Solid-State Kinetic Modeling and Experimental Validation of Cu-Fe Bimetallic Catalyst Synthesis and Its Application to Furfural Hydrogenation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. Preparation of CuFe Samples
2.3. Preparation of CuFe Supported over Al2O3 Samples
2.4. Characterization of CuFe and CuFe/Al2O3
2.5. Catalytic Test: Hydrogenation of Furfural
3. Results and Discussion
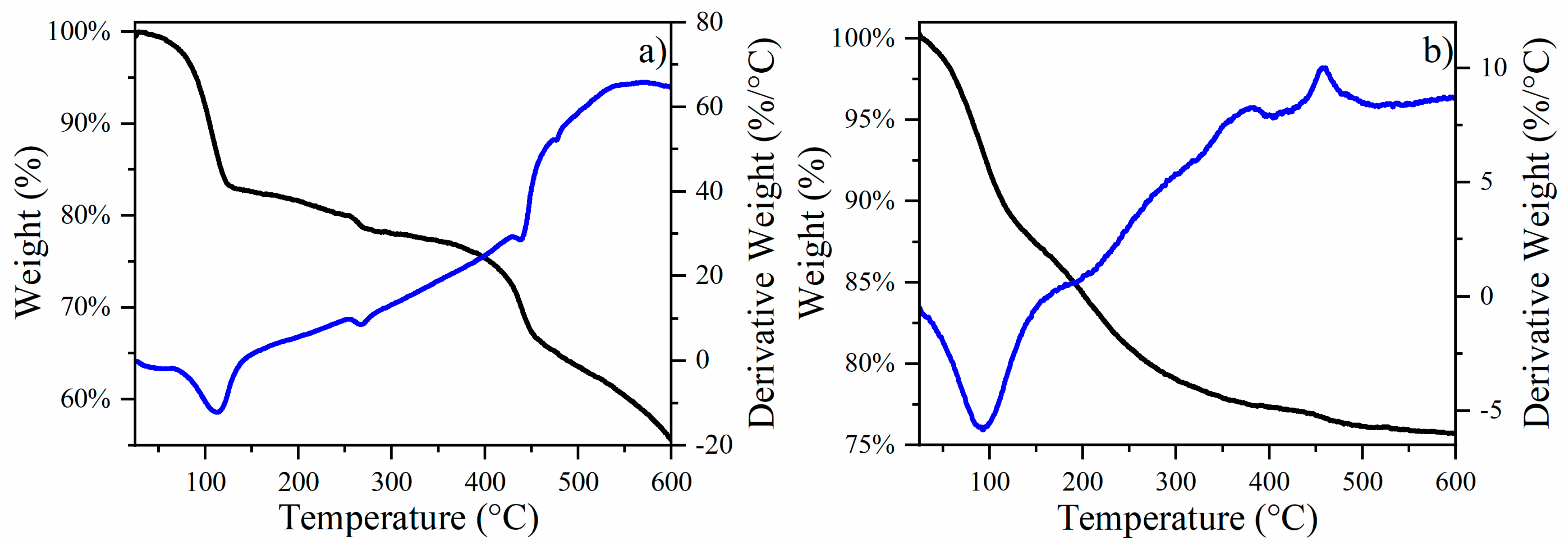
3.1. Thermal Analysis and Structural
3.2. XRD Analysis and Thermal Evolution of CuFe-prec, CuFe-cT, CuFe/Al2O3-prec and CuFe/Al2O3-cT Samples
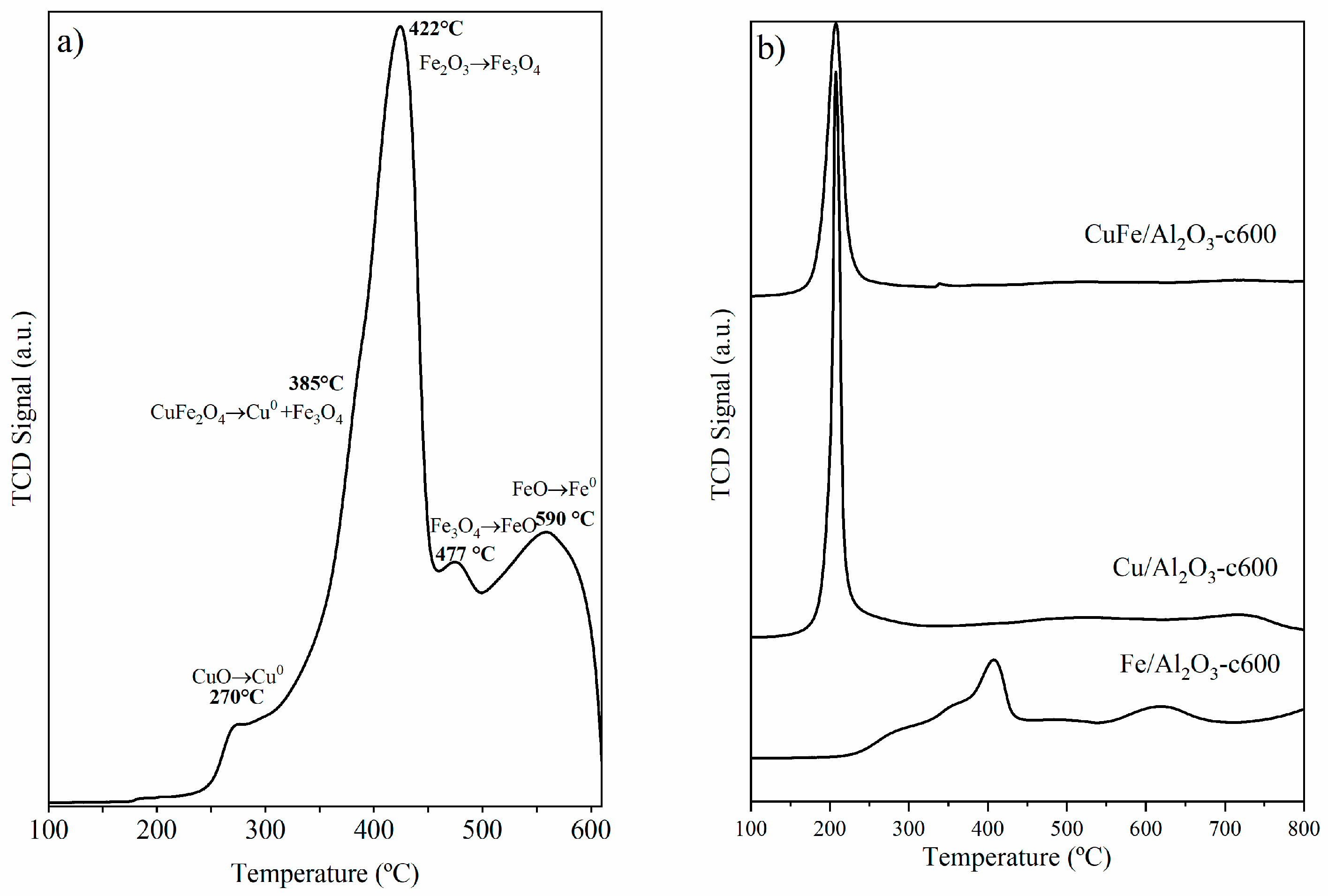
3.3. TPR Analysis: Reduction Behavior of CuFe-c600 and CuFe/Al2O3-c600 Samples
3.4. XRD Analysis and Thermal Evolution of CuFe-c600, CuFe-rT, CuFe/Al2O3-c600, and CuFe/Al2O3-rT Samples
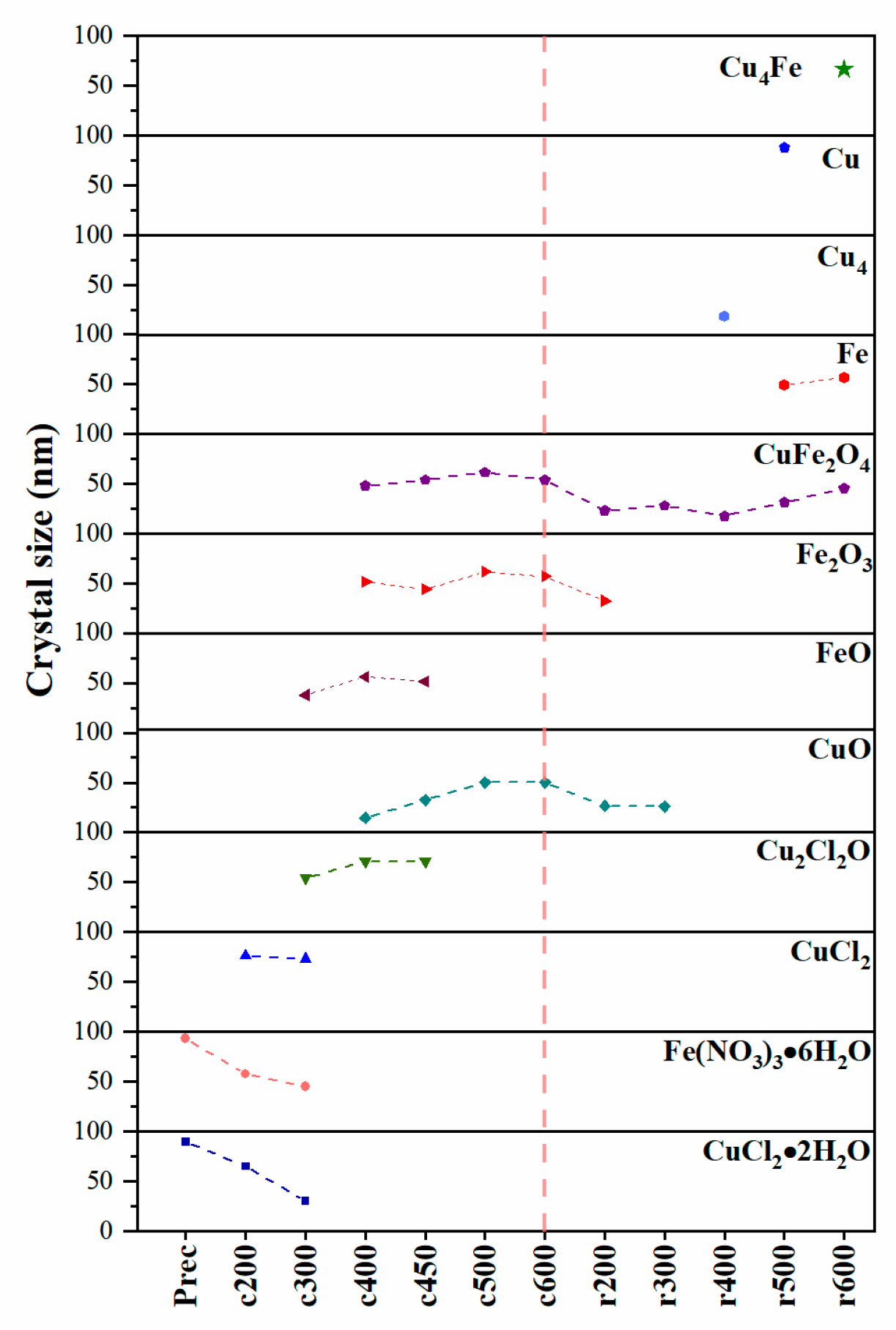
3.5. Mean Crystal Size as a Function of Treatment Temperature
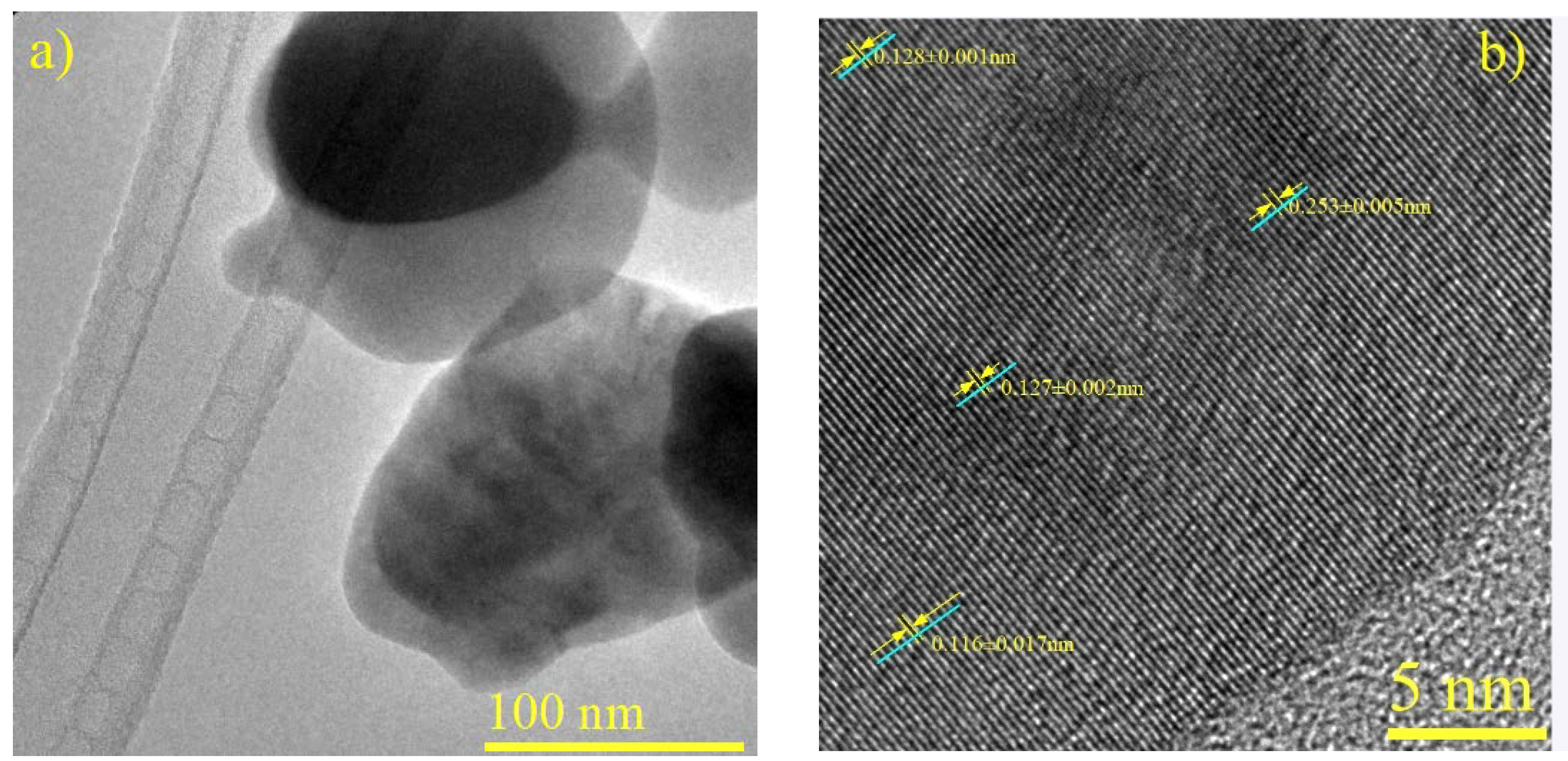
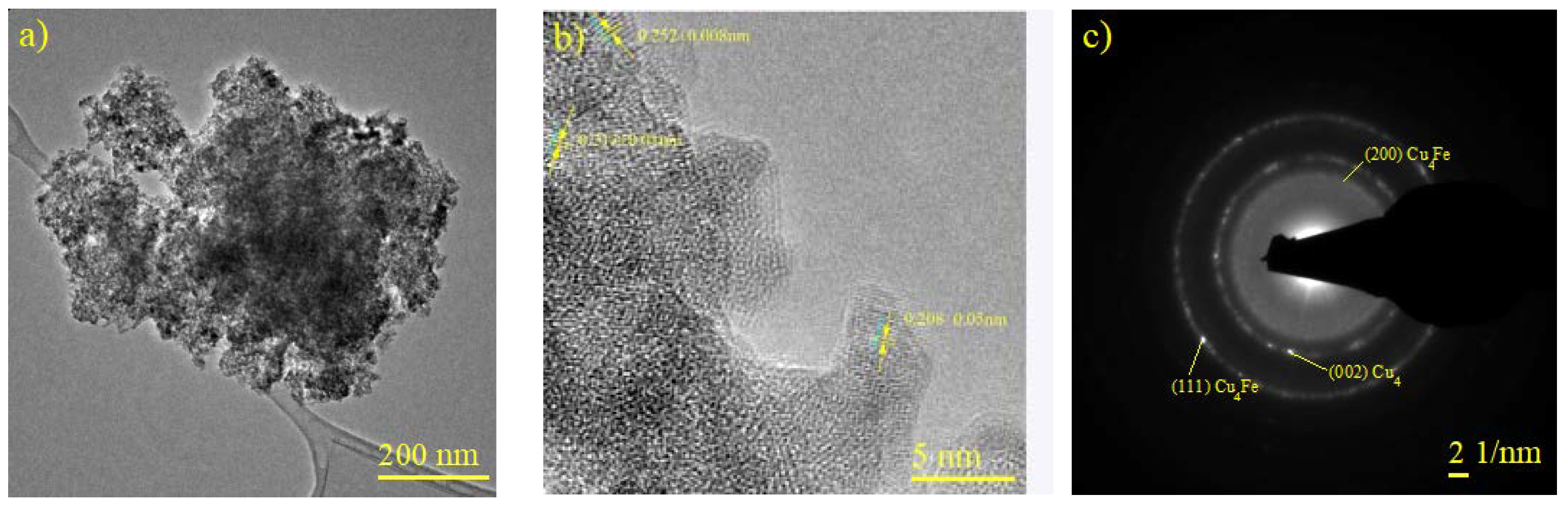
3.6. Crystallographic Analysis of CuFe Catalysts and CuFe/Al2O3 Supported Catalysts
3.7. Comprehensive Analysis and Reaction Dynamics in CuFe Bulk Formation
3.8. Catalytic Hydrogenation of Furfural: Conversion and Yield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalong, M.; Srifa, A.; Hongmanorom, P.; Cholsuk, C.; Klysubun, W.; Ratchahat, S.; Koo-amornpattana, W.; Khemthong, P.; Assabumrungrat, S.; Kawi, S. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol and 2-Methylfuran over CuFe Catalysts: Ex Situ Observation of Simultaneous Structural Phase Transformation. Fuel Process. Technol. 2022, 231, 107256. [Google Scholar] [CrossRef]
- Shen, X.; Wu, D.; Fu, X.-Z.; Luo, J.-L. Highly Selective Conversion of Methane to Ethanol over CuFe2O4-Carbon Nanotube Catalysts at Low Temperature. Chin. Chem. Lett. 2022, 33, 390–393. [Google Scholar] [CrossRef]
- Schöllhorn, R. Solid-State Chemistry: Restoring the Balance. Angew. Chem. (Int. Ed. Engl.) 1996, 35, 2338. [Google Scholar] [CrossRef]
- Morales-Leal, F.J.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Bustos Martínez, D.; De Haro Del Rio, D.A.; Garza-Navarro, M.A.; Martínez-Vargas, D.X.; Garcia, C.D. Comparison between the Catalytic and Photocatalytic Activities of Cu/Al2O3 and TiO2 in the Liquid–Phase Oxidation of Methanol–Ethanol Mixtures: Development of a Kinetic Model for the Preparation of Catalyst. Appl. Catal. A Gen 2018, 562, 184–197. [Google Scholar] [CrossRef]
- Kohlmann, H. Looking into the Black Box of Solid-State Synthesis. Eur. J. Inorg. Chem. 2019, 2019, 4174–4180. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, X.; Dai, S.; Wang, K.W. Tin as a Co-Catalyst for Electrocatalytic Oxidation and Reduction Reactions. Inorg. Chem. Front. 2023, 11, 1019–1047. [Google Scholar] [CrossRef]
- Arias, K.S.; Liu, L.; Garcia-Ortiz, A.; Climent, M.J.; Concepcion, P.; Iborra, S.; Corma, A. Bimetallic CuFe Nanoparticles as Active and Stable Catalysts for Chemoselective Hydrogenation of Biomass-Derived Platform Molecules. Catal. Sci. Technol. 2021, 11, 3353–3363. [Google Scholar] [CrossRef]
- Resasco, D.E.; Sitthisa, S.; Faria, J.; Prasomsri, T.; Ruiz, M.P. Furfurals as Chemical Platform for Biofuels Production. In Solid Waste as a Renewable Resource: Methodologies; Taylor & Francis: London, UK, 2015; pp. 103–144. ISBN 9781771882392. [Google Scholar]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural Over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and Mechanism of Hydrogenation of Furfural on Cu/SiO2 Catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)Furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Dimas-Rivera, G.L.; de la Rosa, J.R.; Lucio-Ortiz, C.J.; de los Reyes Heredia, J.A.; González, V.G.; Hernández, T. Desorption of Furfural from Bimetallic Pt-Fe Oxides/Alumina Catalysts. Materials 2014, 7, 527–541. [Google Scholar] [CrossRef]
- Maderuelo-Solera, R.; Jiménez-Gómez, C.P.; Cecilia, J.A.; García-Sancho, C.; Moreno-Tost, R.; Jesús Maireles-Torres, P. Catalysts Based on Ni and Mg Oxides for the One-Pot Production of High Added-Value Products from Furfural. Adv. Sustain. Syst. 2024, 8, 2300638. [Google Scholar] [CrossRef]
- Wang, Z.; Marin, G.; Naterer, G.F.; Gabriel, K.S. Thermodynamics and Kinetics of the Thermal Decomposition of Cupric Chloride in Its Hydrolysis Reaction. J. Therm. Anal. Calorim. 2015, 119, 815–823. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K.; Kozak, A.J. Thermal Decomposition of Fe(NO3)3·9H2O. J. Therm. Anal. Calorim. 1999, 58, 647–651. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; zeng Jia, D.; Pan, W. Simple Synthesis of CuFe2O4 Nanoparticles as Gas-Sensing Materials. Sens. Actuators B Chem. 2007, 125, 144–148. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jha, A.; Jang, W.J.; Han, W.B.; Roh, H.S. Performance of Spinel Ferrite Catalysts Integrated with Mesoporous Al2O3 in the High Temperature Water-Gas Shift Reaction. Chem. Eng. J. 2015, 265, 100–109. [Google Scholar] [CrossRef]
- Melnikov, P.; Nascimento, V.A.; Arkhangelsky, I.V.; Zanoni Consolo, L.Z.; de Oliveira, L.C.S. Thermal Decomposition Mechanism of Iron(III) Nitrate and Characterization of Intermediate Products by the Technique of Computerized Modeling. J. Therm. Anal. Calorim. 2014, 115, 145–151. [Google Scholar] [CrossRef]
- Małecka, B.; Łącz, A.; Drozdz, E.; Małecki, A. Thermal Decomposition of D-Metal Nitrates Supported on Alumina. J. Therm. Anal. Calorim. 2015, 119, 1053–1061. [Google Scholar] [CrossRef]
- Dissanayake, D.M.S.N.; Mantilaka, M.M.M.G.P.G.; Pitawala, H.M.T.G.A. Synthesis of Low-Cost Magnetite Nano-Architectures from Sri Lankan Laterites. J. Geol. Soc. Sri Lanka 2020, 21, 91. [Google Scholar] [CrossRef]
- Singh, R.V.; Pai, M.R.; Banerjee, A.M.; Thomas, D.; Patkare, G.S.; Phapale, S.; Nayak, C.; Bhasin, V.; Tripathi, A.K. Studies on Reaction Products, Byproducts, and Intermediates in Thermal Steps of the Cu-Cl Thermochemical Cycle for Hydrogen Generation. Energy Fuels 2023, 37, 15206–15221. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef] [PubMed]
- Mdletshe, L.S.; Makgwane, P.R.; Ray, S.S. Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates. Nanomaterials 2019, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.P.; Vidal, H.; García-Cabeza, A.L.; Hernández-Garrido, J.C.; Guerra, F.M.; Cifredo, G.A.; González-Leal, J.M.; Gatica, J.M. Low Temperature Prepared Copper-Iron Mixed Oxides for the Selective CO Oxidation in the Presence of Hydrogen. Appl. Catal. A Gen. 2018, 552, 58–69. [Google Scholar] [CrossRef]
- Kumar, A.; Malvi, B. Iron Oxide Based Catalysts: A Temperature Programmed Reduction Study. Res. J. Chem. Sci. 2021, 11, 39–45. [Google Scholar]
- Zhu, X.; Zhang, J.; Yan, J.; Shen, L. Characteristic Evaluation and Process Simulation of CuFe2O4as Oxygen Carriers in Coal Chemical Looping Gasification. ACS Omega 2021, 6, 4783–4792. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, K.; Wu, W.; Cui, X.; Li, Y. Magnetic Properties of Nanocrystalline CuFe2O4 and Kinetics of Thermal Decomposition of Precursor. J. Therm. Anal. Calorim. 2013, 111, 9–16. [Google Scholar] [CrossRef]
- Chawla, A.K.; Chandra, R. Synthesis and Structural Characterization of Nanostructured Copper. J. Nanopart. Res. 2009, 11, 297–302. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bao, H.; Li, Y.; Bai, H.; Ma, F. Atomic Scale Insights into the Rapid Crystallization and Precipitation Behaviors in FeCu Binary Alloys. J. Alloys Compd. 2021, 882, 160725. [Google Scholar] [CrossRef]
- Ou, X. Molecular Dynamics Simulations of Fcc-to-Bcc Transformation in Pure Iron: A Review. Mater. Sci. Technol. 2017, 33, 822–835. [Google Scholar] [CrossRef]
- Pugachev, V.M.; Zaharov, Y.A.; Datiy, K.A.; Popova, A.N.; Bogomyakov, A.S. The Temperature Effect on Properties of Fe-Co-Ni Nanostructured System. Eurasian Chem.-Technol. J. 2015, 17, 193–200. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Frade, J.R.; Cable, M. Reexamination of the Basic Theoretical Model for the Kinetics of Solid–State Reactions. J. Am. Ceram. Soc. 1992, 75, 1949–1957. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef] [PubMed]
- Ašperger, S. Chemical Kinetics and Reaction Mechanisms. Mcgraw-Hill: New York, NY, USA, 2003; ISBN 9781461348719. [Google Scholar]
- House, J.E. Principles of Chemical Kinetics. Academic Press: Cambridge, MA, USA, 2007; ISBN 9780123567871. [Google Scholar]
- Galwey, A.K. Eradicating Erroneous Arrhenius Arithmetic. Thermochim Acta 2003, 399, 1–29. [Google Scholar] [CrossRef]
- Sorokova, N.; Variny, M.; Pysmennyy, Y.; Kol’chik, Y. Mathematical Model and Numerical Method of Calculating the Dynamics of High-Temperature Drying of Milled Peat for the Production of Fuel Briquettes. Computation 2023, 11, 53. [Google Scholar] [CrossRef]
- Strydom, C.A.; Hudson-Lamb’, D.L.; Potgieter, J.H.; Dagg, E. Thermochimica Acta The Thermal Dehydration of Synthetic Gypsum*. Thermochim Acta 1995, 269, 631–638. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Ababii, N.; Hoppe, M.; Cretu, V.; Tiginyanu, I.; Sontea, V.; Pauporté, T.; Viana, B.; Adelung, R. Influence of CuO Nanostructures Morphology on Hydrogen Gas Sensing Performances. Microelectron. Eng. 2016, 164, 63–70. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J.W. Studies in Gas-Solid Reactions: Part I. A Structural Model for the Reaction of Porous Oxides with a Reducing Gas. Metall. Trans. 1971, 2, 1691–1698. [Google Scholar] [CrossRef]
- Halim, K.S.A.; Khedr, M.H.; Zaki, A.H. Kinetics and Mechanisms of the Reduction of Cu0.5Zn0.5Fe2O4 with Hydrogen at 400-600 °C for the Production of Metallic Nanoparticles. J. Anal. Appl. Pyrolysis 2007, 80, 346–352. [Google Scholar] [CrossRef]
- Khedr, M.H.; Farghali, A.A.; Abdel-Khalek, A.A. Microstructure, Kinetics and Mechanisms of Nano-Crystalline CuFe2O4 Reduction in Flowing Hydrogen at 300-600 °C for the Production of Metallic Nano-Wires. J. Anal. Appl. Pyrolysis 2007, 78, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Liu, J.; Mi, T.-G.; Wu, Y.-W.; Hu, B.; Zhou, X.-Y.; Zhang, B.; Lu, Q. Theoretical Study on the Hydrogenation of Furfural for Furfuryl Alcohol Production over Low Ni Modified Cu Catalysts. Appl. Surf. Sci. 2023, 613, 156106. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, Properties and Catalytic Hydrogenation of Furfural to Fuel Additives and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- García-Sancho, C.; Mérida-Robles, J.M.; Cecilia-Buenestado, J.A.; Moreno-Tost, R.; Maireles-Torres, P.J. The Role of Copper in the Hydrogenation of Furfural and Levulinic Acid. Int. J. Mol. Sci. 2023, 24, 2443. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of 2-Methylfuran by Gas-Phase Hydrogenation of Furfural on Copper Incorporated by Complexation in Mesoporous Silica Catalysts. ChemSusChem 2017, 10, 1448–1459. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Fernandes, A.; Neri, F.; Silva, C.M.; Rocha, S.M.; Ribeiro, M.F.; et al. One-Pot Conversion of Furfural to Useful Bio-Products in the Presence of a Sn,Al-Containing Zeolite Beta Catalyst Prepared via Post-Synthesis Routes. J. Catal. 2015, 329, 522–537. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites for the Production of Γ-Valerolactone from Furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cheng, Y.; Niu, H.; Wang, T.; Liang, C. Efficient Cu/FeOx Catalyst with Developed Structure for Catalytic Transfer Hydrogenation of Furfural. J. Catal. 2022, 413, 575–587. [Google Scholar] [CrossRef]
- Li, F.; Yang, R.; Tian, Z.; Du, Z.; Dai, J.; Wang, X.; Li, N.; Zhang, J. Microwave-Assisted One Pot Cascade Conversion of Furfural to γ-Valerolactone over Sc(OTf)3. Chem.—Eur. J. 2023, 29, e202300950. [Google Scholar] [CrossRef] [PubMed]
- Koranchalil, S.; Lobo Justo Pinheiro, D.; Padilla, R.; Nielsen, M. Homogeneous Catalyzed Direct Conversion of Furfural to Gamma-Valerolactone. ChemSusChem 2024, 17, e202301608. [Google Scholar] [CrossRef] [PubMed]




| Chemical Species | Label | Molecular Weight (g/mol) |
|---|---|---|
| Fe(NO3)3·9H2O | S1 | 337.86 |
| Fe(NO3)3·6H2O | S2 | 289.83 |
| CuCl2·2H2O | S3 | 170.45 |
| CuCl2 | S4 | 134.40 |
| Cu2Cl2O | S5 | 214.00 |
| CuO | S6 | 79.55 |
| FeO | S7 | 71.85 |
| Fe2O3 | S8 | 159.39 |
| CuFe2O4 | S9 | 239.24 |
| Fe | S10 | 55.85 |
| Cu | S11 | 63.55 |
| Cu4Fe | S12 | 310.05 |
| Stage | Proposed Reaction Concerning XRD | Label |
|---|---|---|
| Dry Bulk → Prec | (4) | |
| Prec → c200 | (5) | |
| c200 → c300 | (6) | |
| c300 → c400 | (7) | |
| c400 → c500 | (8) | |
| r200 → r300 | (9) | |
| r300 → r400 | (10) | |
| r400 → r500 | (11) |
| Oxidation Stage | Reduction Stage | ||
|---|---|---|---|
| Parameter | Value | Parameter | Value |
| A1 (min−1) | 9.72 × 104 | A6 (min−1) | 1.05 × 104 |
| Ea1 (kcal/mol) | 10.11 | Ea6 (kcal/mol) | 13.02 |
| A2 (min−1) | 9.57 × 104 | A7 (min−1) | 1.03 × 104 |
| Ea2 (kcal/mol) | 9.84 | Ea7 (kcal/mol) | 14.88 |
| A3 (min−1) | 1.41 × 105 | A8 (min−1) | 1.94 × 106 |
| Ea3 (kcal/mol) | 11.29 | Ea8 (kcal/mol) | 8.17 |
| A4 (min−1) | 3.25 × 104 | ||
| Ea4 (kcal/mol) | 17.06 | ||
| A5 (min−1) | 4.54 × 104 | ||
| Ea5 (kcal/mol) | 13.85 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lino Galarza, B.J.; Rivera De la Rosa, J.; Sánchez Cervantes, E.M.; Lucio-Ortiz, C.J.; Garza-Navarro, M.A.; Maldonado, C.S.; Moreno-Tost, R.; Cecilia-Buenestado, J.A.; Infantes Molina, A. Solid-State Kinetic Modeling and Experimental Validation of Cu-Fe Bimetallic Catalyst Synthesis and Its Application to Furfural Hydrogenation. Technologies 2025, 13, 63. https://doi.org/10.3390/technologies13020063
Lino Galarza BJ, Rivera De la Rosa J, Sánchez Cervantes EM, Lucio-Ortiz CJ, Garza-Navarro MA, Maldonado CS, Moreno-Tost R, Cecilia-Buenestado JA, Infantes Molina A. Solid-State Kinetic Modeling and Experimental Validation of Cu-Fe Bimetallic Catalyst Synthesis and Its Application to Furfural Hydrogenation. Technologies. 2025; 13(2):63. https://doi.org/10.3390/technologies13020063
Chicago/Turabian StyleLino Galarza, Bárbara Jazmín, Javier Rivera De la Rosa, Eduardo Maximino Sánchez Cervantes, Carlos J. Lucio-Ortiz, Marco Antonio Garza-Navarro, Carolina Solís Maldonado, Ramón Moreno-Tost, Juan Antonio Cecilia-Buenestado, and Antonia Infantes Molina. 2025. "Solid-State Kinetic Modeling and Experimental Validation of Cu-Fe Bimetallic Catalyst Synthesis and Its Application to Furfural Hydrogenation" Technologies 13, no. 2: 63. https://doi.org/10.3390/technologies13020063
APA StyleLino Galarza, B. J., Rivera De la Rosa, J., Sánchez Cervantes, E. M., Lucio-Ortiz, C. J., Garza-Navarro, M. A., Maldonado, C. S., Moreno-Tost, R., Cecilia-Buenestado, J. A., & Infantes Molina, A. (2025). Solid-State Kinetic Modeling and Experimental Validation of Cu-Fe Bimetallic Catalyst Synthesis and Its Application to Furfural Hydrogenation. Technologies, 13(2), 63. https://doi.org/10.3390/technologies13020063










