Portable DNA Probe Detector and a New Dry-QCM Approach for SARS-CoV-2 Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Reagents
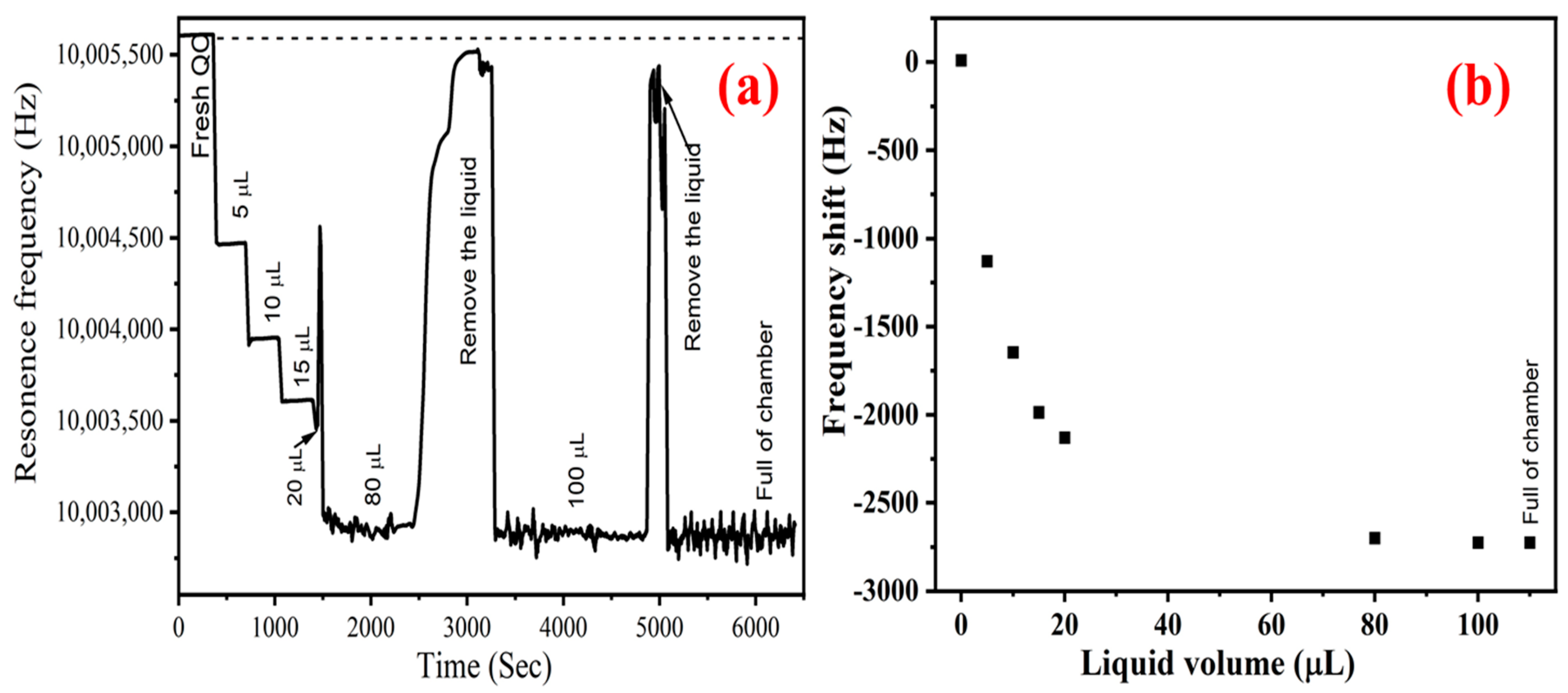
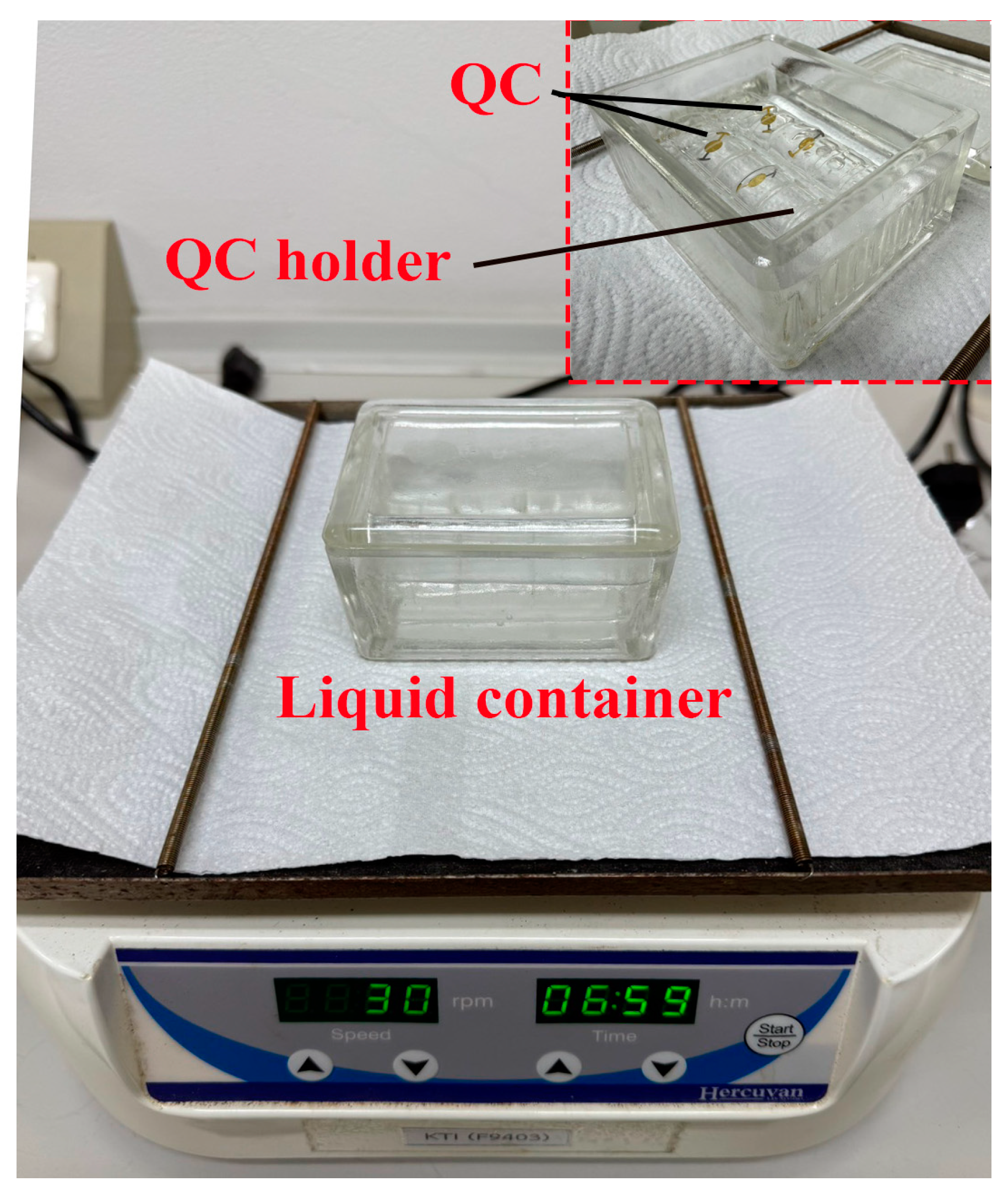
2.3. Apparatus
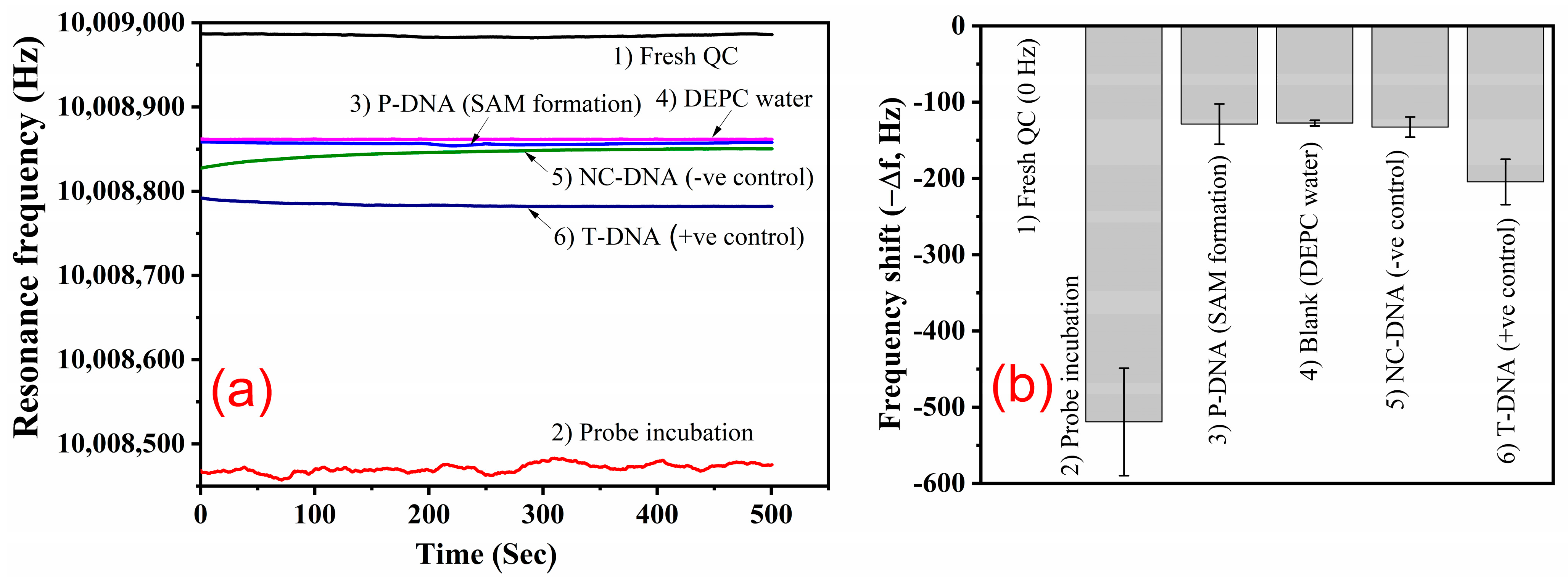
2.4. Methods
3. Results
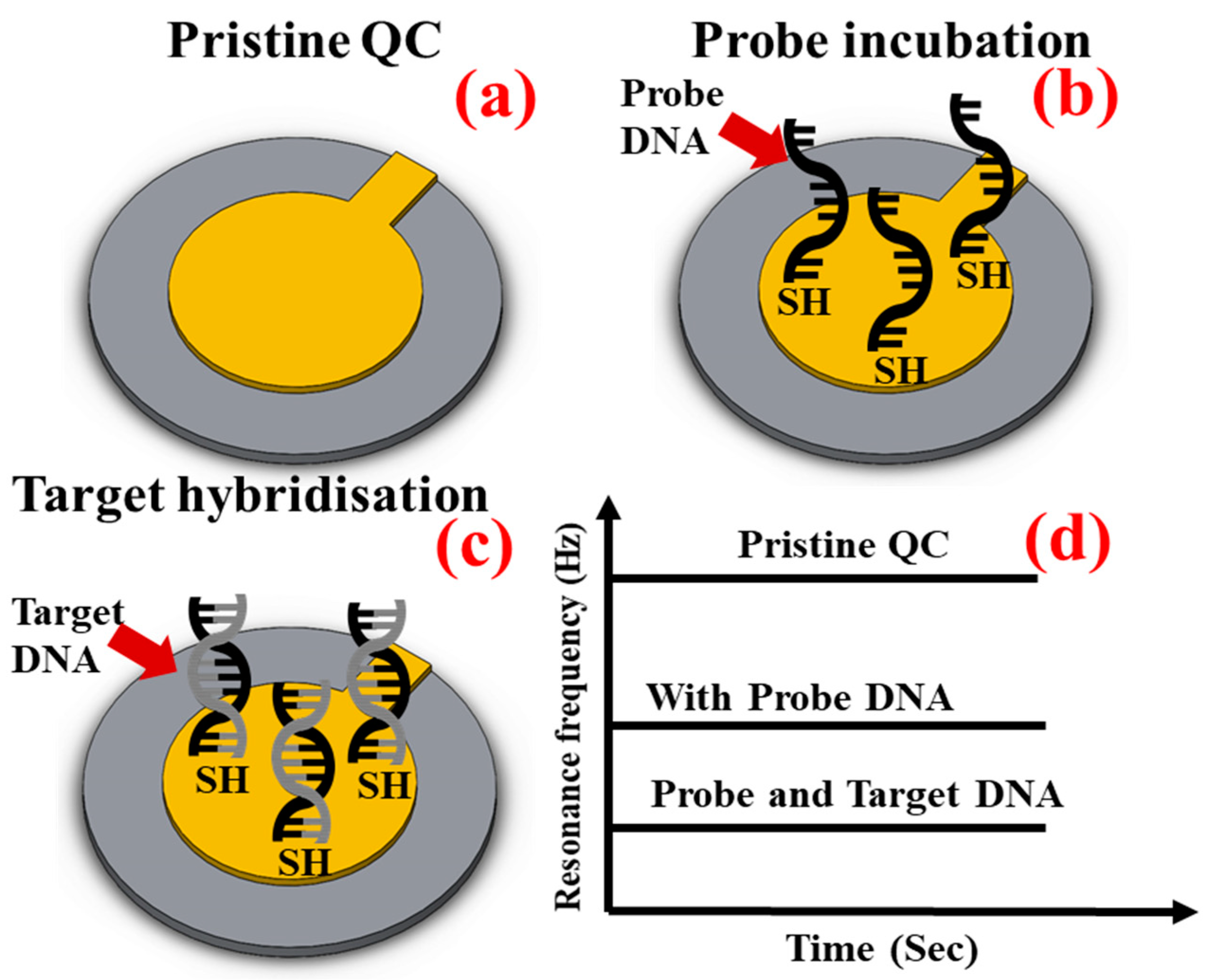
3.1. Theory
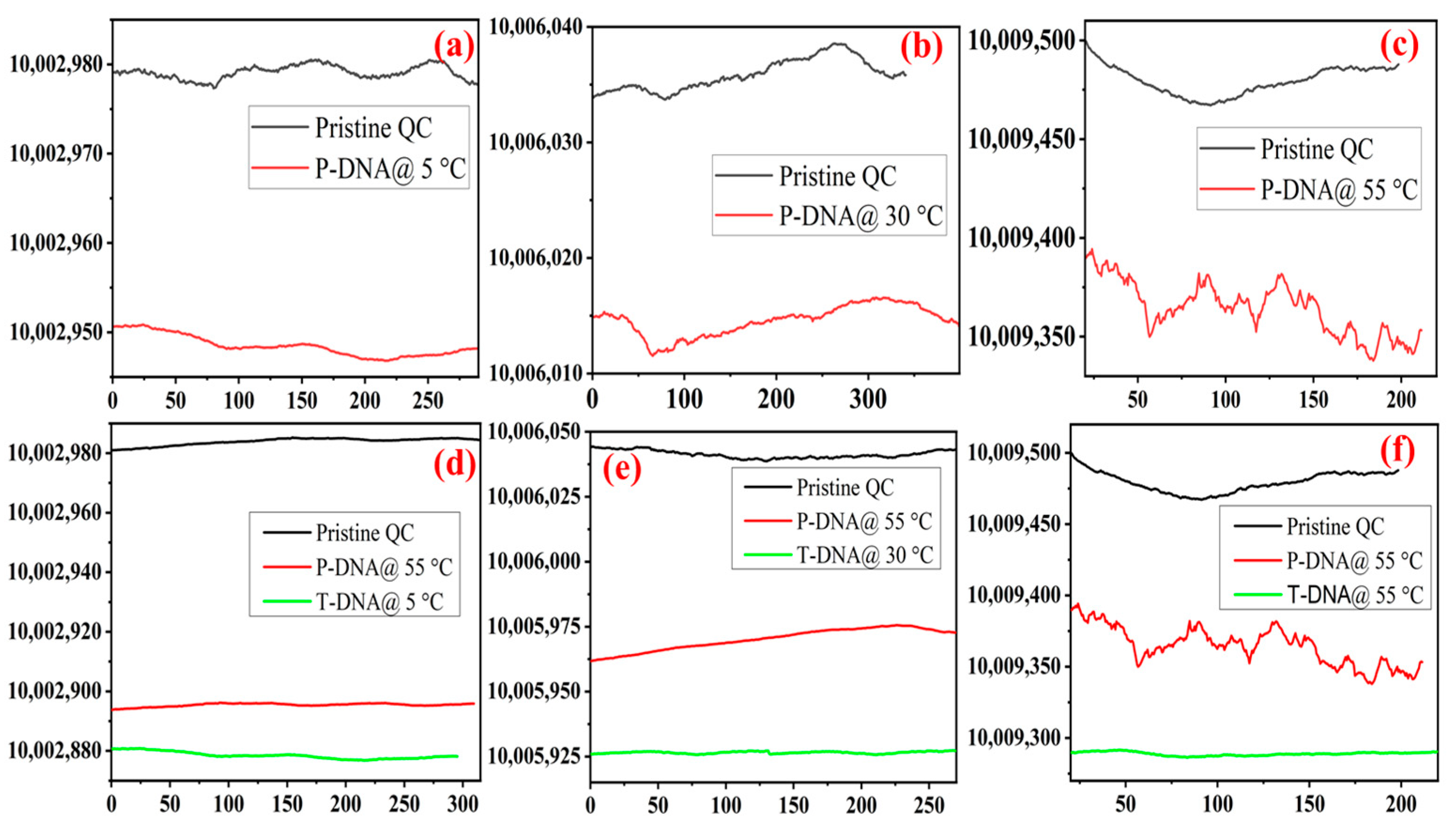
3.2. Effect of Temperature on Probe Incubation and Target Hybridisation
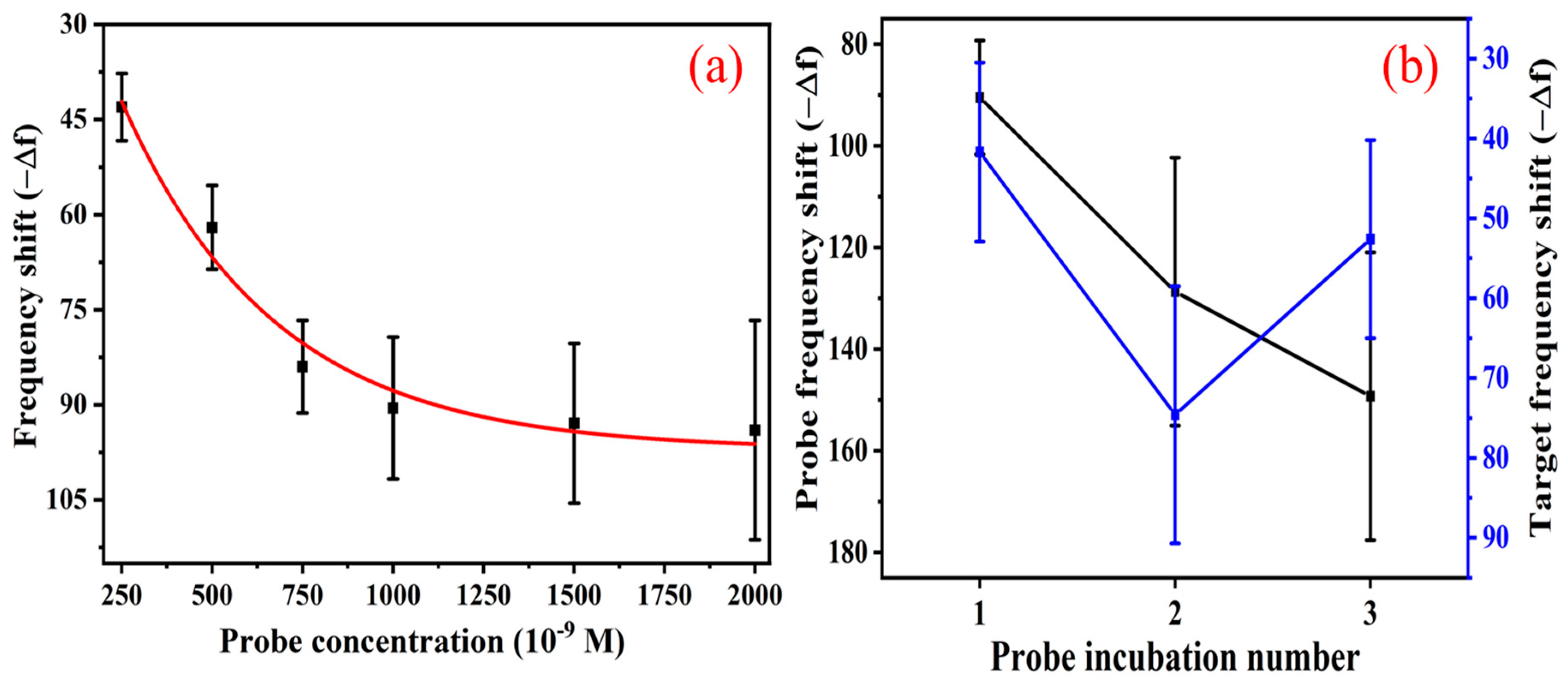
3.3. Effect of Probe Concentration and Double-Probe Approach
3.4. Target Hybridisation and Selectivity
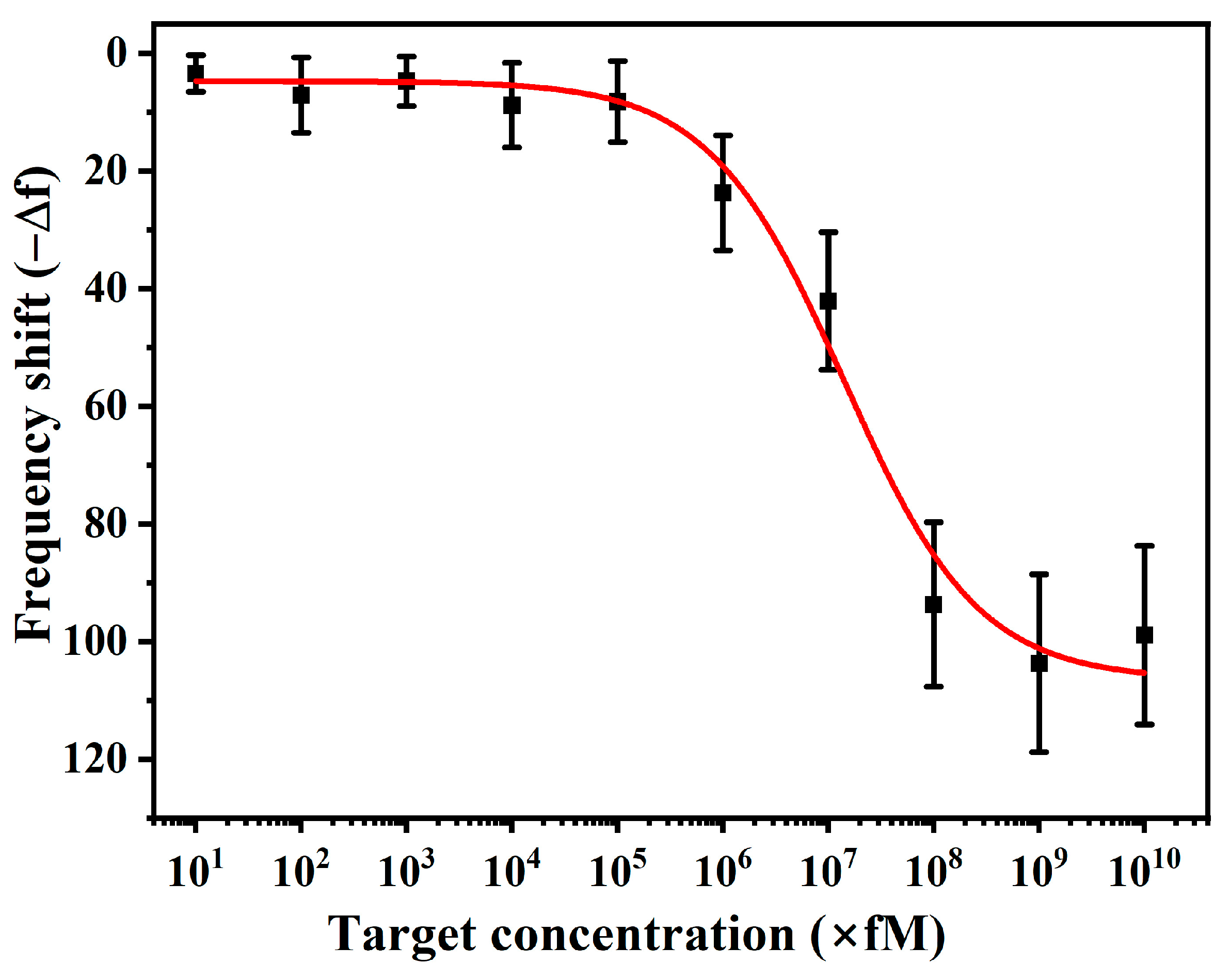
3.5. Sensitivity of Sensor and Limit of Detection (LoD)
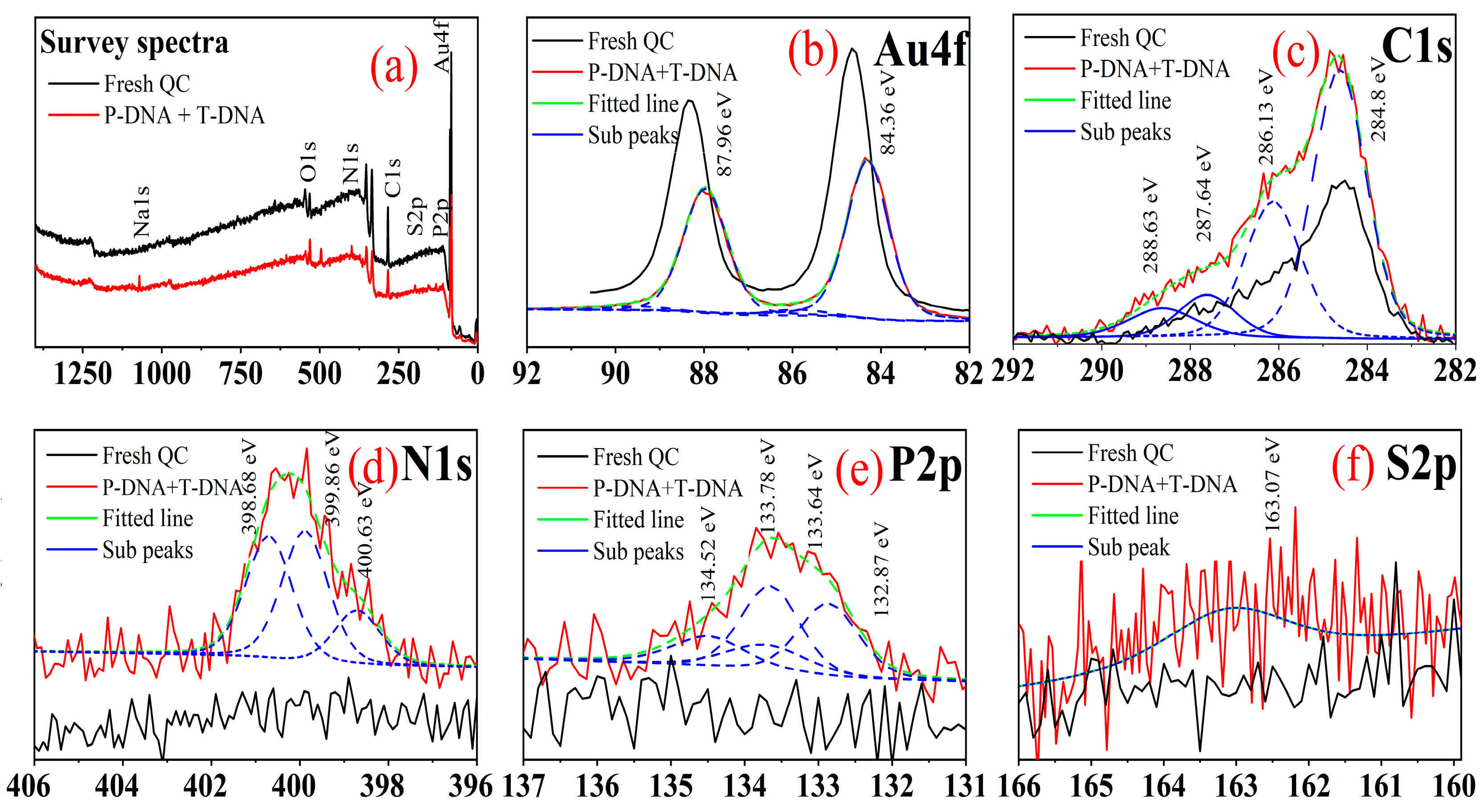
3.6. Surface Characterisations
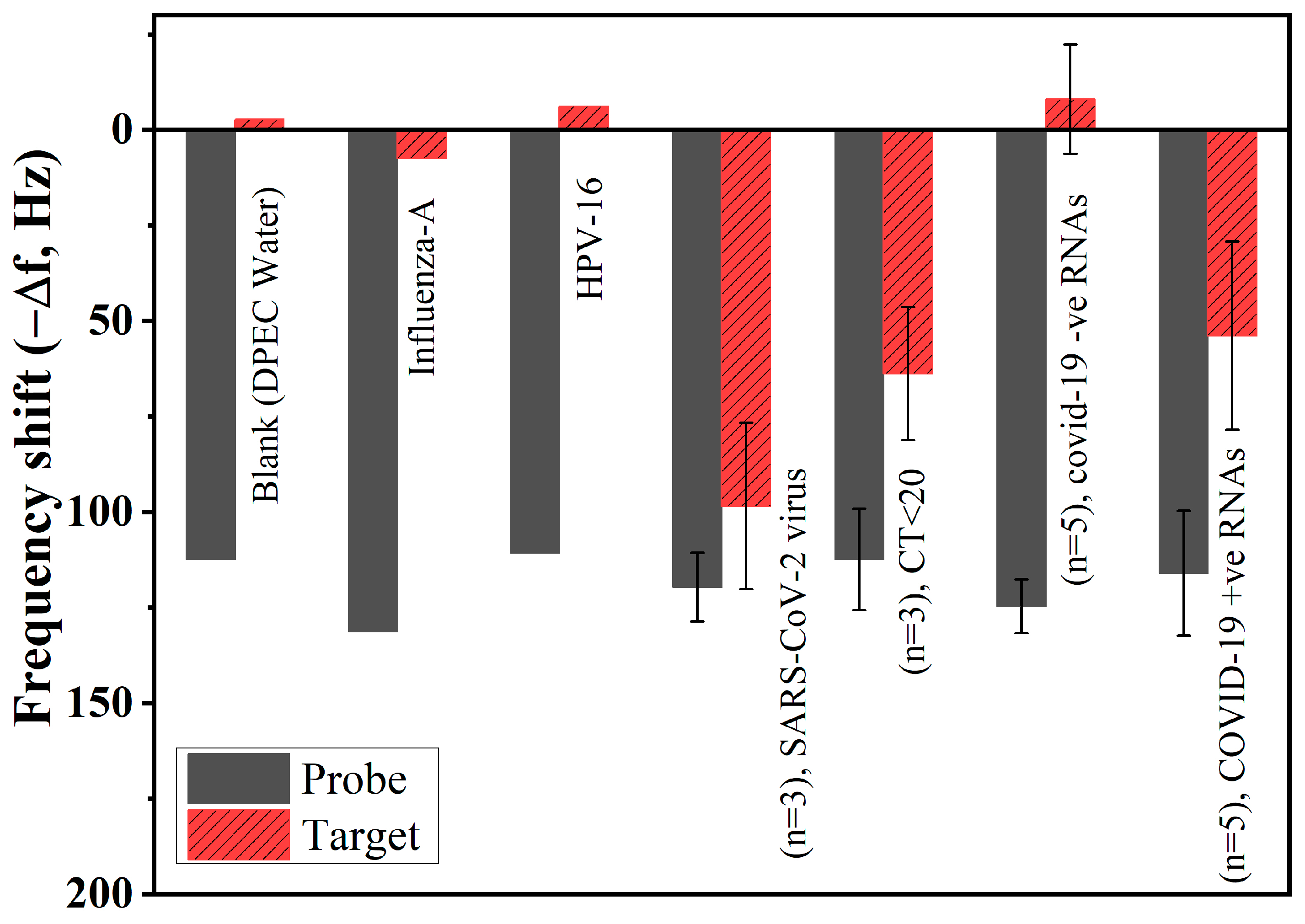
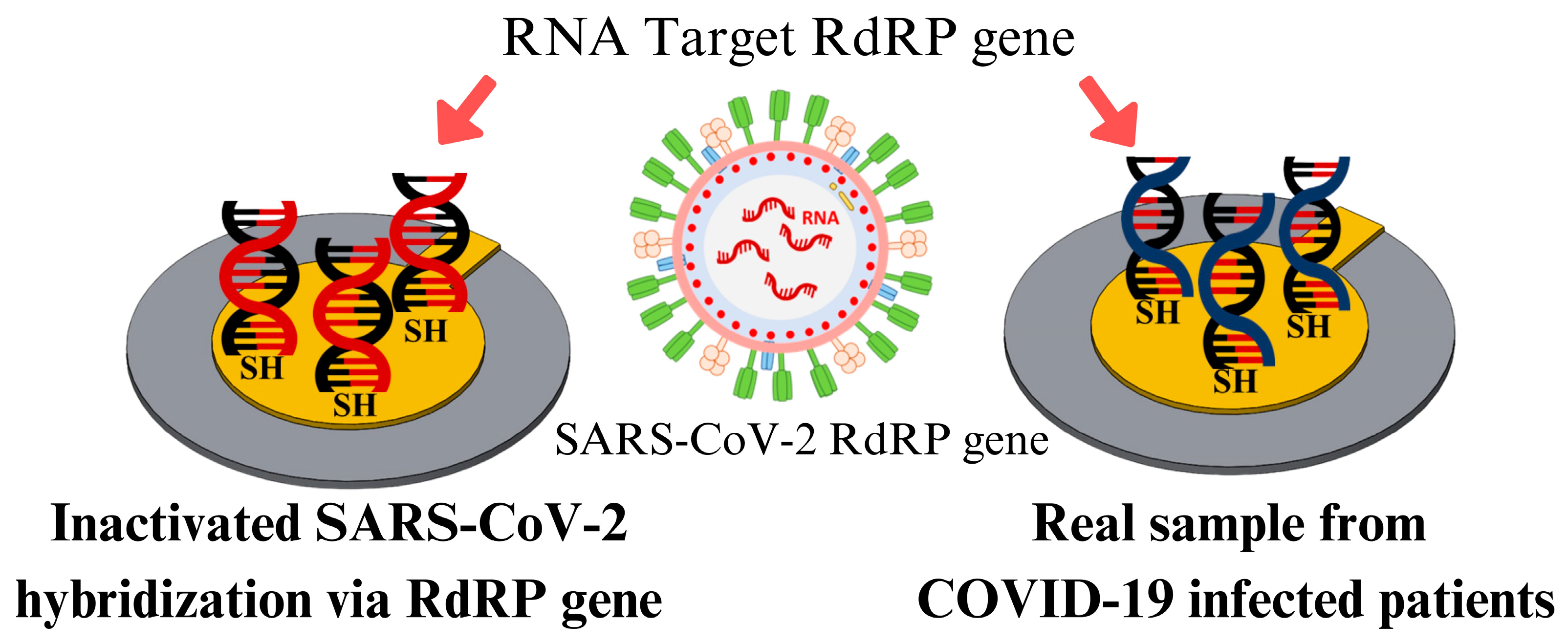
3.7. Specificity and Clinical Validation of Real Samples
3.8. Re-Use and Regeneration of QCM Sensor
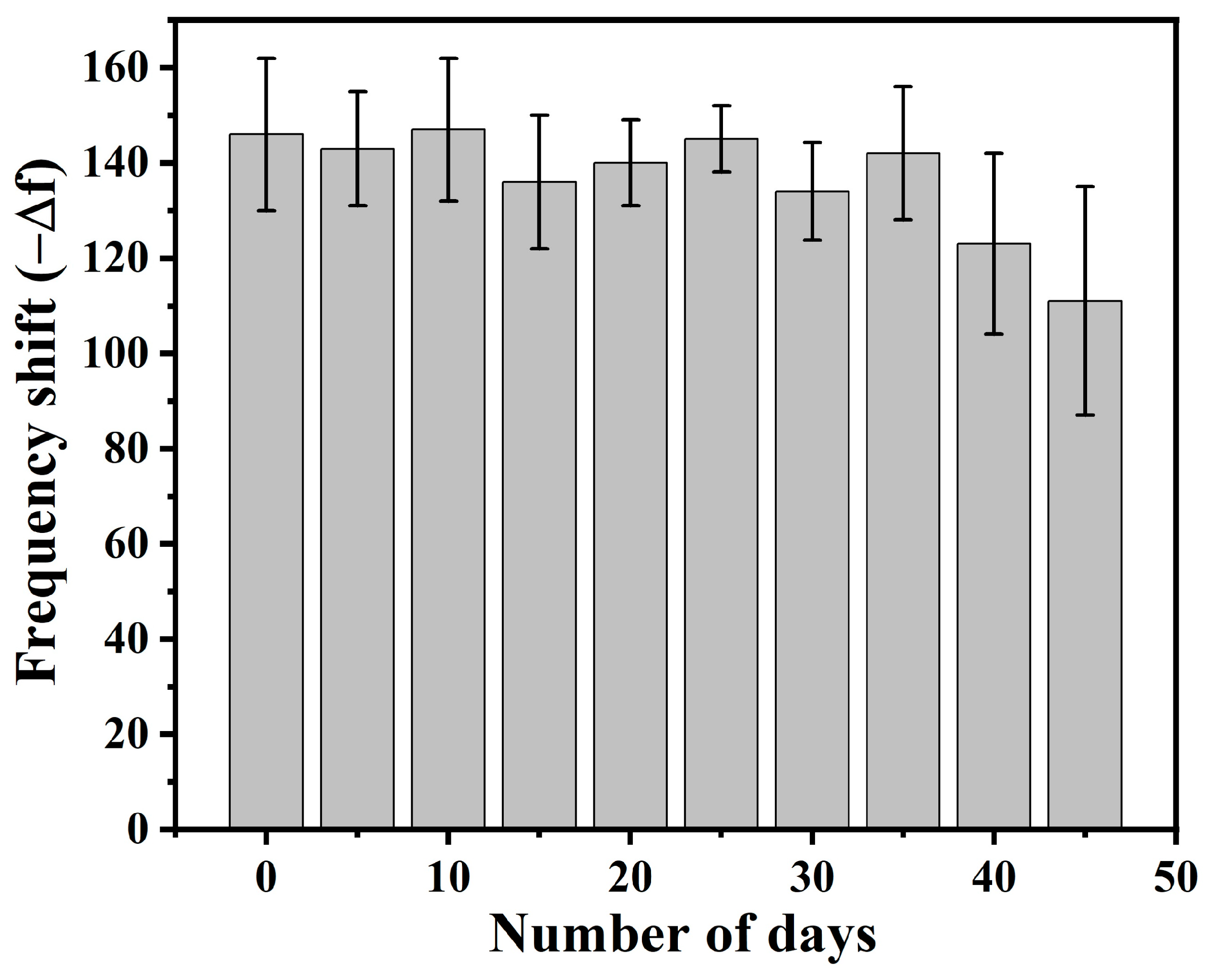
3.9. Shelf-Life of the Developed Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acids |
| RNA | Ribonucleic acid |
| RdRP | RNA-dependent RNA Polymerase |
| SAM | Self-assembled monolayer |
| QCM | Quartz crystal microbalance |
Appendix A


References
- Spiteri, G.; Fielding, J.; Diercke, M.; Campese, C.; Enouf, V.; Gaymard, A.; Bella, A.; Sognamiglio, P.; Sierra Moros, M.J.; Riutort, A.N.; et al. First Cases of Coronavirus Disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Eurosurveillance 2020, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, W.; Zhang, F. Who Was the First Doctor to Report the COVID-19 Outbreak in Wuhan, China? J. Nucl. Med. 2020, 61, 782–783. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, J.W.; Barry, M.A. SARS, the First Pandemic of the 21st Century. Emerg. Infect. Dis. 2004, 10, e26. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. 2024. Available online: https://www.who.int/emergencies/overview/tracking-SARS-CoV-2-Variants (accessed on 1 September 2023).
- COVID-19 Map Johns Hopkins: Coronavirus Resource Center, COVID-19 Dashboard. 2023. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 September 2023).
- Zhu, H.; Wei, L.; Niu, P. The Novel Coronavirus Outbreak in Wuhan, China. Glob. Health Res. Policy 2020, 5, 6. [Google Scholar] [CrossRef]
- Haque, S.M.; Ashwaq, O.; Sarief, A.; Azad John Mohamed, A.K. A Comprehensive Review About SARS-CoV-2. Future Virol. 2020, 15, 625–648. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Dutta, D.; Naiyer, S.; Mansuri, S.; Soni, N.; Singh, V.; Bhat, K.H.; Singh, N.; Arora, G.; Mansuri, M.S. COVID-19 Diagnosis: A Comprehensive Review of the RT-qPCR Method for Detection of SARS-CoV-2. Diagnostics 2022, 12, 1503. [Google Scholar] [CrossRef]
- Munne, K.; Bhanothu, V.; Bhor, V.; Patel, V.; Mahale, S.D.; Pande, S. Detection of SARS-CoV-2 Infection by RT-PCR Test: Factors Influencing Interpretation of Results. VirusDisease 2021, 32, 187–189. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Qi, L.; Liu, M.; Zhao, X.; Kong, L.; Wang, Y.; Chen, F.; Zhang, C.; Cheng, J.; et al. Clinical Evaluation of a New COVID-19 Antigen Rapid Test Kit for Detection of SARS-CoV-2. Diagn. Microbiol. Infect. Dis. 2024, 108, 116136. [Google Scholar] [CrossRef]
- Mak, G.C.; Lau, S.S.; Wong, K.K.; Chow, N.L.; Lau, C.-S.; Lam, E.T.; Ng, K.H.; Chan, R.C. Evaluation of Rapid Antigen Detection Kits for the Detection of SARS-CoV-2 B.1.617.2 Virus. Future Virol. 2022, 17, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Vashist, P. Recent Advances in Quartz Crystal Microbalance-Based Sensors. J. Sens. 2011, 2011, 571405. [Google Scholar] [CrossRef]
- Linares, M.; Pérez-Tanoira, R.; Carrero, A.; Romanyk, J.; Pérez-García, F.; Gómez-Herruz, P.; Arroyo, T.; Cuadros, J. Panbio Antigen Rapid Test Is Reliable to Diagnose SARS-CoV-2 Infection in the First 7 Days After the Onset of Symptoms. J. Clin. Virol. 2020, 133, 104659. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Baldanti, F.; Ganguly, N.K.; Wang, G.; Möckel, M.; O’Neill, L.A.; Renz, H.; Dos Santos Ferreira, C.E.; Tateda, K.; Van Der Pol, B. Choice of SARS-CoV-2 Diagnostic Test: Challenges and Key Considerations for the Future. Crit. Rev. Clin. Lab. Sci. 2022, 59, 445–459. [Google Scholar] [CrossRef]
- Ravina; Kumar, A.; Manjeet; Twinkle; Subodh; Narang, J.; Mohan, H. Analytical Performances of Different Diagnostic Methods for SARS-CoV-2 Virus—A Review. Sens. Int. 2022, 3, 100197. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The Performance Enhancement of Surface Plasmon Resonance Optical Sensors Using Nanomaterials: A Review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Melo, A.F.A.A.; Singh, S.J.; Chinnamuthu, P.; Crespilho, F.N.; Rydzek, G. Magnetically Stimulated Bio- and Electrochemical Systems: State-of-the-Art, Applications, and Future Directions. ChemNanoMat 2023, 9, e202300192. [Google Scholar] [CrossRef]
- Sun, C.; Wang, X.; Auwalu, M.A.; Cheng, S.; Hu, W. Organic Thin Film Transistors-Based Biosensors. EcoMat 2021, 3, e12094. [Google Scholar] [CrossRef]
- Fawcett, N.C.; Evans, J.A.; Chien, L.-C.; Flowers, N. Nucleic Acid Hybridization Detected by Piezoelectric Resonance. Anal. Lett. 1988, 21, 1099–1114. [Google Scholar] [CrossRef]
- Cadoni, E.; Manicardi, A.; Madder, A. PNA-Based MicroRNA Detection Methodologies. Molecules 2020, 25, 1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz Crystal Microbalance: Sensing Cell-Substrate Adhesion and Beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef]
- Zuo, C.; Wen, Y.; Chen, D.; Ouyang, J.; Li, P.; Dong, T. Dynamic Monitoring of Biomolecular Hydrodynamic Dimensions by Magnetization Motion on Quartz Crystal Microbalance. Anal. Chem. 2024, 96, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Rawle, R.J.; Selassie, C.R.D.; Johal, M.S. Creation of Mammalian Single- and Double-Stranded DNA Surfaces: A Real-Time QCM-D Study. Langmuir 2007, 23, 9563–9566. [Google Scholar] [CrossRef] [PubMed]
- Hampitak, P.; Jowitt, T.A.; Melendrez, D.; Fresquet, M.; Hamilton, P.; Iliut, M.; Nie, K.; Spencer, B.; Lennon, R.; Vijayaraghavan, A. A Point-of-Care Immunosensor Based on a Quartz Crystal Microbalance with Graphene Biointerface for Antibody Assay. ACS Sens. 2020, 5, 3520–3532. [Google Scholar] [CrossRef]
- Nilebäck, E.; Feuz, L.; Uddenberg, H.; Valiokas, R.; Svedhem, S. Characterization and Application of a Surface Modification Designed for QCM-D Studies of Biotinylated Biomolecules. Biosens. Bioelectron. 2011, 28, 407–413. [Google Scholar] [CrossRef]
- Zuo, B.; Li, S.; Guo, Z.; Zhang, J.; Chen, C. Piezoelectric Immunosensor for SARS-Associated Coronavirus in Sputum. Anal. Chem. 2004, 76, 3536–3540. [Google Scholar] [CrossRef]
- Albano, D.R.B.; Shum, K.; Tanner, J.A.; Fung, Y.S. BS5.3—Piezoelectric Quartz Crystal Aptamer Biosensor for Detection and Quantification of SARS CoV Helicase Protein. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS 2018, Vienna, Austria, 15–19 July 2018; AMA Service GmbH: Wunstorf, Germany, 2018; pp. 211–213. [Google Scholar] [CrossRef]
- Owen, T.W.; Al-Kaysi, R.O.; Bardeen, C.J.; Cheng, Q. Microgravimetric Immunosensor for Direct Detection of Aerosolized Influenza A Virus Particles. Sens. Actuators B Chem. 2007, 126, 691–699. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Callaway, Z.T.; Lu, H.; Huang, T.J.; Li, Y. A Nanowell-Based QCM Aptasensor for Rapid and Sensitive Detection of Avian Influenza Virus. Sens. Actuators B Chem. 2017, 240, 934–940. [Google Scholar] [CrossRef]
- Forinova, M.; Pilipenco, A.; Visova, I.; Kuncak, J.; Lynn, N.S.; Yudin, P.; Dostalek, J.; Honig, V.; Palus, M.; Maskova, H.; et al. Biosensor for Rapid Detection of SARS-CoV-2 in Real-World Samples. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October–3 November 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Forinová, M.; Pilipenco, A.; Víšová, I.; Lynn, N.S.; Dostálek, J.; Mašková, H.; Hönig, V.; Palus, M.; Selinger, M.; Kočová, P.; et al. Functionalized Terpolymer-Brush-Based Biointerface with Improved Antifouling Properties for Ultra-Sensitive Direct Detection of Virus in Crude Clinical Samples. ACS Appl. Mater. Interfaces 2021, 13, 60612–60624. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.H.; Al-Majdoub, M.; Ibrahim, A.; Aseel, O.; Suriyanarayanan, S.; Andersson, L.; Fostock, S.; Aastrup, T.; Tjernberg, I.; Rydén, I.; et al. Quartz Crystal Microbalance Platform for SARS-CoV-2 Immuno-Diagnostics. Int. J. Mol. Sci. 2023, 24, 16705. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L.M. Design of Engineered Surfaces for Prospective Detection of SARS-CoV-2 Using Quartz Crystal Microbalance-Based Techniques. Expert Rev. Proteom. 2020, 17, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Nemčeková, K.; Korčeková, J.; Svitková, V.; Baraniak, D.; Domšicová, M.; Melníková, E.; Hornychová, M.; Szebellaiová, V.; Gál, M.; Poturnayová, A. Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection. Biosensors 2024, 14, 431. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, S.; Ko, J.; Ko, M.; Seo, H.W. Identification of Conserved Regions from 230,163 SARS-CoV-2 Genomes and Their Use in Diagnostic PCR Primer Design. Genes Genom. 2022, 44, 899–912. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung Dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a Quartz Microbalance in Contact with Liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous Frequency and Dissipation Factor QCM Measurements of Biomolecular Adsorption and Cell Adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef]
- Researcher Formula for Copy Number Calculation|IDT. Available online: https://sg.idtdna.com/pages/education/decoded/article/calculations-converting-from-nanograms-to-copy-number (accessed on 25 June 2024).
- Xu, J.; Craig, S.L. Thermodynamics of DNA Hybridization on Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 13227–13231. [Google Scholar] [CrossRef]
- Wetmur, J.G.; Davidson, N. Kinetics of Renaturation of DNA. J. Mol. Biol. 1968, 31, 349–370. [Google Scholar] [CrossRef]
- Morrison, L.E.; Stols, L.M. Sensitive Fluorescence-Based Thermodynamic and Kinetic Measurements of DNA Hybridization in Solution. Biochemistry 1993, 32, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, Y.; Xie, B. Hybrid Temperature Effect on a Quartz Crystal Microbalance Resonator in Aqueous Solutions. Chin. Phys. B 2017, 26, 067704. [Google Scholar] [CrossRef]
- Petrovykh, D.Y.; Kimura-Suda, H.; Whitman, L.J.; Tarlov, M.J. Quantitative Analysis and Characterization of DNA Immobilized on Gold. J. Am. Chem. Soc. 2003, 125, 5219–5226. [Google Scholar] [CrossRef]
- Roelfs, B.; Bunge, E.; Schröter, C.; Solomun, T.; Meyer, H.; Nichols, R.J.; Baumgärtel, H. Adsorption of Thymine on Gold Single-Crystal Electrodes. J. Phys. Chem. B 1997, 101, 754–765. [Google Scholar] [CrossRef]
- Suvanasuthi, R.; Cheewasatheinchaiyaporn, T.; Wat-aksorn, K.; Promptmas, C. Nucleic Acid Amplification Free-QCM-DNA Biosensor for Burkholderia Pseudomallei Detection. Curr. Microbiol. 2023, 80, 376. [Google Scholar] [CrossRef]
- Peterson, A.W.; Wolf, L.K.; Georgiadis, R.M. Hybridization of Mismatched or Partially Matched DNA at Surfaces. J. Am. Chem. Soc. 2002, 124, 14601–14607. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, J.; Sanjeeva Rao, K.; Lin, W.-H.; Liao, J.-D.; Yoshimura, M. Low Energy Synthesis of Nitrogen Functionalized Graphene/Nanoclay Hybrid via Submerged Liquid Plasma Approach. Carbon 2014, 78, 446–454. [Google Scholar] [CrossRef]
- Dementjev, A.P.; De Graaf, A.; Van De Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray Photoelectron Spectroscopy Reference Data for Identification of the C3N4 Phase in Carbon–Nitrogen Films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Johansson, Å.; Stafström, S. A Δ-Self-Consistent-Field Study of the Nitrogen 1s Binding Energies in Carbon Nitrides. J. Chem. Phys. 1999, 111, 3203–3208. [Google Scholar] [CrossRef]
- Senthilnathan, J.; Rao, K.S.; Lin, W.-H.; Ting, J.-M.; Yoshimura, M. Formation of Reusable Au-Acetonitrile Polymers and N-Doped Graphene Catalyst Under UV Light via Submerged Liquid Plasma Process. J. Mater. Chem. A 2015, 3, 3035–3043. [Google Scholar] [CrossRef]
- Zhang, B.; Kaziz, S.; Li, H.; Wodka, D.; Malola, S.; Safonova, O.; Nachtegaal, M.; Mazet, C.; Dolamic, I.; Llorca, J.; et al. Pd2 Au36 (SR)24 Cluster: Structure Studies. Nanoscale 2015, 7, 17012–17019. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in Different Environmental Conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Mannelli, I.; Minunni, M.; Tombelli, S.; Wang, R.; Michela Spiriti, M.; Mascini, M. Direct Immobilisation of DNA Probes for the Development of Affinity Biosensors. Bioelectrochemistry 2005, 66, 129–138. [Google Scholar] [CrossRef]
- Lazerges, M.; Perrot, H.; Zeghib, N.; Antoine, E.; Compere, C. In Situ QCM DNA-Biosensor Probe Modification. Sens. Actuators B Chem. 2006, 120, 329–337. [Google Scholar] [CrossRef]
- Dupont-Filliard, A.; Billon, M.; Livache, T.; Guillerez, S. Biotin/Avidin System for the Generation of Fully Renewable DNA Sensor Based on Biotinylated Polypyrrole Film. Anal. Chim. Acta 2004, 515, 271–277. [Google Scholar] [CrossRef]
- Le, H.T.N.; Phan, L.M.T.; Cho, S. Removal of Thiol-SAM on a Gold Surface for Re-Use of an Interdigitated Chain-Shaped Electrode. Materials 2022, 15, 2218. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karri, R.R.; Koduru, J.R.; Manickam, S.; Tyagi, I.; Mubarak, N.M. Suhas Recent Trends in the Applications of Sonochemical Reactors as an Advanced Oxidation Process for the Remediation of Microbial Hazards Associated with Water and Wastewater: A Critical Review. Ultrason. Sonochem. 2023, 94, 106302. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munthala, D.; Sonklin, T.; Chanlek, N.; Mathur, A.; Roy, S.; Avasthi, D.K.; Suksaweang, S.; Pojprapai, S. Portable DNA Probe Detector and a New Dry-QCM Approach for SARS-CoV-2 Detection. Technologies 2025, 13, 114. https://doi.org/10.3390/technologies13030114
Munthala D, Sonklin T, Chanlek N, Mathur A, Roy S, Avasthi DK, Suksaweang S, Pojprapai S. Portable DNA Probe Detector and a New Dry-QCM Approach for SARS-CoV-2 Detection. Technologies. 2025; 13(3):114. https://doi.org/10.3390/technologies13030114
Chicago/Turabian StyleMunthala, Dhanunjaya, Thita Sonklin, Narong Chanlek, Ashish Mathur, Souradeep Roy, Devash Kumar Avasthi, Sanong Suksaweang, and Soodkhet Pojprapai. 2025. "Portable DNA Probe Detector and a New Dry-QCM Approach for SARS-CoV-2 Detection" Technologies 13, no. 3: 114. https://doi.org/10.3390/technologies13030114
APA StyleMunthala, D., Sonklin, T., Chanlek, N., Mathur, A., Roy, S., Avasthi, D. K., Suksaweang, S., & Pojprapai, S. (2025). Portable DNA Probe Detector and a New Dry-QCM Approach for SARS-CoV-2 Detection. Technologies, 13(3), 114. https://doi.org/10.3390/technologies13030114






