific thorax phantom with realistic heterogenous bone radiopacity using filament printer technology. Z. Med. Phys. 2022, 32, 438–452. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Fuh, J.; Choo, Y.; Lu, L.; Nee, A.; Wong, Y.; Wang, W.; Miyazawa, T.; Ho, S. Post-cure shrinkage of photo-sensitive material used in laser lithography process. J. Mech. Work. Technol. 1997, 63, 887–891. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, F.; Zhang, Y.; Yao, J.; Zhang, H. Improving the strength and toughness of photopolymerizable resin by blending with a long-chain molecule. Polymer 2017, 128, 100–108. [Google Scholar]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Ikejimba, L.C.; Graff, C.G.; Rosenthal, S.; Badal, A.; Ghammraoui, B.; Lo, J.Y.; Glick, S.J. A novel physical anthropomorphic breast phantom for 2D and 3D x-ray imaging. Med. Phys. 2017, 44, 407–416. [Google Scholar] [CrossRef]
- Jahnke, P.; Limberg, F.R.P.; Gerbl, A.; Pardo, G.L.A.; Braun, V.P.B.; Hamm, B.; Scheel, M. Radiopaque Three-dimensional Printing: A Method to Create Realistic CT Phantoms. Radiology 2017, 282, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, P.; Schwarz, S.; Ziegert, M.; Schwarz, F.B.; Hamm, B.; Scheel, M. Paper-based 3D printing of anthropomorphic CT phantoms: Feasibility of two construction techniques. Eur. Radiol. 2019, 29, 1384–1390. [Google Scholar] [CrossRef]
- Mayer, R.; Liacouras, P.; Thomas, A.; Kang, M.; Lin, L.; Simone, C.B. 3D printer generated thorax phantom with mobile tumor for radiation dosimetry. Rev. Sci. Instrum. 2015, 86, 074301. [Google Scholar] [CrossRef] [PubMed]
- Alssabbagh, E.A.; Tajuddin, M.B.; Manap, R.; Zainon, R. Evaluation of nine 3D printing materials as tissue equivalent materials in terms of mass attenuation coefficient and mass density. Int. J. Adv. Appl. Sci. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Dancewicz, O.; Sylvander, S.; Markwell, T.; Crowe, S.; Trapp, J. Radiological properties of 3D printed materials in kilovoltage and megavoltage photon beams. Phys. Medica 2017, 38, 111–118. [Google Scholar] [CrossRef]
- Ma, X.; Buschmann, M.; Unger, E.; Homolka, P. Classification of X-Ray Attenuation Properties of Additive Manufacturing and 3D Printing Materials Using Computed Tomography From 70 to 140 kVp. Front. Bioeng. Biotechnol. 2021, 9, 763960. [Google Scholar] [CrossRef]
- Hatamikia, S.; Gulyas, I.; Birkfellner, W.; Kronreif, G.; Unger, A.; Oberoi, G.; Lorenz, A.; Unger, E.; Kettenbach, J.; Figl, M.; et al. Realistic 3D printed CT imaging tumor phantoms for validation of image processing algorithms. Phys. Medica 2023, 105, 102512. [Google Scholar] [CrossRef] [PubMed]
- Bibb, R.; Thompson, D.; Winder, J. Computed tomography characterisation of additive manufacturing materials Med. Eng. Phys. 2011, 33, 590–596. [Google Scholar] [CrossRef]
- Shin, J.; Sandhu, R.S.; Shih, G. Imaging Properties of 3D Printed Materials: Multi-Energy CT of Filament Polymers. J. Digit. Imaging 2017, 30, 572–575. [Google Scholar] [CrossRef]
- Craft, D.F.; Kry, S.F.; Balter, P.; Salehpour, M.; Woodward, W.; Howell, R.M. Material matters: Analysis of density uncertainty in 3D printing and its consequences for radiation oncology. Med. Phys. 2018, 45, 1614–1621. [Google Scholar] [CrossRef]
- Okkalidis, N.; Chatzigeorgiou, C.; Okkalides, D. Assessment of 11 Available Materials with Custom Three-Dimensional-Printing Patterns for the Simulation of Muscle, Fat, and Lung Hounsfield Units in Patient-Specific Phantoms. J. Med. Diagn. 2018, 1, 011003. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A Systematic Review on 3D-Printed Imaging and Dosimetry Phantoms in Radiation Therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef] [PubMed]
- Hamedani, B.A.; Melvin, A.; Vaheesan, K.; Gadani, S.; Pereira, K.; Hall, A.F. Three-dimensional printing CT-derived objects with controllable radiopacity. J. Appl. Clin. Med. Phys. 2018, 19, 317–328. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Brandt, M.; Leary, M.; Kron, T. The interlace deposition method of bone equivalent material extrusion 3D printing for imaging in radiotherapy. Mater. Des. 2021, 199, 109439. [Google Scholar] [CrossRef]
- Okkalidis, N.; Marinakis, G. Accurate replication of soft and bone tissues with 3D printing. Med. Phys. 2020, 47, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, P.F.; Colborn, R.E.; Edic, P.M.; Lambert, J.W.; Bonitatibus, P.J.; Yeh, B.M. Liquid tissue surrogates for X-ray and CT phantom studies. Med. Phys. 2017, 44, 6251–6260. [Google Scholar] [CrossRef]
- Ruschin, M.; Davidson, S.R.H.; Phounsy, W.; Yoo, T.S.; Chin, L.; Pignol, J.-P.; Ravi, A.; McCann, C. Technical Note: Multipurpose CT, ultrasound, and MRI breast phantom for use in radiotherapy and minimally invasive interventions. Med. Phys. 2016, 43, 2508–2514. [Google Scholar] [CrossRef]
- Okkalidis, N.; Bliznakova, K.; Kolev, N. A filament 3D printing approach for CT-compatible bone tissues replication. Phys. Medica 2022, 102, 96–102. [Google Scholar] [CrossRef]
- Esposito, G.; Mettivier, G.; Bliznakova, K.; Bliznakov, Z.; Bosmans, H.; Bravin, A.; Buliev, I.; Di Lillo, F.; Ivanov, D.; Minutillo, M.; et al. Investigation of the refractive index decrement of 3D printing materials for manufacturing breast phantoms for phase contrast imaging. Phys. Med. Biol. 2019, 64, 075008. [Google Scholar] [CrossRef]
- Okkalidis, N.; Bliznakova, K. A voxel-by-voxel method for mixing two filaments during a 3D printing process for soft-tissue replication in an anthropomorphic breast phantom. Phys. Med. Biol. 2022, 67, 245019. [Google Scholar] [CrossRef] [PubMed]
- Kozee, M.; Weygand, J.; Andreozzi, J.M.; Hunt, D.; Perez, B.A.; Graham, J.A.; Redler, G. Methodology for computed tomography characterization of commercially available 3D printing materials for use in radiology/radiation oncology. J. Appl. Clin. Med. Phys. 2023, 24, e13999. [Google Scholar] [CrossRef] [PubMed]
- Doner, S.; Paswan, R.; Das, S. The influence of metallic particulate inclusions on the mechanical and thermal performance of 3D printable acrylonitrile-butadiene-styrene/thermoplastic polyurethane fused polymer blends. Mater. Today Commun. 2023, 35, 106111. [Google Scholar] [CrossRef]
- Hayashi, N.; Ogawa, Y.; Kubota, K.; Okino, K.; Akima, R.; Morita-Tokuhiro, S.; Tsuzuki, A.; Yaogawa, S.; Nishioka, A.; Miyamura, M. Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy? Cancers 2016, 8, 43. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Lee, H.J.; Han, S.W.; Lee, J.E.; Lee, S.M. Prognostic Significance of CT-Attenuation of Tumor-Adjacent Breast Adipose Tissue in Breast Cancer Patients with Surgical Resection. Cancers 2019, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Burkett, G.; Boone, J.M. A breast-specific, negligible-dose scatter correction technique for dedicated cone-beam breast CT: A physics-based approach to improve Hounsfield Unit accuracy. Phys. Med. Biol. 2014, 59, 6487–6505. [Google Scholar] [CrossRef]
- Capaccione, K.; Desperito, E.; Asiimwe, A.C.; Salvatore, M. Can Hounsfield units on chest CT characterize breast nodules as cystic or solid? Transl. Breast Cancer Res. 2023, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Rennert, J.; Brochhausen, C.; Stroszczynski, C.; Jung, E. Suspicious breast lesions incidentally detected on chest computer tomography with histopathological correlation. Breast J. 2021, 27, 715–722. [Google Scholar] [CrossRef]
- Desperito, E.; Schwartz, L.; Capaccione, K.M.; Collins, B.T.; Jamabawalikar, S.; Peng, B.; Patrizio, R.; Salvatore, M.M. Chest CT for Breast Cancer Diagnosis. Life 2022, 12, 1699. [Google Scholar] [CrossRef]
- Urata, M.; Kijima, Y.; Hirata, M.; Shinden, Y.; Arima, H.; Nakajo, A.; Koriyama, C.; Arigami, T.; Uenosono, Y.; Okumura, H.; et al. Computed tomography Hounsfield units can predict breast cancer metastasis to axillary lymph nodes. BMC Cancer 2014, 14, 730. [Google Scholar] [CrossRef]
- Wienbeck, S.; Fischer, U.; Perske, C.; Wienke, A.; Meyer, H.J.; Lotz, J.; Surov, A. Cone-beam Breast Computed Tomography: CT Density Does Not Reflect Proliferation Potential and Receptor Expression of Breast Carcinoma. Transl. Oncol. 2017, 10, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Bliznakova, K.; Okkalidis, N.; Okkalidis, F.; Chatzigeorgiou, C.; Dukov, N.; Milev, M.; Bliznakov, Z. A Pre-Manufacturing Approach for Verification of Radiological Quality of Anthropomorphic Phantoms, ECR2024. Available online: https://epos.myesr.org/poster/esr/ecr2024/C-19033/methods%20and%20materials (accessed on 31 July 2024).
- Dukov, N.; Bliznakova, K.; Okkalidis, N.; Teneva, T.; Encheva, E.; Bliznakov, Z. Thermoplastic 3D printing technology using a single filament for producing realistic patient-derived breast models. Phys. Med. Biol. 2022, 67, 045008. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, S.; Okkalidis, N.; Boone, J.M.; Marinov, S.; Bliznakov, Z.; Mettivier, G.; Bosmans, H.; Russo, P.; Bliznakova, K. An-thropomorphic physical breast phantom based on patient breast CT data: Preliminary results. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing—Medicon, Coimbra, Portugal, 26–28 September 2019; Volume 76, pp. 367–374. [Google Scholar] [CrossRef]
- Sharma, S.; Mudgal, D.; Gupta, V. Advancement in biological and mechanical behavior of 3D printed poly lactic acid bone plates using polydopamine coating: Innovation for healthcare. J. Mech. Behav. Biomed. Mater. 2023, 143, 105929. [Google Scholar] [CrossRef]
- Giacomini, G.O.; Dotto, G.N.; Mello, W.M.; Dutra, V.; Liedke, G.S. Three-Dimensional printed model for preclinical training in oral radiology. Eur. J. Dent. Educ. 2023, 27, 280–286. [Google Scholar] [CrossRef]
- Kalender, W. Variables and Procedures for Sequential CT; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Lev, M.H.; Gonzalez, R.G. 17—CT Angiography and CT Perfusion Imaging. In Brain Mapping: The Methods, 2nd ed.; Toga, A.W., Mazziotta, J.C., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 427–484. [Google Scholar]
- Varallo, A.; Sarno, A.; Castriconi, R.; Mazzilli, A.; Loria, A.; del Vecchio, A.; Orientale, A.; Pilotti, I.A.; D’Andria, P.; Bliznakova, K.; et al. Fabrication of 3D printed patient-derived anthropomorphic breast phantoms for mammography and digital breast tomosynthesis: Imaging assessment with clinical X-ray spectra. Phys. Medica 2022, 98, 88–97. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Dyer, B.A.; Boone, J.M.; Liu, S.; Chen, T.; Zheng, F.; Zhu, Y.; Sun, Y.; Rong, Y.; et al. 3D-printed breast phantom for multi-purpose and multi-modality imaging. Quant. Imaging Med. Surg. 2019, 9, 63–74. [Google Scholar] [CrossRef] [PubMed]




| No. | Material | Name | Material Binder/Material Filler |
|---|---|---|---|
| 1 | ABS-CSH | ABS/Cesium Sulfate Hydrate | 80% ABS/20% Cesium Sulfate Hydrate (Roth) |
| 2 | ABS-CE | ABS/Cement | 80% ABS/20% Cement (Isomat white 50.5) |
| 3 | ABS-S | ABS/Silica gel | 80% ABS/20% Silica Gel (Valerus) |
| 4 | ABS-SB20 | ABS/Barium Sulfate | 80% ABS/20% Barium Sulfate (Danhson) |
| 5 | ABS-SB10 | ABS/Barium Sulfate | 90% ABS/10% Barium Sulfate (Danhson) |
| 6 | ABS-G | ABS/Gypsum | 80% ABS/20% Gypsum (Knauf Gipsopiia) |
| 7 | PLA-SSB | Stonefil/Barium Sulfate | 90% Stonefil/10% Barium Sulfate (Danhson) |
| 8 | PETG-HD | HD glass (Formfutura, Nijmegen, The Netherlands) | NA |
| 9 | PEG-PP | Pegasus PP (Formfutura, The Netherlands) | NA |
| 10 | TPC-F | Flexifil (Formfutura, The Netherlands) | NA |
| 11 | PETG-C | Carbonfil (Formfutura, The Netherlands) | 85% PETG/15% Carbon fibers |
| 12 | PLA-XT | XT-CF20 (Colorfabb, Belfeld, The Netherlands) | NA |
| 13 | ASA-A | ApolloX (Formfutura, The Netherlands) | NA |
| 14 | ABS-E | Easyfil ABS (Formfutura, The Netherlands) | NA |
| 15 | HIPS-E | Easyfil Hips (Formfutura, The Netherlands) | NA |
| 16 | TPU-P | Python flex (Formfutura, The Netherlands) | NA |
| 17 | PLA-C | Easycork (Formfutura, The Netherlands) | 70%PLA/30% Cork |
| 18 | PLA-E | Easyfil PLA (Formfutura, The Netherlands) | NA |
| 19 | PLA-S | Stonefil (Formfutura, The Netherlands) | 50% PLA/50% Stone |
| 20 | BVOH | BVOH (Formfutura, The Netherlands) | NA |
| 21 | PLA-W | Easywood (Formfutura, The Netherlands) | 60% PLA/40% Wood |
| 22 | PLA-CC | Corkfill (Colorfabb, The Netherlands) | NA |
| 23 | PLA-WC | Woodfill (Colorfabb, The Netherlands) | NA |
| 24 | ABS-C | Conductive ABS (SainSmart, Lenexa, KS, USA) | NA |
| 25 | PLA-MC | Metalfil copper (Formfutura, The Netherlands) | 20% PLA/80% Copper |
| 26 | REFL | Reflect-o-Lay (Lay Filaments, Cologne, Germany) | Reflective particles |
| 27 | PLA-I | Iron-filled PLA (Protopasta, Vancouver, WA, USA) | 55% PLA/45%Iron |
| 28 | PLA-ST | Steelfill (Colorfabb, The Netherlands) | NA |
| 29 | PLA-G | Glowfill (Colorfabb, The Netherlands) | PLA/PHA (Phosphorescent pigment) |
| Material | PT (°C) | PS (mm/s) | FT (°C) | Material | PT (°C) | PS (mm/s) | FT (°C) |
|---|---|---|---|---|---|---|---|
| ABS-CSH | 240 | 20 | 240 | TPU-P | 230 | 10 | 200 |
| ABS-CE | 240 | 20 | 240 | PLA-C | 230 | 40 | 220 |
| ABS-S | 240 | 15 | 240 | PLA | 180 | 40 | 180 |
| ABS-SB20 | 240 | 20 | 240 | PLA-S | 200 | 40 | 190 |
| ABS-SB10 | 240 | 20 | 240 | BVOH | 220 | 40 | 200 |
| ABS-G | 240 | 20 | 240 | PLA-W | 220 | 40 | 210 |
| PLA-SSB | 200 | 15 | 190 | PLA-CC | 200 | 20 | 170 |
| PETG-HD | 240 | 40 | 210 | PLA-WC | 200 | 20 | 180 |
| PEG-PP | 230 | 8 | 210 | ABS-C | 200 | 20 | 240 |
| TPC-F | 230 | 10 | 210 | PLA-MC | 210–230 | 15 | 180 |
| PETG-C | 230 | 40 | 210 | REFL | 230 | 40 | 200 |
| PLA-XT | 250 | 15 | 230 | PLA-I | 200 | 40 | 200 |
| ASA-A | 240 | 40 | 220 | PLA-ST | 200 | 20 | 180 |
| ABS-E | 200 | 40 | 210 | PLA-G | 200 | 20 | 180 |
| HIPS-E | 230 | 40 | 220 |
| Material | Phantom Application | HU Values, at 120 kV |
|---|---|---|
| ABS-SB10 | Cortical bone | +1272 ± 5 HU (printed) |
| ABS-CE | 50/50 glandular/adipose tissue Bone tissue | −25 ± 3 HU (printed) +212 ± 3 HU (melted) |
| PLA-S | Dense bone tissue | +563 ± 11 HU (printed) +1030 ± 2 HU (melted) |
| PLA-E | Tumor tissue Bone tissue | +138 ± 1 HU (printed) +192 ± 2 HU (melted) |
| PLA-G | Bone tissue | +290 ± 2 HU (printed) +353 ± 4 HU (melted) |
| PETG-C | 50/50 glandular/adipose tissue Bone tissue | −29 ± 3 HU (printed) +158 ± 2 HU (melted) |
| PETG-HD | Tumor tissue | +106 ± 2 HU (printed) +181 ± 3 (melted) |
| PLA-W | 50/50 glandular/adipose tissue Bone tissue | −31 ± 1 HU (printed) +176 ± 6 HU (melted) |
| ABS-CSH | Bone tissue | +2208 ± 46 HU (printed) +1951 ± 52 HU (melted) |
| TPU-P | Adipose tissue Tumor tissue | −86 ± 7 HU (printed) +96 ± 10 HU (melted) |
| PLA-WC | Adipose tissue Tumor tissue | −124 ± 12 HU (printed) +93 ± 5 HU (melted) |
| ABS-G | Soft tissue, glandular, and tumor tissues | +46 ± 6 HU (melted) |
| PLA-XT | Soft tissue (glandular tissue) Bone tissue | +16 ± 6 HU (printed) +128 ± 5 HU (melted) |
| PLA-CC | Tumor tissue Bone tissue | +119 ± 4 HU (printed) +210 ± 2 HU (melted) |
| PLA-C | Soft tissue, glandular tissue Bone Tissue | +25 ± 1 HU (printed) +183 ± 2 HU (melted) |
| ASA-A | Soft tissue, glandular tissue Tumor tissue | +3 ± 1 HU (printed) +65 ± 8 HU (melted) |
| HIPS-E | Adipose tissue 50/50 glandular/adipose tissue | −132 ± 2 HU (printed) −32 ± 2 HU (melted) |
| BVOH | Adipose tissue | −43 ± 24 HU (melted) |
| PLA-SSB | Bone tissue | +2536 ± 8 HU (printed) +3042 ± 15 HU (melted) |
| ABS-E | Adipose tissue 50/50 glandular/adipose tissue | −89 ± 2 HU (printed) −20 ± 4 HU (melted) |
| ABS-C | 50/50 glandular/adipose tissue Adipose tissue | −36 ± 4 HU (printed) −41 ± 12 HU (melted) |
| TPC-F | Adipose tissue Soft tissue, glandular tissue | −70 ± 4 HU (printed) +28 ± 31 HU (melted) |
| ABS-S | 50/50 glandular/adipose tissue | −32 ± 2 HU (melted) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okkalidis, F.; Chatzigeorgiou, C.; Okkalidis, N.; Dukov, N.; Milev, M.; Bliznakov, Z.; Mettivier, G.; Russo, P.; Bliznakova, K. Characterization of Commercial and Custom-Made Printing Filament Materials for Computed Tomography Imaging of Radiological Phantoms. Technologies 2024, 12, 139. https://doi.org/10.3390/technologies12080139
Okkalidis F, Chatzigeorgiou C, Okkalidis N, Dukov N, Milev M, Bliznakov Z, Mettivier G, Russo P, Bliznakova K. Characterization of Commercial and Custom-Made Printing Filament Materials for Computed Tomography Imaging of Radiological Phantoms. Technologies. 2024; 12(8):139. https://doi.org/10.3390/technologies12080139
Chicago/Turabian StyleOkkalidis, Filippos, Chrysoula Chatzigeorgiou, Nikiforos Okkalidis, Nikolay Dukov, Minko Milev, Zhivko Bliznakov, Giovanni Mettivier, Paolo Russo, and Kristina Bliznakova. 2024. "Characterization of Commercial and Custom-Made Printing Filament Materials for Computed Tomography Imaging of Radiological Phantoms" Technologies 12, no. 8: 139. https://doi.org/10.3390/technologies12080139
APA StyleOkkalidis, F., Chatzigeorgiou, C., Okkalidis, N., Dukov, N., Milev, M., Bliznakov, Z., Mettivier, G., Russo, P., & Bliznakova, K. (2024). Characterization of Commercial and Custom-Made Printing Filament Materials for Computed Tomography Imaging of Radiological Phantoms. Technologies, 12(8), 139. https://doi.org/10.3390/technologies12080139







