Heuristics Hindering the Development of Understanding of Molecular Structures in University Level Chemistry Education: The Lewis Structure as an Example
Abstract
:1. Introduction
1.1. Understanding Molecular Structures Utilizing Lewis Model Suggests Systemic Understanding
1.2. High-Level Chemistry Learning Requires Conceptual Change
1.3. Heuristics May Induce Misconceptions in Chemistry Education
1.4. Research Questions
- How do students’ concepts and sub-concepts of molecular structures generally develop during a chemistry basics course, and what kind of systematic mistakes and misrepresentations exist?
- How do the used heuristics affect students’ learning of molecular structures?
2. Materials and Methods
2.1. Participants
2.2. Context and Design
2.3. Measures
2.4. Data Analysis
3. Results
3.1. Understanding of Molecular Structures in Multiple-Choice Tasks
3.2. The Development of Students’ General Understanding and the Conceptual Change Based on the Drawings
3.3. Heuristics Used in the Multiple-Choice Tasks and Drawings
4. Discussion
4.1. Difficulties in Going beyond Basic Organic Compounds
4.2. Heuristics Hindering the Understanding of Lewis Model
4.3. Systemic Understanding and Conceptual Change in the Understanding of Lewis Model
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, M.M.; Corley, L.M.; Underwood, S.M. An investigation of college chemistry students’ understanding of structure-property relationships. J. Res. Sci. Teach. 2013, 50, 699–721. [Google Scholar] [CrossRef]
- McClary, L.; Talanquer, V. Heuristic reasoning in chemistry: Making decisions about acid strength. Int. J. Sci. Educ. 2011, 33, 1433–1454. [Google Scholar] [CrossRef]
- Murtonen, M.; Salmento, H. Broadening the theory of scientific thinking for higher education. In Redefining Scientific Thinking for Higher Education: Higher-Order Thinking, Evidence-Based Reasoning and Research Skills; Murtonen, M., Balloo, K., Eds.; Palgrave Macmillan: London, UK, 2019; pp. 3–29. [Google Scholar]
- Lewis, G.N. The atom and the molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.M.; Grove, N.; Underwood, S.M. Lost in Lewis structures: An investigation of student difficulties in developing representational competence. J. Chem. Ecol. 2010, 87, 869–874. [Google Scholar] [CrossRef]
- Cooper, M.M.; Underwood, S.M.; Hilley, C.Z.; Klymkowsky, M.W. Development and assessment of a molecular structure and properties learning progression. J. Chem. Educ. 2012, 89, 1351–1359. [Google Scholar] [CrossRef]
- Suidan, L.; Badenhoop, J.K.; Glendening, E.D.; Weinhold, F. Common textbook and teaching misrepresentations of Lewis structures. J. Chem. Educ. 1995, 72, 583–586. [Google Scholar] [CrossRef]
- Purser, G.H. Lewis structures are models for predicting molecular structure, not electronic structure. J. Chem. Educ. 1999, 76, 1013–1018. [Google Scholar] [CrossRef]
- Eilam, B. System thinking and feeding relations: Learning with a live ecosystem model. Instr. Sci. 2012, 40, 213–239. [Google Scholar] [CrossRef]
- Wadouh, J.; Liu, N.; Sandmann, A.; Neuhaus, B.J. The effect of knowledge linking levels in biology lessons upon students’ knowledge structure. Int. J. Sci. Math. Educ. 2014, 12, 25–47. [Google Scholar] [CrossRef]
- Barak, J.; Sheva, B.; Gorodetsky, M.A.; Gurion, B. As ‘process’ as it can get: Students’ understanding of biological processes. Int. J. Sci. Educ. 1999, 21, 1281–1292. [Google Scholar] [CrossRef]
- Verhoeff, R.P.; Waarlo, A.J.; Boersma, K.T. Systems modelling and the development of coherent understanding of cell biology. Int. J. Sci. Educ. 2008, 30, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Brandstädter, K.; Harms, U.; Großschedl, J. Assessing system thinking through different concept-mapping practices. Int. J. Sci. Educ. 2012, 34, 2147–2170. [Google Scholar] [CrossRef]
- Bransford, J.; Brown, A.; Cocking, R. How People Learn: Brain, Mind, Experience and School: Expanded Edition of Sciences; The National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Sinatra, G.M.; Mason, L. Beyond knowledge: Learner characteristics influencing conceptual change. In International Handbook on Conceptual Change Research; Vosniadou, S., Ed.; Routledge: New York, NY, USA, 2013; pp. 377–394. [Google Scholar]
- Broughton, S.H.; Sinatra, G.M.; Nussbaum, E.M. “Pluto has been a planet my whole life!” Emotions, attitudes, and conceptual change in elementary students’ learning about Pluto’s reclassification. Res. Sci. Educ. 2013, 43, 529–550. [Google Scholar] [CrossRef]
- Carey, S. Conceptual Change in Childhood; MIT Press: Cambridge, UK, 1985; ISBN 0-262-03110-8. [Google Scholar]
- Chinn, C.A.; Brewer, W.F. The role of anomalous data in knowledge acquisition: A theoretical framework and implications for science instruction. Rev. Educ. Res. 1993, 63, 1–49. [Google Scholar] [CrossRef]
- Diakidoy, I.-A.N.; Kendeou, P.; Ioannides, C. Reading about energy: The effects of text structure in science learning and conceptual change. Contemp. Educ. Psychol. 2003, 28, 335–356. [Google Scholar] [CrossRef]
- Duit, R.; Treagust, D.F. Conceptual change: A powerful framework for improving science teaching and learning. Int. J. Sci. Educ. 2003, 25, 671–688. [Google Scholar] [CrossRef]
- Limón, M. On the cognitive conflict as an instructional strategy for conceptual change: A critical appraisal. Learn. Instr. 2001, 11, 357–380. [Google Scholar] [CrossRef]
- Mason, L. Responses to anomalous data on controversial topics and theory change. Learn. Instr. 2001, 11, 453–483. [Google Scholar] [CrossRef]
- Vosniadou, S. Capturing and modeling the process of conceptual change. Learn. Instr. 1994, 4, 45–69. [Google Scholar] [CrossRef]
- Vosniadou, S.; Skopeliti, I. Developmental shifts in children’s categorization of the earth. In Proceedings of the XXVII Annual Conference of the Cognitive Science Society, Stresa, Italy, 21–23 July 2005; Bara, B.G., Barsalou, L., Bucciarelli, M., Eds.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2005; pp. 2325–2330. [Google Scholar]
- Carey, S. Science education as conceptual change. J. Appl. Dev. Psychol. 2000, 21, 13–19. [Google Scholar] [CrossRef]
- Amin, T.; Levrini, O. Converging Perspectives on Conceptual Change; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Chi, M.T.H. Two kinds and four sub-types of misconceived knowledge, ways to change it, and the learning outcomes. In International Handbook of Research on Conceptual Change; Vosniadou, S., Ed.; Routledge: New York, NY, USA, 2013; pp. 49–70. [Google Scholar]
- Limón, M.; Mason, L. Prologue. In Reconsidering Conceptual Change: Issues in Theory and Practice; Limón, M., Mason, L., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 15–20. [Google Scholar]
- Posner, G.J.; Strike, K.A.; Hewson, P.W.; Gertzog, W.A. Accommodation of a scientific conception: Toward a theory of conceptual change. Sci. Educ. 1982, 66, 211–227. [Google Scholar] [CrossRef]
- Potvin, P.; Nenciovici, L.; Malenfant-Robichaud, G.; Thibault, F.; Sy, O.; Mahhou, M.A.; Bernard, A.; Allaire-Duquette, G.; Blanchette Sarrasin, J.; Brault Foisy, L.-M.; et al. Models of conceptual change in science learning: Establishing an exhaustive inventory based on support given by articles published in major journals. Stud. Sci. Educ. 2020, 56, 1–55. [Google Scholar] [CrossRef]
- Chi, M.T.H. Three types of conceptual change: Belief revision, mental model transformation, and categorical shift. In Handbook of Research on Conceptual Change; Vosniadou, S., Ed.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 2008; pp. 61–82. [Google Scholar]
- Chi, M.T.H. Quantifying qualitative analyses of verbal data: A practical guide quantifying qualitative analyses of verbal data: A practical guide. J. Learn. Sci. 1997, 6, 271–315. [Google Scholar] [CrossRef]
- Chi, M.T.H. Commonsense conceptions of emergent processes: Why some misconceptions are robust. J. Learn. Sci. 2005, 14, 161–199. [Google Scholar] [CrossRef]
- Vosniadou, S.; Brewer, W.F. Mental models of the earth: A study of conceptual change in childhood. Cogn. Psychol. 1992, 24, 535–585. [Google Scholar] [CrossRef]
- Flaig, M.; Simonsmeier, B.A.; Mayer, A.-K.; Rosman, T.; Gorges, J.; Schneider, M. Conceptual change and knowledge integration as learning processes in higher education: A latent transition analysis. Learn. Individ. Differ. 2018, 62, 49–61. [Google Scholar] [CrossRef]
- Södervik, I.; Virtanen, V.; Mikkilä-Erdmann, M. Challences in understanding photosynthesis in a university introductory biociences class. Int. J. Sci. Math. Educ. 2015, 13, 733–750. [Google Scholar] [CrossRef]
- Treagust, D.F.; Duit, R. Conceptual change: A discussion of theoretical, methodological and practical challenges for science education. Cult. Stud. Sci. Educ. 2008, 3, 297–328. [Google Scholar] [CrossRef]
- Duit, R.; Treagust, D.F. Conceptual change: Still a powerful framework for improving the practice of science instruction. In Issues and Challenges in Science Education Research: Moving Forward; Tan, K.C.D., Kim, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 43–54. ISBN 9789400739802. [Google Scholar]
- Tiettmeyer, J.M.; Coleman, A.F.; Balok, R.S.; Gampp, T.W.; Duffy, P.L.; Mazzarone, K.M.; Grove, N.P. Unraveling the complexities: An investigation of the factors that induce load in chemistry students constructing Lewis structures. J. Chem. Educ. 2017, 94, 282–288. [Google Scholar] [CrossRef]
- Guzzetti, B.J.; Snyder, T.E.; Glass, G.V.; Gamas, W.S. Promoting conceptual change in science: A comparative meta-analysis of instructional interventions from reading education and science education. Read. Res. Q. 1993, 28, 116–159. [Google Scholar] [CrossRef]
- Üce, M.; Ceyhan, İ. Misconception in chemistry education and practices to eliminate them: Literature analysis. J. Educ. Train. Stud. 2019, 7, 202–208. [Google Scholar] [CrossRef]
- Aw, J.K.; Boellaard, K.C.; Tan, T.K.; Yap, J.; Loh, Y.P.; Colasson, B.; Blanc, É.; Lam, Y.; Fung, F.M. Interacting with three-dimensional molecular structures using an augmented reality mobile app. J. Chem. Educ. 2020, 97, 3877–3881. [Google Scholar] [CrossRef]
- Mason, L.; Gava, M.; Boldrin, A. On warm conceptual change: The interplay of text, epistemological beliefs, and topic interest. J. Educ. Psychol. 2008, 100, 291–309. [Google Scholar] [CrossRef]
- Mikkilä-Erdmann, M. Text comprehension and conceptual change: Interaction between text design and levels of text comprehension. In Reframing the Processes of Conceptual Change: Integrating Theory and Practice; Mason, L., Limón, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 337–356. [Google Scholar]
- Tippett, C.D. Refutation text in science education: A review of two decades of research. Int. J. Sci. Math. Educ. 2010, 8, 951–970. [Google Scholar] [CrossRef]
- Nkosi, T.; Mnguni, L. The impact of physical molecular models on students’ visuo-semiotic reasoning skills related to the lewis structure and ball & stick model of ammonia. J. Balt. Sci. Educ. 2020, 19, 594–604. [Google Scholar]
- Erman, E. Factors contributing to students’ misconceptions in learning covalent bonds. J. Res. Sci. Teach. 2017, 54, 520–537. [Google Scholar] [CrossRef]
- Joki, J.; Aksela, M. The challenges of learning and teaching chemical bonding at different school levels using electrostatic interactions instead of the octet rule as a teaching model. Chem. Educ. Res. Pract. 2018, 19, 932–953. [Google Scholar] [CrossRef] [Green Version]
- Bagnoli, F. Bursting money bins, the ice and water structure. Europhys. News 2015, 46, 15–16. [Google Scholar] [CrossRef] [Green Version]
- Hague, G.R.J. The magic of chemistry: Learning it is fun! J. Chem. Educ. 1983, 60, 741–743. [Google Scholar] [CrossRef]
- Laing, M. No rabbit ears on water. The structure of the water molecule: What should we tell the students? J. Chem. Educ. 1987, 64, 124–128. [Google Scholar] [CrossRef]
- Williamson, V.M.; Hegarty, M.; Deslongchamps, G.; Williamson, K.C.; Shultz, M.J. Identifying student use of ball-and-stick images versus electrostatic potential map images via eye tracking. J. Chem. Educ. 2013, 90, 159–164. [Google Scholar] [CrossRef]
- Hinze, S.R.; Rapp, D.N.; Williamson, V.M.; Shultz, M.J.; Deslongchamps, G.; Williamson, K.C. Beyond ball-and-stick: Students’ processing of novel STEM visualizations. Learn. Instr. 2013, 26, 12–21. [Google Scholar] [CrossRef]
- Ferk, V.; Vrtacnik, M.; Blejec, A.; Gril, A. Students ’ understanding of molecular structure representations. Int. J. Sci. Educ. 2003, 25, 1227–1245. [Google Scholar] [CrossRef]
- Criswell, B. Do you see what I see? Lessons about the use of models in high school chemistry classes. J. Chem. Educ. 2011, 88, 415–419. [Google Scholar] [CrossRef]
- Harrison, A.G.; Treagust, D.F. Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry. Sci. Educ. 2000, 84, 352–381. [Google Scholar] [CrossRef]
- Kerber, R.C. If it’s resonance, what is resonating? J. Chem. Educ. 2006, 83, 223–227. [Google Scholar] [CrossRef]
- Lastusaari, M.; Laakkonen, E.; Murtonen, M. Persistence in studies in relation to learning approaches and first-year grades: A study of university chemistry students in Finland. Chem. Educ. Res. Pract. 2019, 20, 452–467. [Google Scholar] [CrossRef]
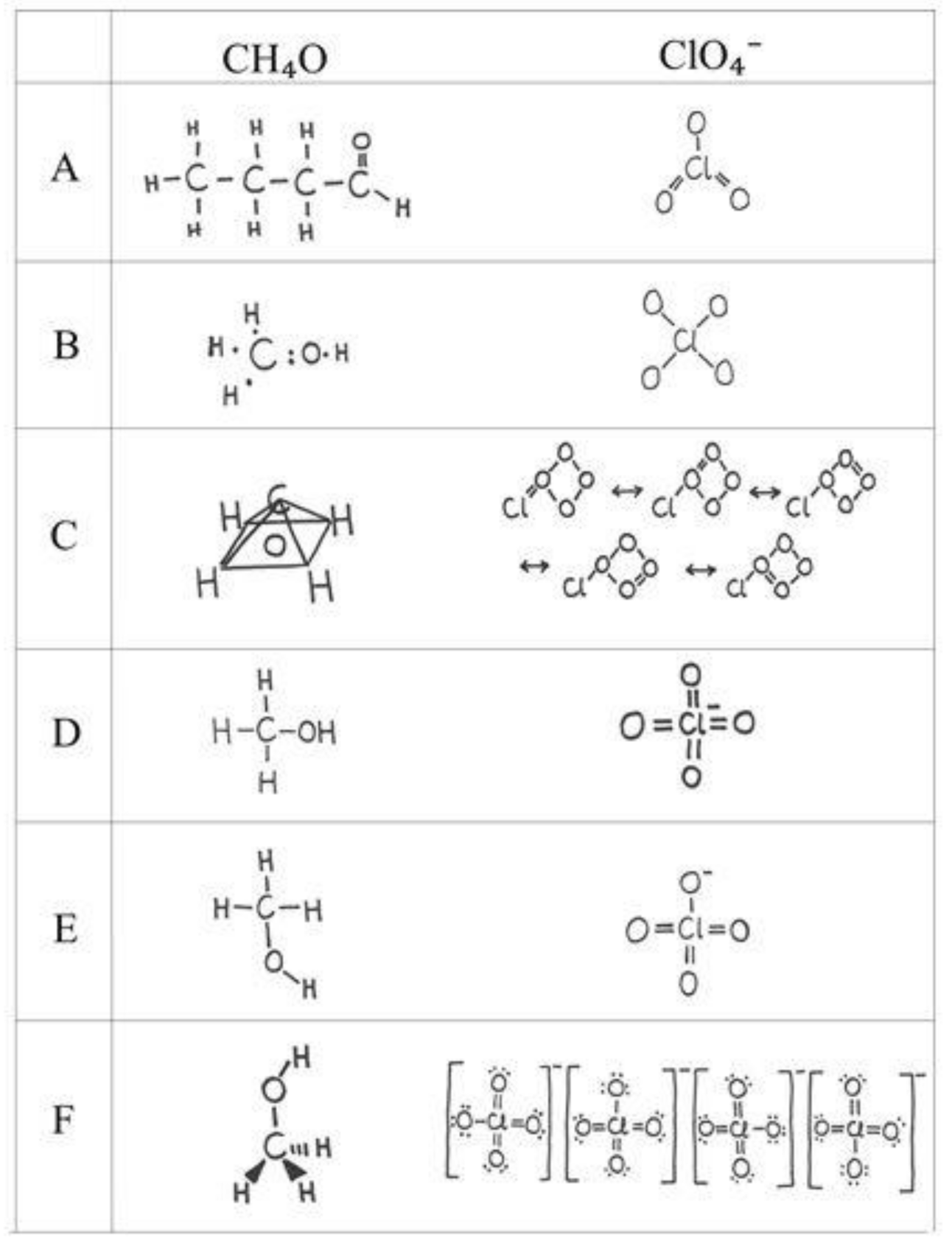

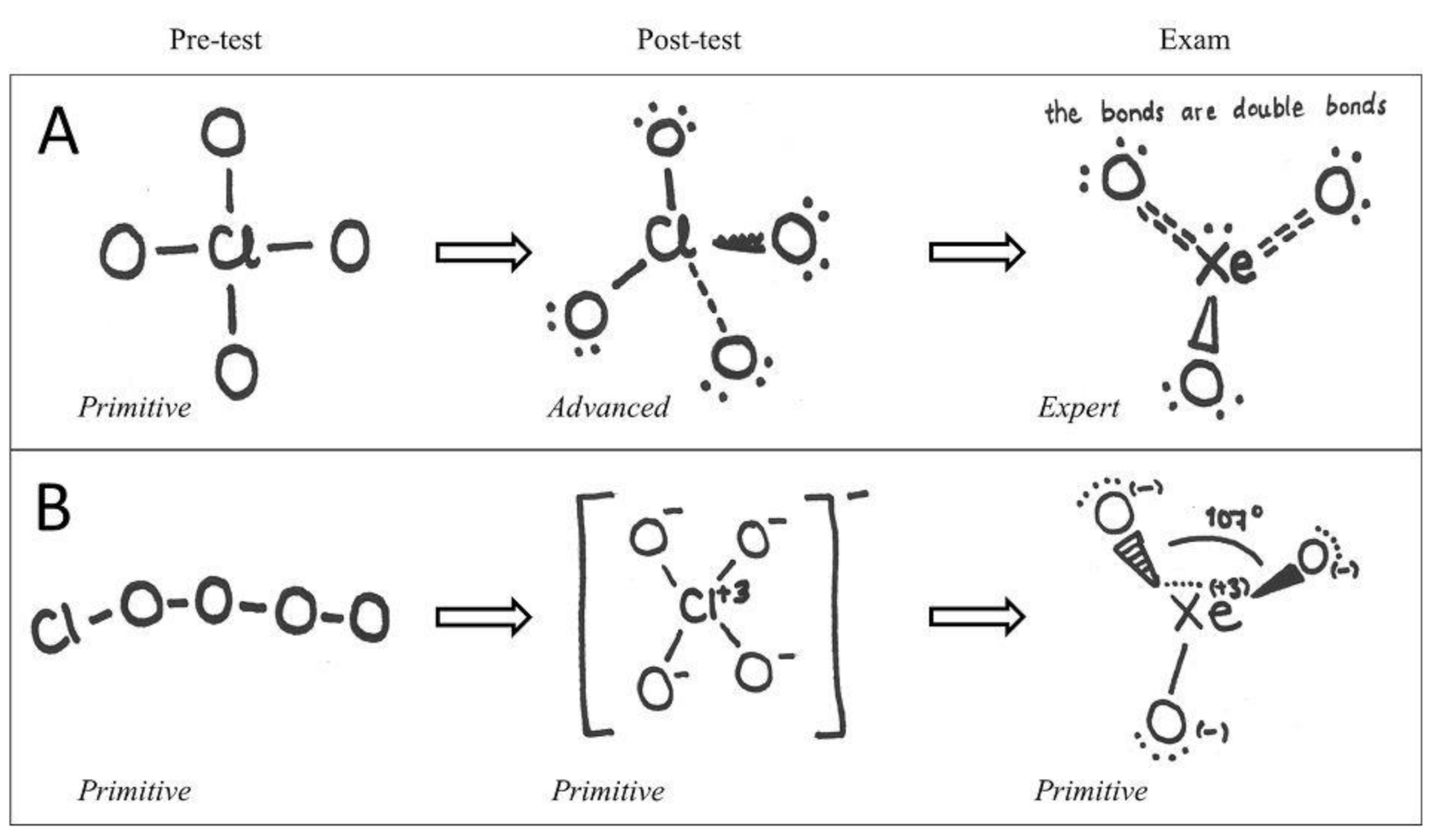
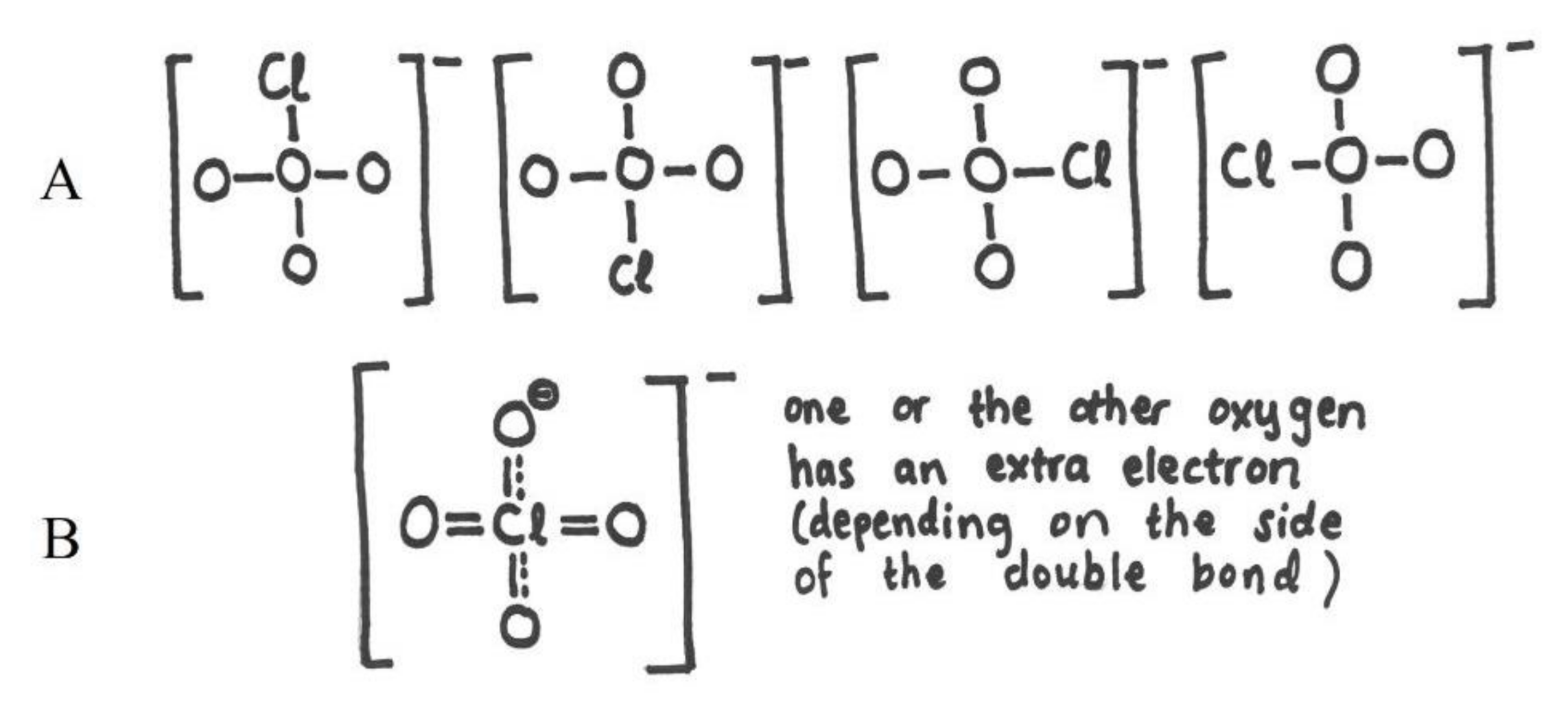
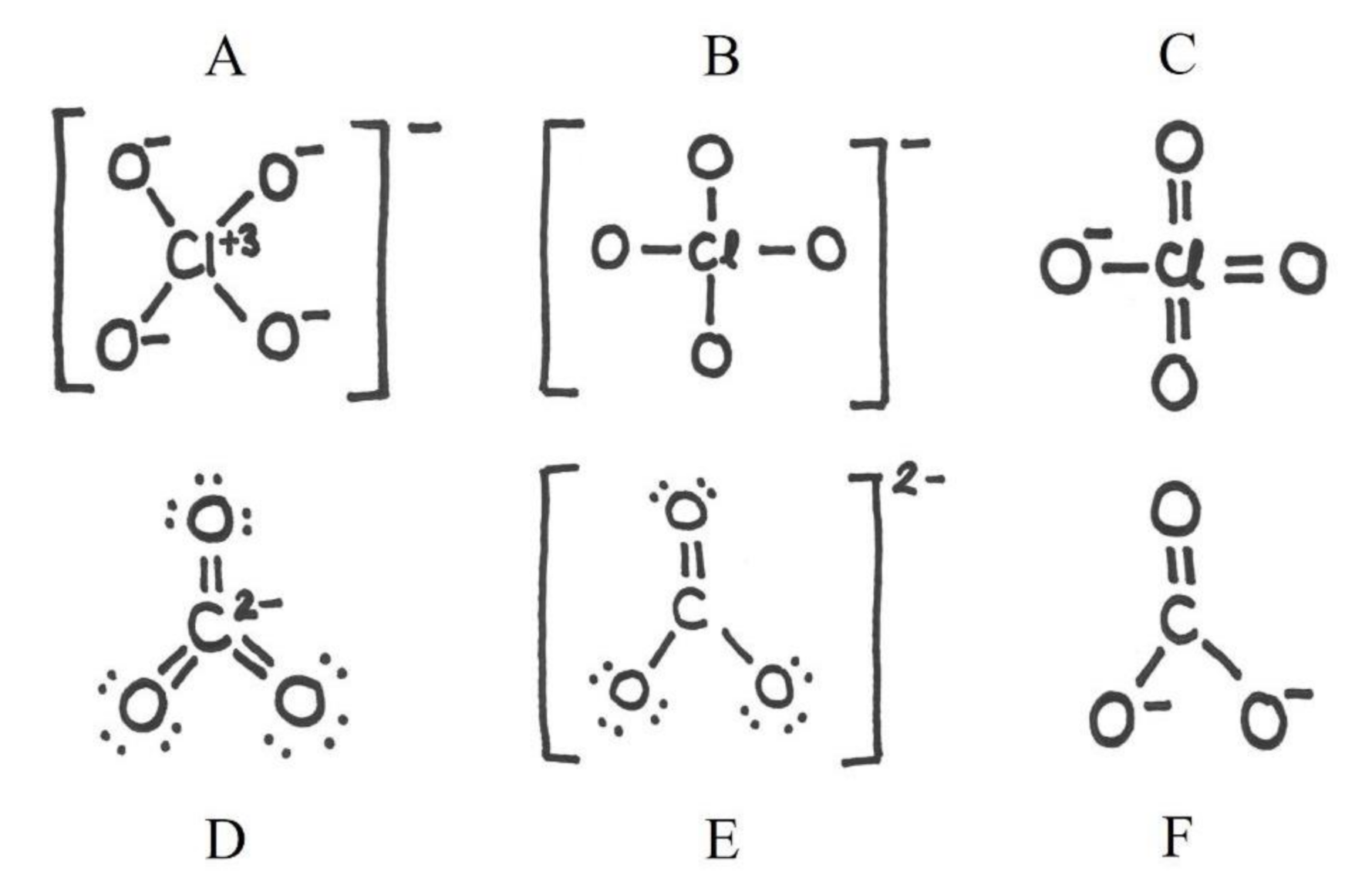

| Reason for Our Choice | Needed Student Accomplishment | |
|---|---|---|
| 1 | valence bond | understanding the nature of a bond |
| 2 | valence electrons | ability to obtain information from the periodical table for the atoms O, C and Al; subtasks a, b and c, respectively |
| 3 | electron octet | understanding the concept of electron octet |
| 4 | number and nature of chemical bonds | understanding what compounds are possible based on reasoning. Subtasks: (a) oxygen with four bonds without resonance or net charge, (b) tetraoxygen in square form, (c) oxygen with three bonds without resonance or net charge, (d) halogen compound with three bonds in the central atom, (e) noble gas compound, (f) carbon exceeding the octet, (g) sulfur exceeding the octet, (h) fluoride as the central atom |
| 5 | bond order | understanding the bond order of diatomic compounds: H2, F2, O2 and N2; subtasks a, b, c and d, respectively |
| 6 | geometry | understanding the geometry of molecules. Subtasks: (a) phosgene given as T-shape in 2d, (b) methane given as formula, (c) ammonia given as formula |
| 7 | polarity | understanding the polarity of molecules given as 2D planar drawings, subtasks (a) CH4, (b) CH3Cl, (c) CH2Cl2, and given as molecular formula subtasks (d) H2O, (e) CO2, (f) NH3 |
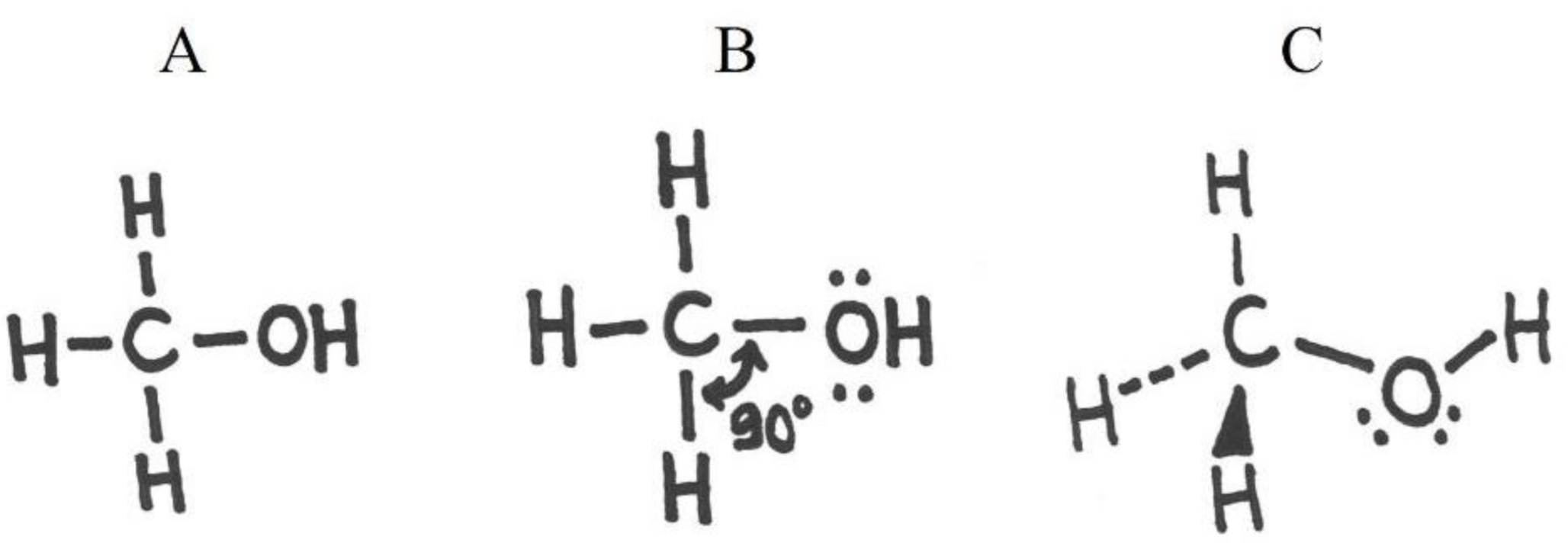
| H2O | octet rule, valence electron repulsion model | ability to apply basic bond rules of second-row elements and to resolve geometry based on the bond structure and free electron pairs of the central atom |
| CH2O | octet rule, valence electron repulsion model | ability to apply basic bond rules of second-row elements and to resolve geometry based on the bond structure of the central atom |
| N2H2 | geometry, octet rule, valence electron repulsion model | ability to apply basic bond rules of second-row elements on a non-carbon molecule and to resolve geometry when information about free electron pairs are needed |
| O3 | formal charge, geometry, resonance, valence electron repulsion model | ability to adapt to situation where the oxygen does not form two bonds. Ability to understand resonance and formal charge in order to decide the bond order and to resolve geometry when information about free electron pairs is needed |
| CH4O | geometry, octet rule, valence electron repulsion model | ability to apply basic bond rules when atoms are given in an unconventional order and to resolve geometry based on the bond structure and free electron pairs of the central atoms |
| HCN | geometry, octet rule, valence electron repulsion model | ability to apply basic bond rules of second-row elements and to resolve geometry based on the bond structure of the central atom |
| CO₃2ˉ | bond order, formal charge, geometry, resonance, valence electron repulsion model | ability to understand several concepts at the same time. Ability to adapt into situation where the oxygen does not form two bonds. Ability to understand resonance and formal charge in order to decide the bond order and to associate between formal charge and net charge. Ability to resolve geometry based on the bond structure of the central atom |
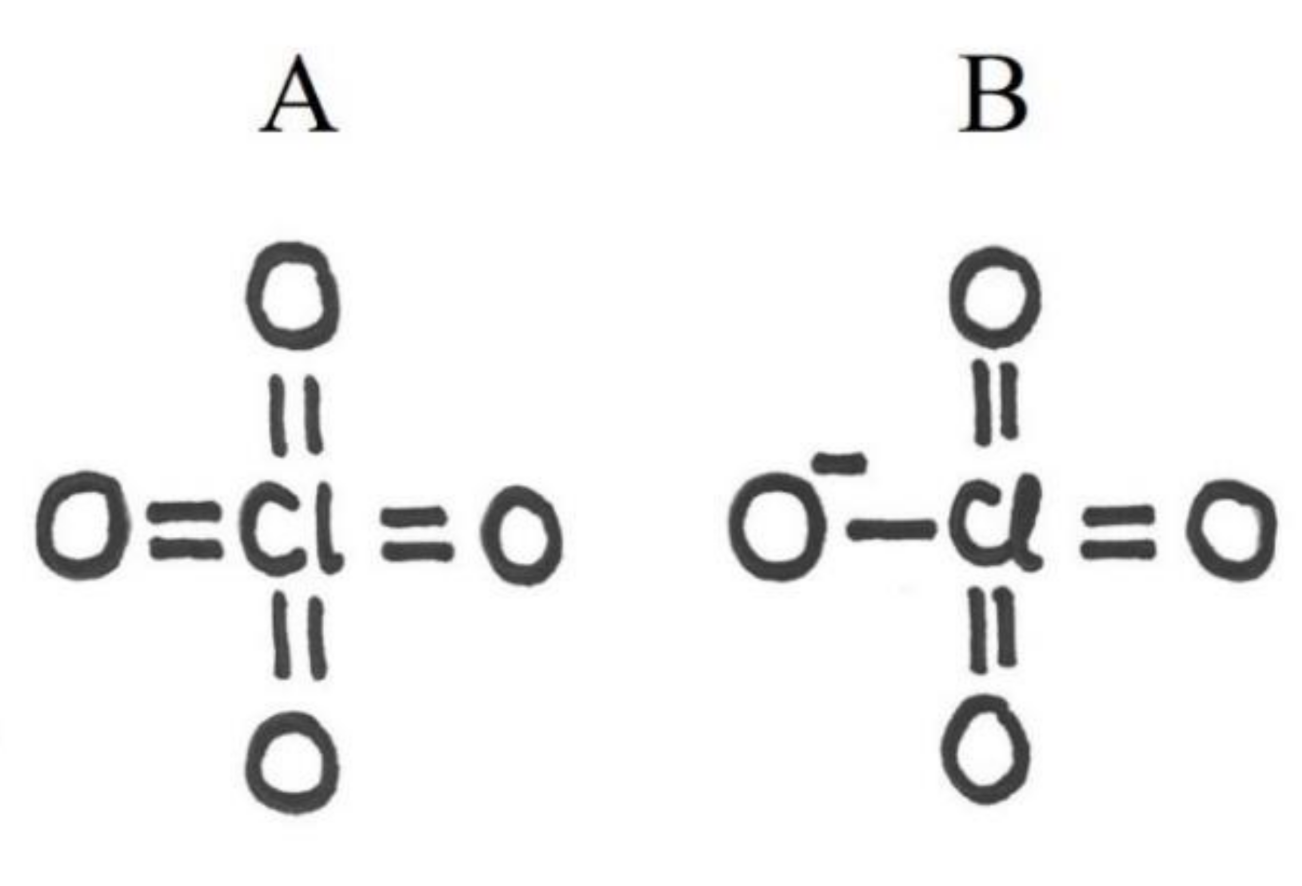
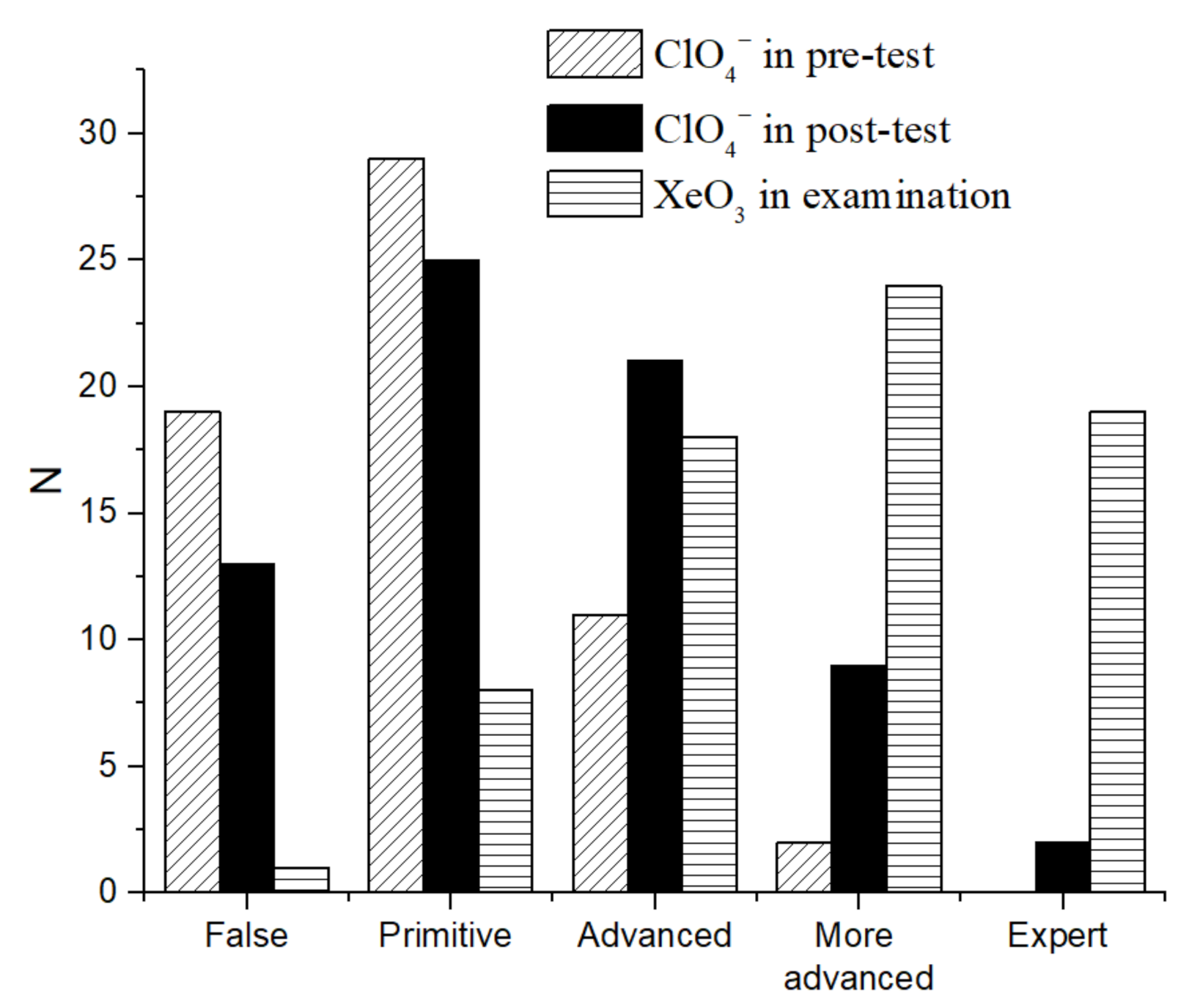
| ClO4ˉ | expanded octet, formal charge, geometry, resonance, valence electrons, valence electron repulsion model | ability to understand several concepts at the same time. Ability to calculate the total number of valence electrons and match that with the final structure. Ability to adapt into situation where oxygen does not form two bonds and where the octet rule does not apply, because of d-orbitals of heavy central atom. Ability to understand resonance and formal charge in order to decide the bond order and to associate between formal charge and net charge. Ability to resolve geometry based on the bond structure of the central atom |
| XeO3 | expanded octet, formal charge, geometry, resonance, valence electrons, valence electron repulsion model | ability to understand several concepts at the same time. Ability to calculate the total number of valence electrons and match that with the final structure. Ability to adapt into situation where the basic bond rules might not apply and where the octet rule does not apply, because of d-orbitals of heavy central atom. Ability to understand resonance and formal charge in order to decide the bond order and to associate between formal charge and net charge. Ability to resolve geometry based on the bond and free electron structure of the central atom |
| CH2O | N2H2 | O3 | CH4O | HCN | CO32ˉ | ClO4ˉ | XeO3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Exam | |
| false | 6 | 3 | 6 | 6 | 6 | 1 | 8 | 7 | 7 | 3 | 19 | 11 | 33 | 16 | 1 |
| primitive | 3 | 1 | 13 | 17 | 18 | 14 | 9 | 4 | 7 | 6 | 20 | 13 | 32 | 30 | 8 |
| advanced | 15 | 21 | 47 | 42 | 47 | 39 | 43 | 39 | 9 | 5 | 33 | 31 | 12 | 22 | 18 |
| more advanced | 55 | 54 | 13 | 14 | 7 | 15 | 17 | 26 | 56 | 65 | 7 | 17 | 2 | 9 | 24 |
| expert | N/A | N/A | N/A | N/A | 1 | 10 | 2 | 3 | N/A | N/A | 0 | 7 | 0 | 2 | 19 |
| Pre-Test (n = 200) | Post-Test (n = 79) | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Wrong | Do Not Know | Right | Wrong | Do Not Know | Both Right | Improved | Not Improved | Declined | |
| covalent bond (1) | 80 | 17 | 3 | 100 | 0 | 0 | 89 | 11 | 0 | 0 |
| valence electrons of O (2a) | 62 | 26 | 13 | 96 | 4 | 0 | 73 | 23 | 4 | 0 |
| valence electrons of C (2b) | 75 | 13 | 13 | 97 | 3 | 0 | 85 | 13 | 1 | 1 |
| valence electrons of Al (2c) | 68 | 18 | 15 | 94 | 4 | 3 | 76 | 18 | 5 | 1 |
| octet (3) | 49 | 24 | 28 | 56 | 27 | 18 | 41 | 22 | 16 | 22 |
| possible compound? | ||||||||||
 | 79 | 14 | 7 | 86 | 13 | 1 | 75 | 13 | 6 | 6 |
| (4a) | ||||||||||
 | 53 | 31 | 17 | 46 | 37 | 18 | 35 | 13 | 33 | 19 |
| (4b) | ||||||||||
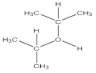 | 73 | 24 | 4 | 78 | 19 | 3 | 71 | 10 | 5 | 14 |
| (4c) | ||||||||||
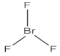 | 15 | 74 | 12 | 61 | 35 | 4 | 11 | 52 | 29 | 8 |
| (4d) | ||||||||||
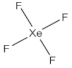 | 7 | 79 | 15 | 48 | 51 | 1 | 8 | 42 | 43 | 8 |
| (4e) | ||||||||||
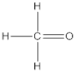 | 81 | 17 | 3 | 82 | 16 | 1 | 75 | 8 | 8 | 10 |
| (4f) | ||||||||||
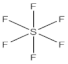 | 27 | 57 | 17 | 82 | 18 | 0 | 23 | 59 | 10 | 8 |
| (4g) | ||||||||||
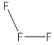 | 74 | 10 | 16 | 73 | 15 | 11 | 65 | 10 | 3 | 23 |
| (4h) | ||||||||||
| bond order of H2 (5a) | 92 | 4 | 5 | 99 | 1 | 0 | 95 | 4 | 0 | 1 |
| bond order of F2 (5b) | 79 | 8 | 13 | 97 | 1 | 1 | 81 | 16 | 1 | 1 |
| bond order of O2 (5c) | 92 | 2 | 7 | 97 | 3 | 0 | 96 | 1 | 0 | 3 |
| bond order of N2 (5d) | 86 | 6 | 9 | 95 | 4 | 1 | 90 | 5 | 1 | 4 |
| geometry of COCl2 (6a) | 30 | 57 | 13 | 47 | 51 | 3 | 15 | 32 | 33 | 20 |
| geometry of CH4 (6b) | 91 | 6 | 4 | 97 | 3 | 0 | 95 | 3 | 0 | 3 |
| geometry of NH3 (6c) | 44 | 51 | 5 | 77 | 23 | 0 | 43 | 34 | 14 | 9 |
| polarity of CH4 (7a) | 84 | 5 | 12 | 95 | 4 | 1 | 89 | 6 | 1 | 4 |
| polarity of CH3Cl (7b) | 75 | 9 | 17 | 85 | 11 | 4 | 68 | 18 | 4 | 10 |
| polarity of CH2Cl2 (7c) | 26 | 56 | 19 | 24 | 71 | 5 | 13 | 13 | 47 | 28 |
| polarity of H2O (7d) | 82 | 9 | 10 | 96 | 3 | 1 | 87 | 10 | 3 | 0 |
| polarity of CO2 (7e) | 71 | 17 | 12 | 95 | 4 | 1 | 72 | 23 | 4 | 1 |
| polarity of NH3 (7f) | 56 | 24 | 21 | 72 | 22 | 6 | 47 | 27 | 8 | 19 |
| average | 63 | 25 | 12 | 80 | 17 | 3 | 62 | 19 | 11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karonen, M.; Murtonen, M.; Södervik, I.; Manninen, M.; Salomäki, M. Heuristics Hindering the Development of Understanding of Molecular Structures in University Level Chemistry Education: The Lewis Structure as an Example. Educ. Sci. 2021, 11, 258. https://doi.org/10.3390/educsci11060258
Karonen M, Murtonen M, Södervik I, Manninen M, Salomäki M. Heuristics Hindering the Development of Understanding of Molecular Structures in University Level Chemistry Education: The Lewis Structure as an Example. Education Sciences. 2021; 11(6):258. https://doi.org/10.3390/educsci11060258
Chicago/Turabian StyleKaronen, Maarit, Mari Murtonen, Ilona Södervik, Marianna Manninen, and Mikko Salomäki. 2021. "Heuristics Hindering the Development of Understanding of Molecular Structures in University Level Chemistry Education: The Lewis Structure as an Example" Education Sciences 11, no. 6: 258. https://doi.org/10.3390/educsci11060258
APA StyleKaronen, M., Murtonen, M., Södervik, I., Manninen, M., & Salomäki, M. (2021). Heuristics Hindering the Development of Understanding of Molecular Structures in University Level Chemistry Education: The Lewis Structure as an Example. Education Sciences, 11(6), 258. https://doi.org/10.3390/educsci11060258







