Detection of Odorant Molecules in the Gaseous Phase Using ?-, ?-, and ?-Cyclodextrin Films on a Quartz Crystal Microbalance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of α-, β-, and γ-CD Films on QCM Sensor Surfaces
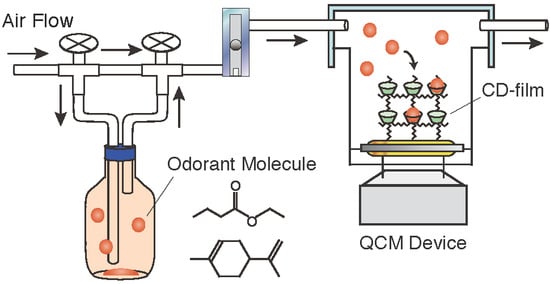
2.3. QCM Setup and Odorant Molecule Detection
3. Results and Discussion
3.1. Reaction Time for CD-Film Preparation
3.2. QCM Response during Odorant-Molecule Detection
3.3. Concentration Dependence of Odorant-Molecule Adsorption
3.4. Response to Different Odorant Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, J.W.; Bartlet, P.N. A brief history of electronic noses. Sens. Actuators B 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 114, 103–111. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gebicki, J.; Kamysz, W. Bioelectronic nose: Current status and perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile organic compound optical fiber sensors: A review. Sensors 2006, 6, 1440–1465. [Google Scholar] [CrossRef]
- Tasaltin, C.; Basarir, F. Preparation of flexible VOC sensor based on carbon nanotubes and gold nanoparticles. Sens. Actuators B 2014, 194, 173–179. [Google Scholar] [CrossRef]
- Hyodo, T.; Hashimoto, T.; Ueda, T.; Nakagoe, O.; Kamada, K.; Sasahara, T.; Tanabe, S.; Shimizu, T. Adsorption/combustion-type VOC sensors employing mesoporous γ-alumina co-loaded with noble-metal and oxide. Sens. Actuators B 2015, 220, 1091–1104. [Google Scholar] [CrossRef]
- Song, J.; Fomey, C.F. Flavor volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Plotto, A.; Goodner, K. Shelf-life versus flavour-life for fruits and vegetables: How to evaluate this complex trait. Stewart Postharvest Rev. 2007, 1, 1–10. [Google Scholar] [CrossRef]
- Ding, B.; Kim, J.; Miyazaki, Y.; Shiratori, S. Electrospun nanofibrous membranes coated quartz crystal microbalance as gas sensor for NH3 detection. Sens. Actuators B 2004, 101, 373–380. [Google Scholar] [CrossRef]
- Ding, B.; Yamazaki, M.; Shiratori, S. Electrospun fibrous polyacrylic acid membrane-based gas sensors. Sens. Actuators B 2005, 106, 477–483. [Google Scholar] [CrossRef]
- Manesh, K.; Gopalan, A.; Lee, K.; Santhosh, P.; Song, K.; Lee, D. Fabrication of functional nanofibrous ammonia sensor. IEEE Trans. Nanotechnol. 2007, 6, 513–518. [Google Scholar] [CrossRef]
- Pinto, N.; Ramos, I.; Rojas, R.; Wang, P.; Johnson, A., Jr. Electric response of isolated electrospun polyaniline nanofibers to vapors of aliphatic alcohols. Sens. Actuators B 2008, 129, 621–627. [Google Scholar] [CrossRef]
- Luoh, R.; Hahn, H.T. Electrospun nanocomposite fiber mats as gas sensors. Compos. Sci. Technol. 2006, 66, 2436–2441. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas sensors based on electrospun nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Aizawa, H.; Park, J.W. Quartz crystal microbalance immunosensor for highly sensitive 2,3,7,8-tetrachlorodibenzo-p-dioxin detection in fly ash from municipal solid waste incinerators. Analyst 2005, 130, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, F.; Zhang, J.; Gong, H. A room temperature indium tin oxide/quartz crystal microbalance gas sensor for nitric oxide. Sens. Actuators B 2003, 93, 175–180. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Finklea, H.O. Quartz crystal microbalance sensor for organic vapor detection based on molecularly imprinted polymers. Anal. Chem. 2003, 75, 5387–5393. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, S.M.; Yoshino, A.; Nakamoto, T.; Moriizumi, T. Odor approximation of fruit flavors using a QCM odor sensing system. Sens. Actuators B 2007, 123, 1101–1106. [Google Scholar] [CrossRef]
- Saito, H.; Suzuki, Y.; Gessei, T.; Miyajima, K.; Arakawa, T.; Mitsubayashi, K. Bioelectronic sniffer (biosniffer) based on enzyme inhibition of butyrylcholinesterase for toluene detection. Sens. Mater. 2014, 26, 121–129. [Google Scholar]
- Misawa, N.; Mitsuno, H.; Kanzaki, R.; Takeuchi, S. Highly sensitive and selective odorant sensor using living cells expressing insect olfactory receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 15340–15344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.F.; Syu, M.J.; Teng, H.S.; Chou, S.K.; Chang, Y.S. Preparation and identification of cyclodextrin polymer thin film for quartz crystal microbalance sensing of benzene, toluene, and p-xylene. Sens. Actuators B 2008, 132, 319–326. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Allylated cyclodextrins as effective affinity materials in chemical sensing of volatile aromatic hydrocarbons using an optical planar Bragg grating sensor. Anal. Chem. Acta 2013, 791, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ren, F.; Gao, H.; Wu, Q.; Zhu, F.; Tang, B.Z. Bioinspired fluorescent nanosheets for rapid and sensitive detection of organic pollutants in water. ACS Sens. 2016, 1, 1272–1278. [Google Scholar] [CrossRef]
- Celebioglua, A.; Sen, H.S.; Durgun, E.; Uyar, T. Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemoshpere 2016, 144, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Furusawa, H.; Tsuyuki, Y.; Takahashi, S.; Okahata, Y. In-situ monitoring of structural changes during formation of 30S translation initiation complex by energy dissipation measurement using 27-MHz quartz-crystal microbalance. Anal. Chem. 2014, 86, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, H.; Nakayama, H.; Funasaki, M.; Okahata, Y. Kinetic characterization of small DNA-binding molecules interacting with a DNA strand on a quartz crystal microbalance. Anal. Biochem. 2016, 492, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, H.; Sekine, T.; Ozeki, T. Hydration and viscoelastic properties of high- and low-density polymer brushes using a quartz-crystal microbalance based on admittance analysis (QCM-A). Macromolecules 2016, 49, 3463–3470. [Google Scholar] [CrossRef]
- Komiyama, M.; Sugiura, I.; Hirai, H. Immobilized β-cyclodextrin catalyst for selective synthesis of 4-hydroxybenzoic acid. Polym. J. 1985, 17, 1225–1227. [Google Scholar] [CrossRef]
- Pérez, A.G.; Cert, A.; Ríos, J.J.; Olías, J.M. Free and glycosidically bound volatile compounds from two banana cultivars: Valery and Pequeña Enana. J. Agric. Food Chem. 1997, 45, 4393–4397. [Google Scholar] [CrossRef]
- Lebrilla, C.B. The gas-phase chemistry of cyclodextrin inclusion complexes. Acc. Chem. Res. 2001, 34, 653–661. [Google Scholar] [CrossRef] [PubMed]





| Film | ∆mfilm (ng cm−2) | CD Content *1 [normalized] *2 (nmol cm−2) | Ethyl Butyrate *3 | Limonene *3 | ||||
|---|---|---|---|---|---|---|---|---|
| ∆m *4 [normalized] *2 (ng cm−2) | Molecule [normalized] *2 (nmol cm−2) | Occupancy *5 (normalized) *6 | ∆m *4 [normalized] *2 (ng cm−2) | Molecule [normalized] *2 (nmol cm−2) | Occupancy *5 (normalized) *6 | |||
| α-CD | 1500 | 1.23 [2.46] | 14.9 [29.8] | 0.128 [0.256] | 0.10 (1) | 53.3 [107] | 0.391 [0.783] | 0.32 (1) |
| β-CD | 3130 | 2.21 [2.12] | 102 [98.4] | 0.875 [0.847] | 0.40 (4.0) | 283 [274] | 2.08 [2.01] | 0.95 (3.0) |
| γ-CD | 4230 | 2.61 [1.85] | 51.5 [36.8] | 0.443 [0.317] | 0.17 (1.7) | 144 [103] | 1.06 [0.757] | 0.41 (1.3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, K.; Furusawa, H.; Nagamine, K.; Tokito, S. Detection of Odorant Molecules in the Gaseous Phase Using ?-, ?-, and ?-Cyclodextrin Films on a Quartz Crystal Microbalance. Technologies 2018, 6, 63. https://doi.org/10.3390/technologies6030063
Sasaki K, Furusawa H, Nagamine K, Tokito S. Detection of Odorant Molecules in the Gaseous Phase Using ?-, ?-, and ?-Cyclodextrin Films on a Quartz Crystal Microbalance. Technologies. 2018; 6(3):63. https://doi.org/10.3390/technologies6030063
Chicago/Turabian StyleSasaki, Kai, Hiroyuki Furusawa, Kuniaki Nagamine, and Shizuo Tokito. 2018. "Detection of Odorant Molecules in the Gaseous Phase Using ?-, ?-, and ?-Cyclodextrin Films on a Quartz Crystal Microbalance" Technologies 6, no. 3: 63. https://doi.org/10.3390/technologies6030063
APA StyleSasaki, K., Furusawa, H., Nagamine, K., & Tokito, S. (2018). Detection of Odorant Molecules in the Gaseous Phase Using ?-, ?-, and ?-Cyclodextrin Films on a Quartz Crystal Microbalance. Technologies, 6(3), 63. https://doi.org/10.3390/technologies6030063