Using Microwave Energy to Synthesize Light Weight/Energy Saving Magnesium Based Materials: A Review
Abstract
:1. Introduction
1.1. Brief History and Applications of Microwave Heating
1.2. Fundamentals of Microwave Heating

1.3. Microwave Sintering of Metallic Composites
2. Experimental Section
| Element | Size | Supplier |
|---|---|---|
| Magnesium | 60–300 µm | Merck KGaA, Germany |
| SiC | 25 µm | Amet |
| β-SiC | 45–55 nm | Nanostructured & Amorphous Materials, USA |
| Cu | 50 nm | Argonide Corporation, USA |
| Ni | 20 nm | Nanostructured & Amorphous Materials, USA |
| Al2O3 | 0.3 µm | Baikowski, USA |
| Al2O3 | 50 nm | Baikowski, USA |
| Y2O3 | 30–50 nm | Inframat Advanced Materials, USA |
| ZrO2 | 51–65 nm | Nanostructured & Amorphous Materials, USA |
| Ni60Nb40 | - | Prepared by mechanically alloying element Ni and Nb metals |
3. Results and Discussion
3.1. Hybrid Microwave Sintering Technique





| Material | Heating Unit | Power kW | Time h (mins) | Energy Consumption kWh | Energy Savings % |
|---|---|---|---|---|---|
| Magnesium | Tube furnace (Carbolite CTF15/75) | 6 | 0.82 (49) | 4.92 | 86 |
| Microwave (Sharp magnetron) | 1.6 * | 0.42 (25) | 0.67 |
3.2. Physical Properties
| Materials | Theoretical ρ | Experimental ρ | Porosity | Grain Size | Aspect Ratio |
|---|---|---|---|---|---|
| (g/cm3) | (g/cm3) | (%) | (μm) | ||
| Mg Conv | 1.740 | 1.737 ± 0.002 | 0.15 | 33 ± 8 | 1.5 ± 0.3 |
| Mg MW (32 min) | 1.734 ± 0.002 | 0.33 | 36 ± 9 | 1.4 ± 0.3 | |
| Mg MW (25 min) | 1.737 ± 0.001 | 0.17 | 27 ± 7 | 1.6 ± 0.4 | |
| Mg MW (13 min) | 1.738 ± 0.007 | 0.13 | 20 ± 3 | 1.4 ± 0.1 | |
| Composites containing microwave susceptors reinforcement | |||||
| Mg 10 SiC (38 µm) * | 1.888 | 1.865 ± 0.004 | 1.22 | - | - |
| Mg 0.35 β-SiC (45–55 nm) * | 1.745 | 1.735 ± 0.003 | 0.58 | - | - |
| Mg 0.5 βSiC (45–55 nm) * | 1.747 | 1.739 ± 0.002 | 0.48 | - | - |
| Mg 1.0 β-SiC (45–55 nm) * | 1.755 | 1.753 ± 0.007 | 0.11 | - | - |
| Composites containing microwave transparent reinforcements | |||||
| Mg 0.3 Al2O3 (50 nm) * | 1.747 | 1.741 ± 0.004 | 0.32 | - | - |
| Mg 0.6 Al2O3 (50 nm) * | 1.753 | 1.742 ± 0.008 | 0.67 | 24 ± 4 | 1.5 ± 0.4 |
| Mg 1.0 Al2O3 (50 nm) * | 1.762 | 1.748 ± 0.010 | 0.83 | 15 ± 3 | 1.5 ± 0.3 |
| Mg 0.17 Y2O3 (40 nm) * | 1.746 | 1.73 ± 0.01 | 0.87 | 19 ± 3 | 1.4 ± 0.2 |
| Mg 0.7 Y2O3 (40 nm) * | 1.763 | 1.757 ± 0.006 | 0.35 | 18 ± 3 | 1.4 ± 0.2 |
| Mg 0.3 ZrO2 (51–65 nm) * | - | - | - | 24 ± 7 | - |
| Mg 1.0 ZrO2 (51–65 nm) * | - | - | - | 25 ± 4 | - |
| Composites containing metallic reinforcements | |||||
| Mg 0.3 Cu (50 nm) * | 1.762 | 1.758 ± 0.002 | 0.19 | 17 ± 5 | 1.5 ± 0.3 |
| Mg 0.6 Cu (50 nm) * | 1.783 | 1.776 ± 0.006 | 0.41 | 15 ± 4 | 1.5 ± 0.3 |
| Mg 1.0 Cu (50 nm) * | 1.812 | 1.809 ± 0.007 | 0.13 | 15 ± 4 | 1.5 ± 0.3 |
| Composites containing hybrid reinforcements | |||||
| Mg 0.3 ZrO2 0.7 Cu | - | - | - | 9 ± 2 | - |
| Mg 0.7 Y2O3 0.3 Cu | 1.784 | 1.775 ± 0.001 | 0.45 | 9 ± 5 | 1.6 ± 0.4 |
| Mg 0.7 Y2O3 0.6 Cu | 1.806 | 1.792 ± 0.004 | 0.77 | 9 ± 4 | 1.5 ± 0.3 |
| Mg 0.7 Y2O3 0.3 Ni | 1.785 | 1.778 ± 0.002 | 0.34 | 9 ± 3 | 1.4 ± 0.3 |
| Mg 0.7 Y2O3 0.6 Ni | 1.806 | 1.802 ± 0.002 | 0.21 | 6 ± 2 | 1.4 ± 0.3 |
| Mg 0.7 Y2O3 1.0 Ni | 1.835 | 1.829 ± 0.002 | 0.30 | 5 ± 2 | 1.5 ± 0.3 |
3.3. Microstructure
3.4. Mechanical Properties
| Materials | Processing Method | Microhardness HV | 0.2% YS (MPa) | UTS (MPa) | Failure Strain (%) | Ref. |
|---|---|---|---|---|---|---|
| Tensile properties | ||||||
| Mg Conv | Tube furnace | 37 ± 1 | 105 ± 0 | 150 ± 1 | 5.0 ± 0.7 | [18] |
| Mg MW (32 min) | Hybrid microwave sintering | 36 ± 2 | 116 ± 17 | 186 ± 21 | 11.3 ± 1.0 | |
| Mg MW (25 min) | 40 ± 1 | 121 ± 2 | 176 ± 2 | 5.4 ± 0.7 | ||
| Mg MW (13 min) | 47 ± 2 | 134 ± 7 | 193 ± 1 | 6.9 ± 2.5 | ||
| Mg/0.3 Al2O3 | 48 ± 3 | 119 ± 7 | 175 ± 8 | 7.5 ± 0.2 | [21] | |
| Mg/0.6 Al2O3 | 54 ± 3 | 130 ± 5 | 180 ± 7 | 7.4 ± 0.3 | ||
| Mg/1.0 Al2O3 | 60 ± 4 | 154 ± 5 | 213 ± 12 | 6.3 ± 0.4 | ||
| Mg/0.17 Y2O3 | 38 ± 0 | 144 ± 2 | 214 ± 4 | 8.0 ± 2.8 | [22] | |
| Mg/0.7 Y2O3 | 45 ± 2 | 157 ± 10 | 244 ± 1 | 8.6 ± 1.2 | ||
| Mg/0.3 ZrO2 | 40 ± 1 | 85 ± 8 | 139 ± 8 | 8.1 ± 1.6 | [23] | |
| Mg/0.6 ZrO2 | 42 ± 2 | 117 ± 11 | 182 ± 14 | 9.4 ± 2.7 | ||
| Mg/1.0 ZrO2 | 42 ± 2 | 98 ± 6 | 158 ± 12 | 8.6 ± 2.2 | ||
| Mg 10 SiC | 44 ± 1 | 140 ± 2 | 165 ± 2 | 1.5 ± 0.8 | [12] | |
| Mg/0.3 β-SiC | 40 ± 1 | 132 ± 14 | 194 ± 11 | 6.3 ± 1.0 | [20] | |
| Mg/0.5 β-SiC | 42 ± 1 | 144 ± 12 | 194 ± 10 | 7.0 ± 2.0 | ||
| Mg/1.0 β-SiC | 43 ± 2 | 157 ± 22 | 203 ± 22 | 7.6 ± 1.5 | ||
| Mg/0.3 Cu | 49 ± 1 | 188 ± 13 | 218 ± 11 | 5.9 ± 1.1 | [24] | |
| Mg/0.6 Cu | 52 ± 2 | 237 ± 24 | 286 ± 8 | 5.4 ± 1.2 | ||
| Mg/1.0 Cu | 60 ± 3 | 194 ± 17 | 221 ± 17 | 2.9 ± 0.4 | ||
| Mg (0.3 ZrO2 + 0.7 Cu) | 48 ± 1 | 196 ± 16 | 249 ± 8 | 8.2 ± 1.1 | [23] | |
| Mg (0.6 ZrO2 + 0.4 Cu) | 50 ± 1 | 139 ± 22 | 193 ± 21 | 11.4 ± 2.9 | ||
| Mg (0.7 Y2O3 + 0.3 Ni) | 54 ± 4 | 221 ± 7 | 244 ± 1 | 9.0 ± 0.9 | [26] | |
| Mg (0.7 Y2O3 + 0.6 Ni) | 60 ± 4 | 232 ± 8 | 262 ± 6 | 9.5 ± 0.9 | ||
| Mg (0.7 Y2O3 + 1.0Ni) | 63 ± 4 | 228 ± 8 | 272 ± 2 | 5.5 ± 0.7 | ||
| Compressive properties | ||||||
| Pure Mg | Hybrid microwave sintering | - | 109 ± 4 | 284 ± 11 | 23 ± 3 | [23] |
| Mg/0.3 ZrO2 | 40 ± 1 | 109 ± 6 | 273 ± 13 | 19 ± 1 | ||
| Mg/1.0 ZrO2 | 42 ± 2 | 109 ± 5 | 262 ± 18 | 19 ± 4 | ||
| Mg/(0.3 ZrO2 + 0.7 Cu) | 48 ± 1 | 124 ± 7 | 352 ± 18 | 12 ± 3 | ||
| Pure Mg | 43 ± 2 | 70 ± 6 | 265 ± 8 | 16.2 ± 0.8 | [10] | |
| Mg/3 Ni60Nb40 | 62 ± 4 | 85 ± 4 | 283 ± 10 | 17.6 ± 1.1 | ||
| Mg/5 Ni60Nb40 | 84 ± 5 | 130 ± 11 | 320 ± 11 | 18.4 ± 1.3 | ||
| Mg/10 Ni60Nb40 | 95 ± 5 | 90 ± 7 | 322 ± 10 | 17.2 ± 1.6 | ||
| Tensile properties | ||||||
| Pure Mg | PM + hot extrusion | - | 215 | 230 | 7.0 | [28] |
| Mg/3.0 SiC (mixed) | - | 180 | 220 | 3.0 | ||
| Mg/3.0 SiC (milled) | - | 220 | 280 | 2.0 | ||
| Pure Mg | PM + hot extrusion | - | 134 ± 11 | 190 ± 10 | 4.6 ± 0.6 | [31] |
| Mg/0.5 Al | - | 218 ± 16 | 271 ± 11 | 6.2 ± 0.9 | ||
| Mg/1.0 Al | - | 185 ± 9 | 226 ± 12 | 3.3 ± 1.0 | ||
3.5. Fractography
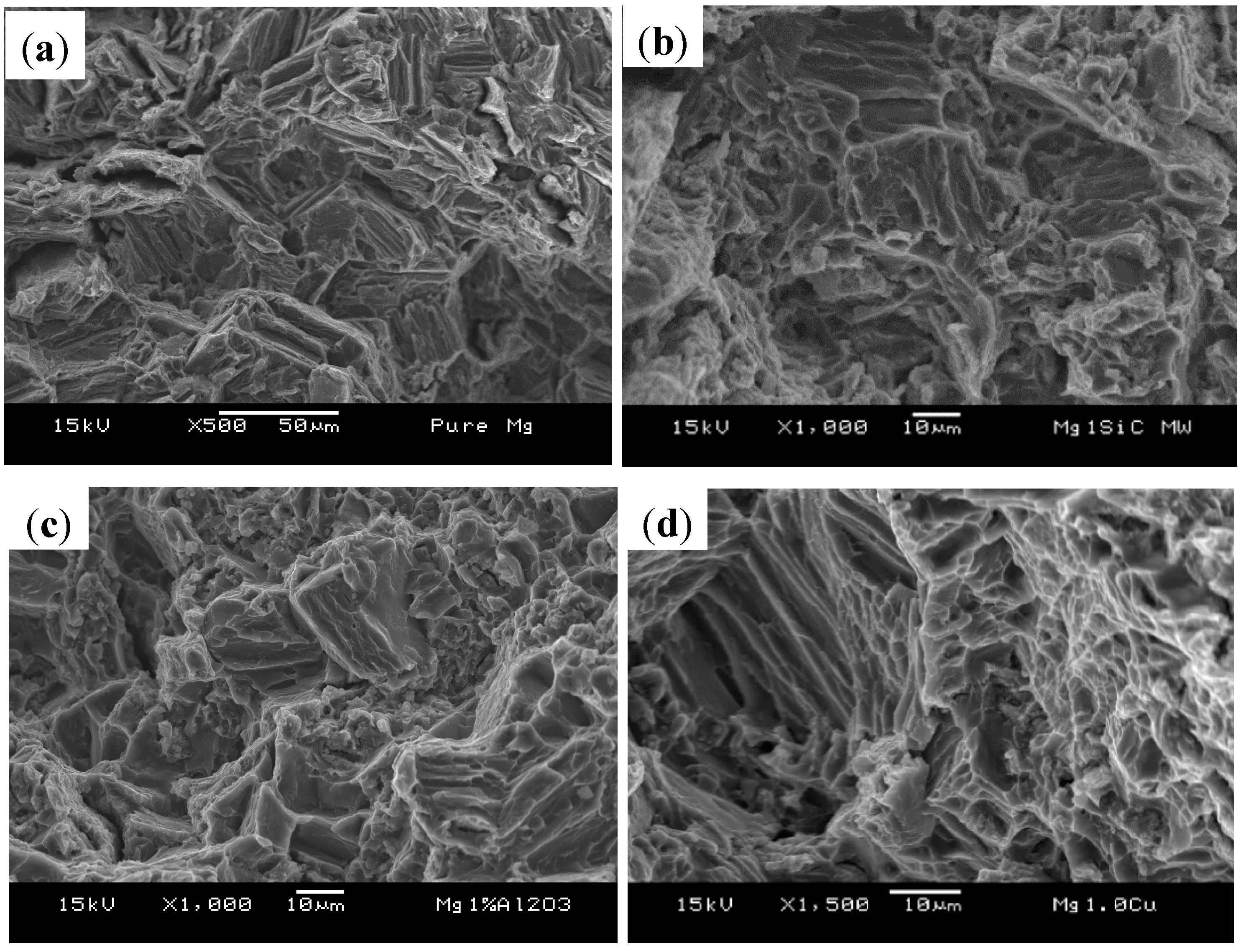

4. Conclusions
- (1)
- It has been shown that pure magnesium and magnesium composites can be synthesized using a hybrid microwave sintering technique that utilizes microwave energy and heat from external susceptors for sintering.
- (2)
- Significant reduction in sintering time was achieved through rapid heating, higher sintering temperature and the elimination of holding time without any detrimental effect on the end properties of the sintered magnesium materials.
- (3)
- Potential cost savings can be realized with the reduction in sintering time and sintering under atmospheric condition without the need for an inert atmosphere.
- (4)
- Microstructural characterization revealed finer microstructure for microwave-sintered magnesium when compared to conventionally sintered magnesium.
- (5)
- The nano-size reinforcements formed a continuous network along the grain boundaries of the matrix.
- (6)
- Mechanical characterization revealed an increase in hardness, 0.2% YS and UTS of magnesium with the addition of nano-size reinforcements. Failure strain was improved with the addition of SiC and Al2O3 ceramic reinforcements but displayed the opposite trend with the addition of metallic copper as reinforcement.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, M.; Wong, W.L.E. Microwaves and Metals; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2007. [Google Scholar]
- Sobol, H.; Fellow, L.; Tomiyasu, K. Milestones of microwaves. IEEE Trans. Microw. Theory Techn. 2002, 50, 594–611. [Google Scholar] [CrossRef]
- National Research Council. Microwave Processing of Materials; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Kasap, S.O. Principles of Electrical Engineering Materials and Devices, 2nd edition; McGraw-Hill Higher Education: New York, NY, USA, 2000. [Google Scholar]
- Walkiewicz, J.W.; Kazonich, G; McGill, S.L. Microwave heating characteristics of selected minerals and compounds. Miner. Metall. Process. 1988, 5, 39–42. [Google Scholar]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Saitou, K. Microwave sintering of iron, cobalt, nickel, copper and stainless steel powders. Scr. Mater. 2006, 54, 875–879. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Gupta, M.; Wong, W.L.E. Enhancing overall mechanical performance of metallic materials using two-directional microwave assisted rapid sintering. Scr. Mater. 2005, 52, 479–483. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Sahu, S.; Sankaranarayanan, S.; Gupta, S.; Gupta, M. Development of novel Mg–Ni60Nb40 amorphous particle reinforced composites with enhanced hardness and compressive response. Mater. Des. 2014, 53, 849–855. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Characteristics of aluminum and magnesium based nanocomposites processed using hybrid microwave sintering. J. Microw. Power Electromagn. Energy 2010, 44, 4–27. [Google Scholar] [PubMed]
- Wong, W.L.E. Development of Advanced Materials Using Microwaves; National University of Singapore: Singapore, 2007. [Google Scholar]
- Tun, K.S.; Gupta, M. Effect of heating rate during hybrid microwave sintering on the tensile properties of magnesium and Mg/Y2O3 nanocomposite. J. Alloys Compd. 2008, 466, 140–145. [Google Scholar] [CrossRef]
- German, R.M. Sintering Theory and Practice; John Wiley & Sons Inc.: New York, NY, USA, 1996; p. 568. [Google Scholar]
- Ripley, E.B.; Eggleston, P.A.; White, T.L. Direct microwave coupling to metals at elevated temperature. In Third World Congress on Microwave and Radio Frequency Processing; Folz, D.C., Booske, J.H., Clarke, D.E., Gerling, J.F., Eds.; The Microwave Working Group Ltd.: Arnold, MD, USA, 2002; pp. 241–246. [Google Scholar]
- Askeland, D.; Fulay, P.; Wright, W. The Science and Engineering of Materials, SI Edition ed; Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- Takayama, S.; Saito, Y.; Sato, M.; Nagasaka, T.; Muroga, T.; Ninomiya, Y. Sintering Behavior of Metal Powders Involving Microwave-Enhanced Chemical Reaction. Jpn. J. Appl. Phys. 2006, 45, 1816. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Tun, K.S.; Gupta, M. Microwave processing of magnesium based materials: A review. Met. Mater. 2011, 49, 219–231. [Google Scholar]
- Wong, W.L.E.; Gupta, M. Effect of hybrid length scales ( micro + nano ) of SiC reinforcement on the properties of magnesium. Solid State Phenom. 2006, 111, 91–94. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Simultaneously improving strength and ductility of magnesium using nano-size SiC particulates and microwaves. Adv. Eng. Mater. 2006, 8, 735–740. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Improving overall mechanical performance of magnesium using nano-alumina reinforcement and energy efficient microwave assisted processing route. Adv. Eng. Mater. 2007, 9, 902–909. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Improving mechanical properties of magnesium using nano-yttria reinforcement and microwave assisted powder metallurgy method. Compos. Sci. Technol. 2007, 67, 2657–2664. [Google Scholar] [CrossRef]
- Tun, K.S.; Wong, W.L.E.; Nguyen, Q.; Gupta, M. Tensile and compressive responses of ceramic and metallic nanoparticle reinforced Mg composites. Materials. 2013, 6, 1826–1839. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Development of Mg/Cu nanocomposites using microwave assisted rapid sintering. Compos. Sci. Technol. 2007, 67, 1541–1552. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M.; Srivatsan, T.S. Investigating influence of hybrid (yttria + copper) nanoparticulate reinforcements on microstructural development and tensile response of magnesium. Mater. Sci. Technol. 2010, 26, 87–94. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Development of magnesium/(yttria+nickel) hybrid nanocomposites using hybrid microwave sintering: Microstructure and tensile properties. J. Alloys Compd. 2009, 487, 76–82. [Google Scholar] [CrossRef]
- Cheng, J.; Roy, R.; Agrawal, D. Radically different effects on materials by separated microwave electric and magnetic fields. Mater. Res. Innov. 2002, 170–177. [Google Scholar]
- Ferkel, H.; Mordike, B.L. Magnesium strengthened by SiC nanoparticles. Mater. Sci. Eng. A 2001, 298, 193–199. [Google Scholar] [CrossRef]
- Kang, Y.-C.; Chan, S.L.-I. Tensile properties of nanometric Al2O3 particulate-reinforced aluminum matrix composites. Mater. Chem. Phys. 2004, 85, 438–443. [Google Scholar] [CrossRef]
- Anklekar, R.M.; Agrawal, D.K.; Roy, R. Microwave sintering and mechanical properties of PM copper steel. Powder Metall. 2001, 44, 355–362. [Google Scholar] [CrossRef]
- Zhong, X.L.; Wong, W.L.E.; Gupta, M. Enhancing strength and ductility of magnesium by integrating it with aluminum nanoparticles. Acta Mater. 2007, 55, 6338–6344. [Google Scholar] [CrossRef]
- Agnew, S.R.; Duygulu, Ö. Plastic anisotropy and the role of non-basal slip in magnesium alloy AZ31B. Int. J. Plast. 2005, 21, 1161–1193. [Google Scholar] [CrossRef]
- Neite, G.; Kubota, K.; Higashi, K.; Hehmann, F. Magnesium-Based Alloys. In Materials Science and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, W.L.E.; Gupta, M. Using Microwave Energy to Synthesize Light Weight/Energy Saving Magnesium Based Materials: A Review. Technologies 2015, 3, 1-18. https://doi.org/10.3390/technologies3010001
Wong WLE, Gupta M. Using Microwave Energy to Synthesize Light Weight/Energy Saving Magnesium Based Materials: A Review. Technologies. 2015; 3(1):1-18. https://doi.org/10.3390/technologies3010001
Chicago/Turabian StyleWong, Wai Leong Eugene, and Manoj Gupta. 2015. "Using Microwave Energy to Synthesize Light Weight/Energy Saving Magnesium Based Materials: A Review" Technologies 3, no. 1: 1-18. https://doi.org/10.3390/technologies3010001
APA StyleWong, W. L. E., & Gupta, M. (2015). Using Microwave Energy to Synthesize Light Weight/Energy Saving Magnesium Based Materials: A Review. Technologies, 3(1), 1-18. https://doi.org/10.3390/technologies3010001








