Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
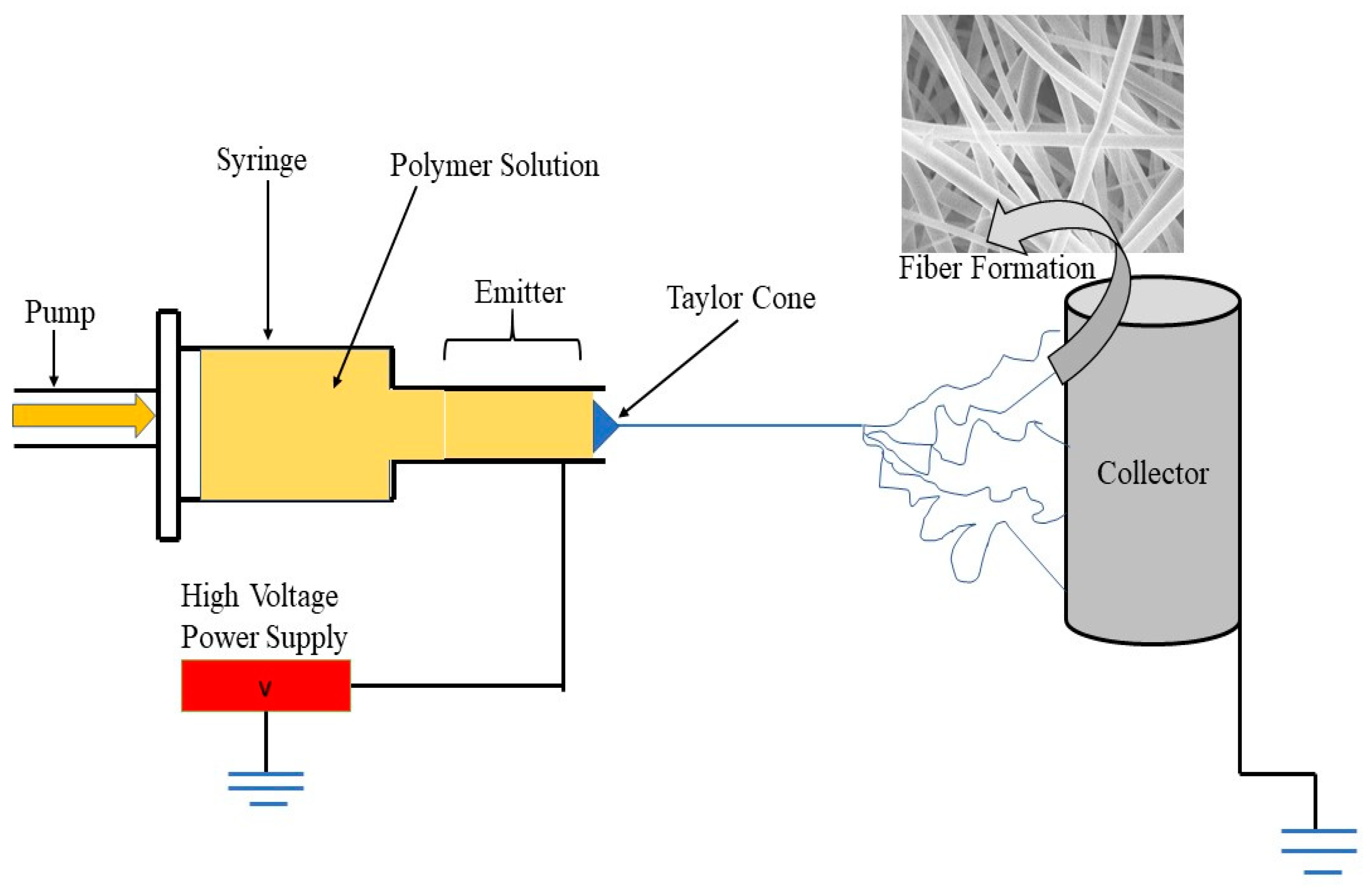
2.2. Electrospinning Setup
2.3. Preparation of the TiO2 Solution
2.4. Characterization
3. Results and Discussion
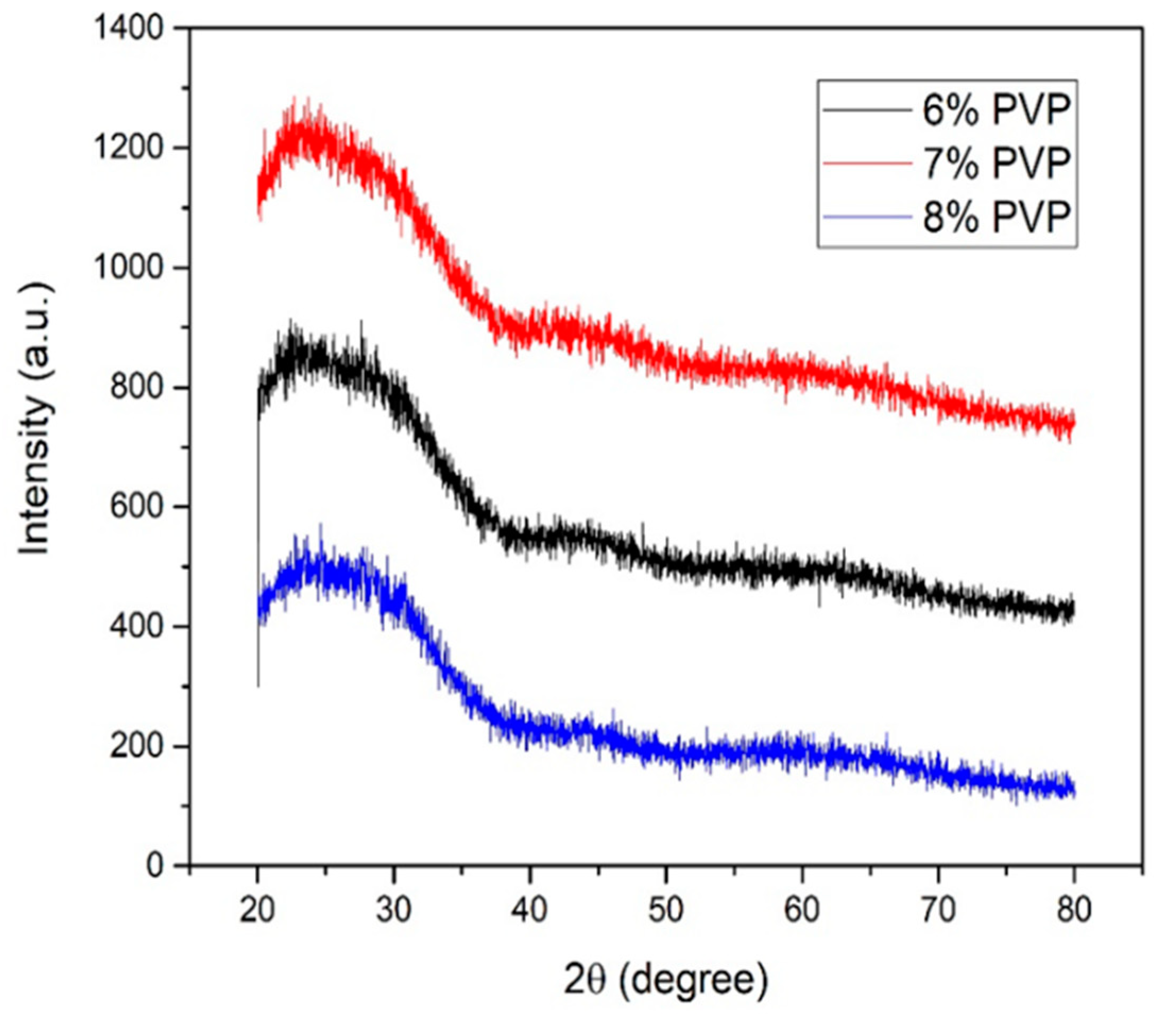
3.1. X-ray Analysis of TiO2/PVP Nanofibers
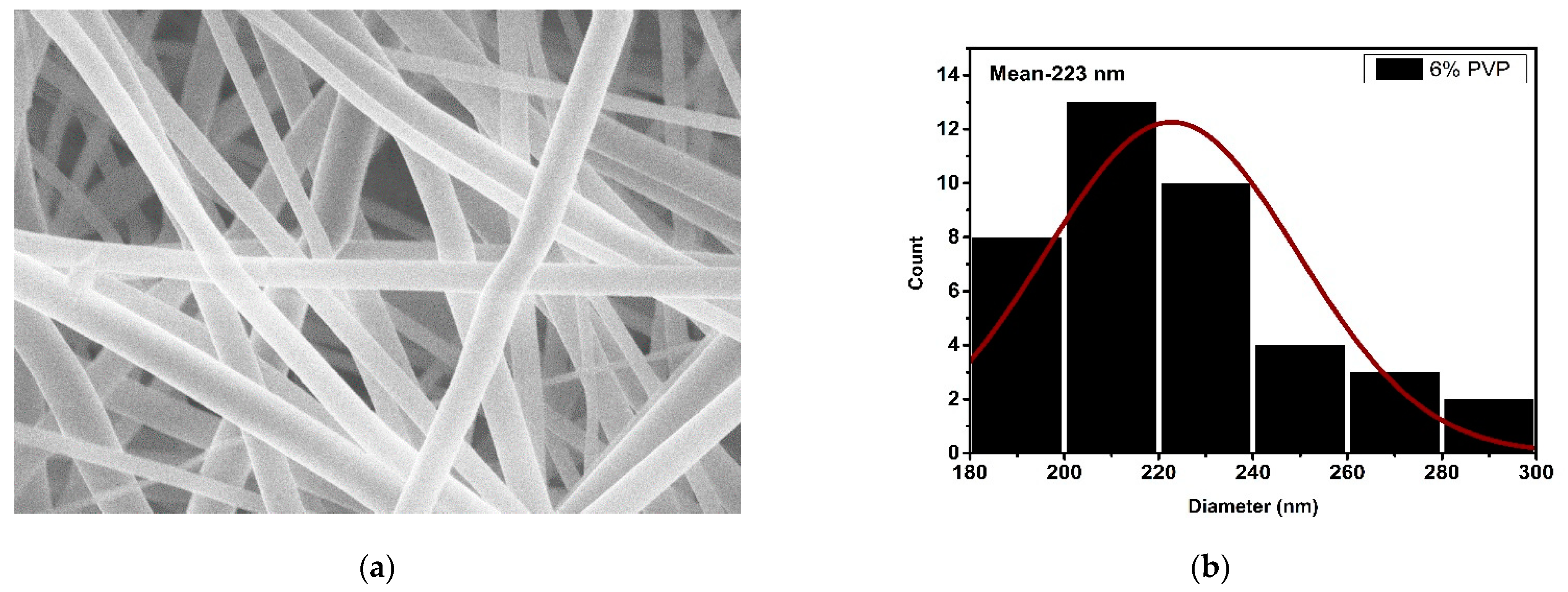
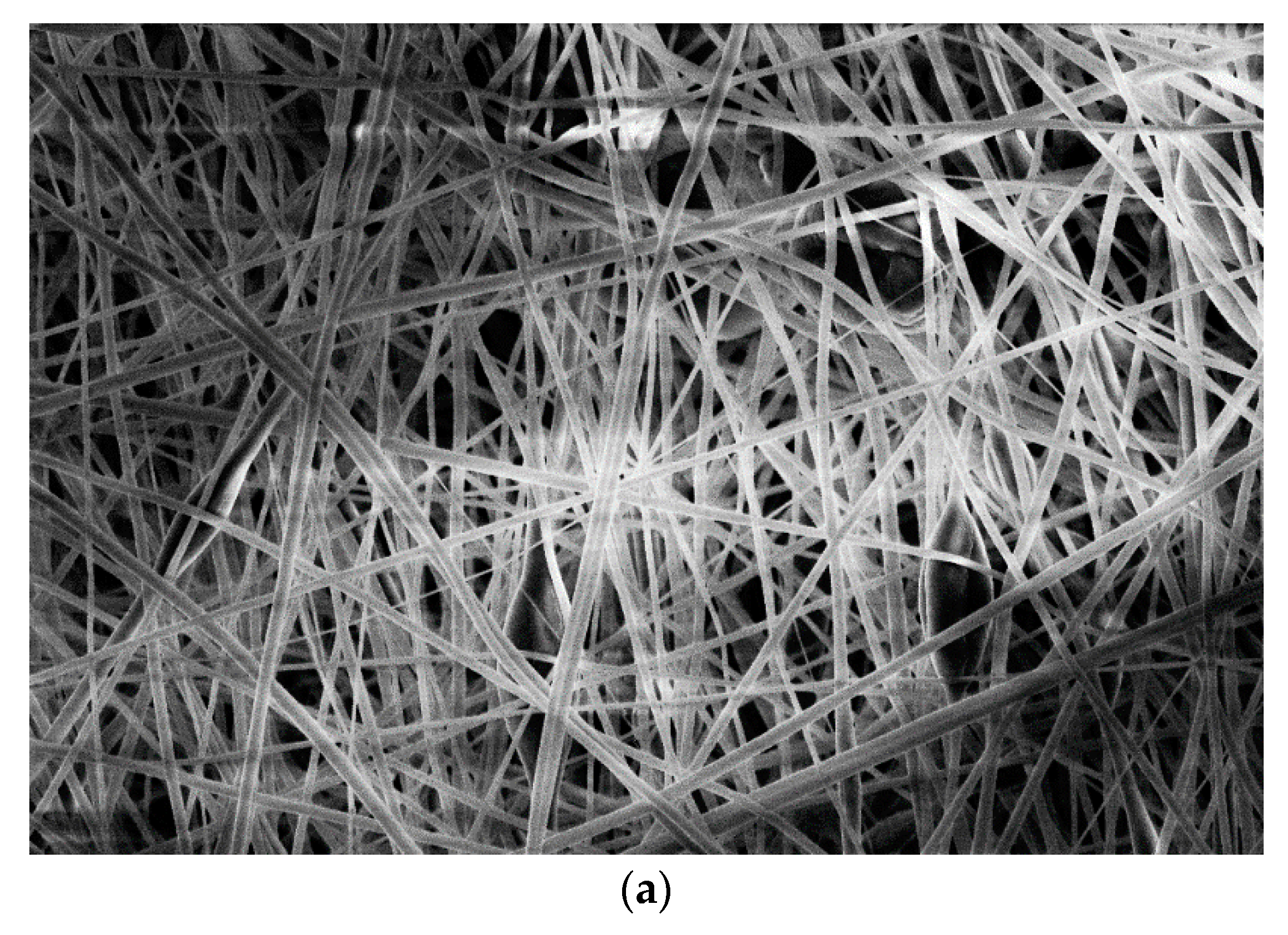
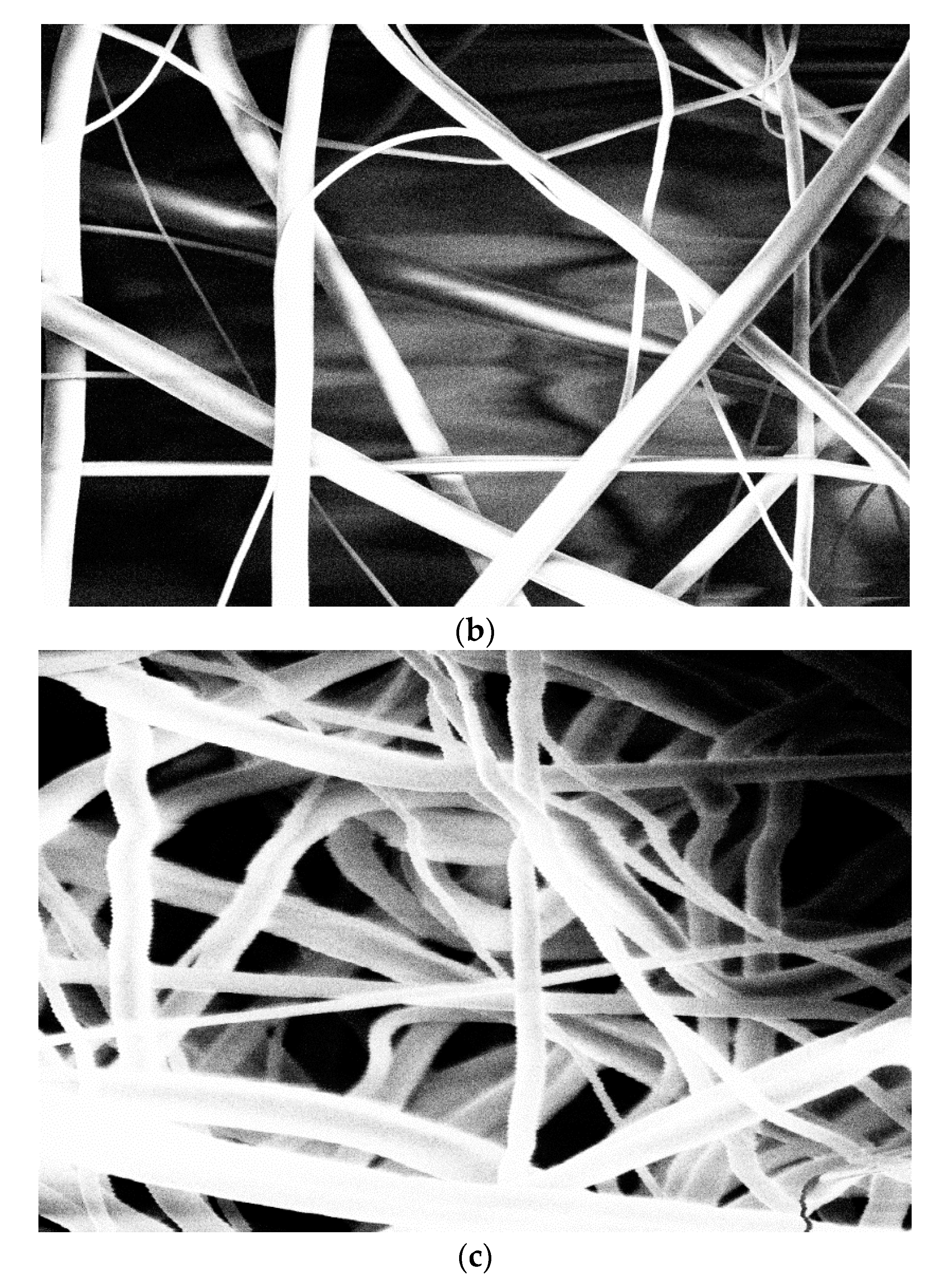
3.2. Microscopic Analysis
3.3. Surface Area and Porosity Analysis
3.4. Adsorption-Desorption Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stoye, E. China coronavirus: How many papers have been published? Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: Covid19.who.int (accessed on 14 September 2021).
- Ivanoska-Dacikj, A.; Stachewicz, U. Smart textiles and wearable technologies–opportunities offered in the fight against pandemics in relation to current COVID-19 state. Rev. Adv. Mater. Sci. 2020, 59, 487–505. [Google Scholar] [CrossRef]
- De Sio, L.; Ding, B.; Focsan, M.; Kogermann, K.; Pascoal-Faria, P.; Petronela, F.; Mitchell, G.; Zussman, E.; Pierini, F. Personalized reusable face masks with smart nano-assisted destruction of pathogens for COVID-19: A visionary road. Chem.–A Eur. J. 2021, 27, 6112–6130. [Google Scholar] [CrossRef] [PubMed]
- Rinoldi, C.; Zargarian, S.S.; Nakielski, P.; Li, X.; Liguori, A.; Petronella, F.; Presutti, D.; Wang, Q.; Costantini, M.; De Sio, L.; et al. Nanotechnology-assisted RNA delivery: From nucleic acid therapeutics to COVID-19 vaccines. Small Methods 2021, 5, 2100402. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. A Collect. Rev. Nat. J. 2010, 452, 337–346. [Google Scholar]
- Tlili, I.; Timoumi, Y.; Nasrallah, S.B. Numerical simulation and losses analysis in a Stirling engine. Int. J. Heat Technol. 2006, 24, 97–105. [Google Scholar]
- Dersch, R.; Liu, T.; Schaper, A.K.; Greiner, A.; Wendorff, J.H. Electrospun nanofibers: Internal structure and intrinsic orientation. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 545–553. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, K.H.; Khil, M.S.; Ho, Y.S.; Kim, H.Y. The effect of molecular weight and the linear velocity of drum surface on the properties of electrospun poly(ethylene terephthalate) nonwovens. Fibers Polym. 2004, 5, 122–127. [Google Scholar] [CrossRef]
- Kansara, S.; Patel, S.; Gan, Y.X.; Jaimes, G.; Gan, J.B. Dye adsorption and electrical property of oxide-loaded carbon fiber made by electrospinning and hydrothermal treatment. Fibers 2019, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Huang, Y.; Zhang, D.; Chen, S. Electrospinning fabricating Au/TiO2 network-like nanofibers as visible light activated photocatalyst. Sci. Rep. 2019, 9, 8008. [Google Scholar] [CrossRef]
- Essa, W.; Yasin, S.; Saeed, I.; Ali, G. Nanofiber-based face masks and respirators as COVID-19 protection: A review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, S.; Aftab, F.; Rauf, A.; Duran, H.; Kirchhoff, K.; Arshad, S.N. P-doped TiO2 nanofibers decorated with Ag nanoparticles for enhanced photocatalytic activity under simulated solar light. ChemistrySelect 2020, 5, 14078–14085. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Osman, M.; Tlili, I. Thermal behavior of auxetic honeycomb structure: An experimental and modeling investigation. J. Energy Resour. Technol. 2018, 140, 122904. [Google Scholar] [CrossRef]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; John Wiley & Sons, Inc.: Brooklyn, NY, USA, 2002. [Google Scholar]
- Hao, Y.; Feng, Z.; He, Z.; Zhang, J.; Liu, X.; Qin, J.; Guo, L.; Bi, K. Gradient design of ultrasmall dielectric nanofillers for PVDF-based high energy-density composite capacitors. Mater. Des. 2020, 189, 108523. [Google Scholar] [CrossRef]
- Arumugam, V.; Moodley, K.G. Mixed metal and metal oxide nanofibers: Preparation, fabrication, and applications. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Geltmeyer, J.; Teixido, H.; Meire, M.; Van Acker, T.; Deventer, K.; Vanhaecke, F.; Van Hulle, S.; De Buysser, K.; De Clerck, K. TiO2 functionalized nanofibrous membranes for removal of organic (micro)pollutants from water. Sep. Purif. Technol. 2017, 179, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-Y.; Lee, I.-H. Relative humidity effect on the preparation of porous electrospun polystyrene fibers. J. Nanosci. Nanotechnol. 2010, 10, 3473–3477. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hwang, K.-J.; Lee, J.-W.; Lee, I.-H. Fabrication and characterization of electrospun Ag doped TiO2 nanofibers for photocatalytic reaction. J. Mater. Sci. 2011, 46, 7240–7246. [Google Scholar] [CrossRef]
- Pan, H.; Feng, Y.P. Semiconductor nanowires and nanotubes: Effects of size and surface-to-volume ratio. ACS Nano 2008, 2, 2410–2414. [Google Scholar] [CrossRef]
- Lolla, D.; Gorse, J.; Kisielowski, C.; Miao, J.; Taylor, P.L.; Chase, G.G.; Reneker, D.H. Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale 2016, 8, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Murillo, N.; Pérez-Márquez, A.; Maudes, J.; Gayoso, J.; Laza, J.M.; Aranzabe, E.; Vilas, J.L. Effect of different types of electrospun polyamide 6 nanofibres on the mechanical properties of carbon fibre/epoxy composites. Polymers 2018, 10, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamnongkan, T.; Wattanakornsiri, A.; Pansila, P.P.; Migliaresi, C.; Kaewpirom, S. Effect of poly(vinyl alcohol)/chitosan ratio on electrospun-nanofiber morphologies. Adv. Mater. Res. 2012, 463-464, 734–738. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A Review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z. A review of polymer nanofibres byelectrospinning and their application in oil-water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Lai, Y.; Meng, M.; Yu, Y.; Wang, X.; Ding, T. Photoluminescence and photocatalysis of the flower-like nano-ZnO photocatalysts prepared by a facile hydrothermal method with or without ultrasonic assistance. Appl. Catal. B Environ. 2011, 105, 335–345. [Google Scholar] [CrossRef]
- Hu, M.; Fang, M.; Tang, C.; Yang, T.; Huang, Z.; Liu, Y.; Wu, X.; Min, X. The effects of atmosphere and calcined temperature on photocatalytic activity of TiO2 nanofibers prepared by electrospinning. Nanoscale Res. Lett. 2013, 8, 548. [Google Scholar] [CrossRef] [Green Version]
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V.; Singh, S. Electrospinning process parameters dependent investigation of TiO2 nanofibers. Results Phys. 2018, 11, 223–231. [Google Scholar] [CrossRef]
- Stefan, M.; Pana, O.; Leostean, C.; Bele, C.; Silipas, D.; Senila, M.; Gautron, E. Synthesis and characterization of Fe3O4–TiO2 core-shell nanoparticles. J. Appl. Phys. 2014, 116, 114312. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloys Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.-N.; Fu, L.; Zhang, B.; Li, F. Preparation of doped TiO2 nanofiber membranes through electrospinning and their application for photocatalytic degradation of malachite green. J. Mater. Sci. 2014, 49, 2303–2314. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.E.A.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibersand textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Lin, T.; Wang, X.G. Controlling the morphologies of electrospun nanofibers. In Nanofibers and Nanotechnology in Textiles; Woodhead Publishing: Sawston, UK, 2007; pp. 90–110. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]




| Experiment No. | PVP wt.% | PVP | Ethanol (10 mL) | Total |
|---|---|---|---|---|
| 1 | 6% | 0.504 g | 7.9 g | 8.404 g |
| 2 | 7% | 0.59 g | 7.9 g | 8.49 g |
| 3 | 8% | 0.68 g | 7.9 g | 8.58 g |
| Experiment No. | TiP-IV | Ethanol | Acetic Acid |
|---|---|---|---|
| 1 | 1 mL | 2 mL | 2 mL |
| 2 | 1 mL | 2 mL | 2 mL |
| 3 | 1 mL | 2 mL | 2 mL |
| Concentration of PVP | Diameter (nm) | Pore Size (nm) |
|---|---|---|
| 6% | 223 | 4.045 |
| 7% | 458 | 2.827 |
| 8% | 813 | 1.664 |
| Concentration of PVP | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cc/g) | |
|---|---|---|---|---|
| Adsorption | Desorption | |||
| 6% | 26.056 | 21.974 | 4.0872 | 0.03232 |
| 7% | 1.961 | 1.777 | 2.74709 | 0.00208 |
| 8% | 36.700 | 22.655 | 1.44705 | 0.02266 |
| Various Elements and Viruses | Size (nm) |
|---|---|
| Obtained Pore Size | 1.44 nm, 2.74 nm, 4.08 nm |
| Size of toxic elements in water (Arsenic, Cobalt, Mercury, Cadmium, Nickel, Chromium, Lead) | 0.121 nm to 0.175 nm |
| Size of Water Molecule | 0.275 nm |
| Size of Ar, N & O | 0.363 nm, 0.305 nm, 0.299 nm |
| Size of toxic elements in air (Asbestos, Mercury, Cadmium, Chromium) | 0.127 to 0.157 nm |
| Size of few harmful viruses (Ebola, COVID-19, SARS-COV-CoV, Bird-flu, Zika, Marburg, Hantavirus) | 50–140 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Pathak, D.; Patil, D.S.; Dhiman, N.; Bhullar, V.; Mahajan, A. Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19. Technologies 2021, 9, 89. https://doi.org/10.3390/technologies9040089
Sharma A, Pathak D, Patil DS, Dhiman N, Bhullar V, Mahajan A. Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19. Technologies. 2021; 9(4):89. https://doi.org/10.3390/technologies9040089
Chicago/Turabian StyleSharma, Ankush, Dinesh Pathak, Deepak S. Patil, Naresh Dhiman, Viplove Bhullar, and Aman Mahajan. 2021. "Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19" Technologies 9, no. 4: 89. https://doi.org/10.3390/technologies9040089
APA StyleSharma, A., Pathak, D., Patil, D. S., Dhiman, N., Bhullar, V., & Mahajan, A. (2021). Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19. Technologies, 9(4), 89. https://doi.org/10.3390/technologies9040089








