Improving the Scientific Literacy of Food Engineering Students in Electrohydrodynamic Processing by Means of Zein Solutions
Abstract
:1. Introduction
2. Objectives
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Zein Solutions
3.3. Characterization of the Zein Solutions
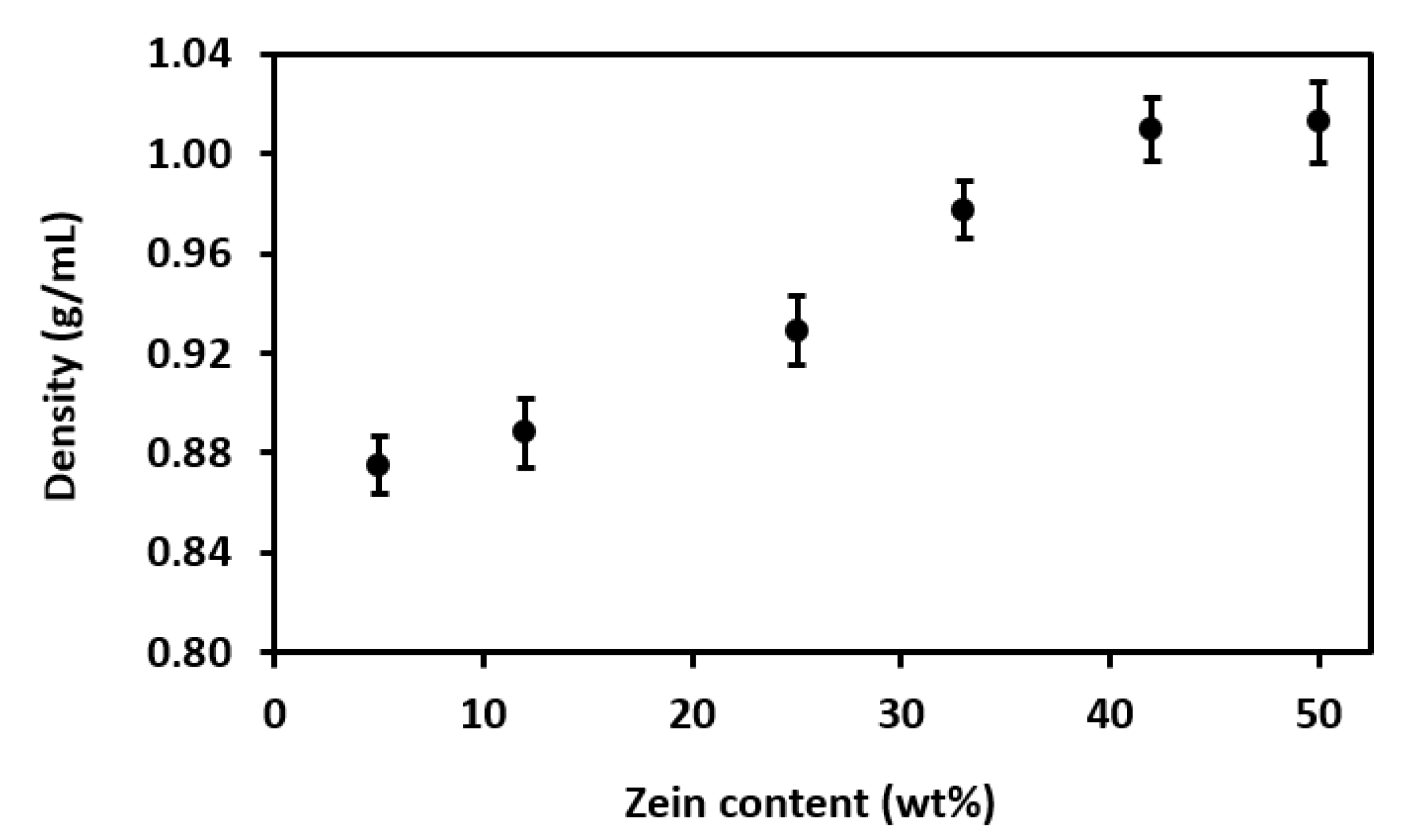
3.3.1. Density
- The empty pycnometer was weighed and its mass (mp) noted.
- The pycnometer was filled up with distilled water and its mass (mp+w) weighed, avoiding the formation of bubbles. When closed, the liquid overflowing through the capillary was removed and the pycnometer was dried.
- The pycnometer was cleaned and filled up with the studied sample solution and its mass (mp+s) weighed, considering the previous cautions.
- Equation (1) was applied to determine the density of the sample.
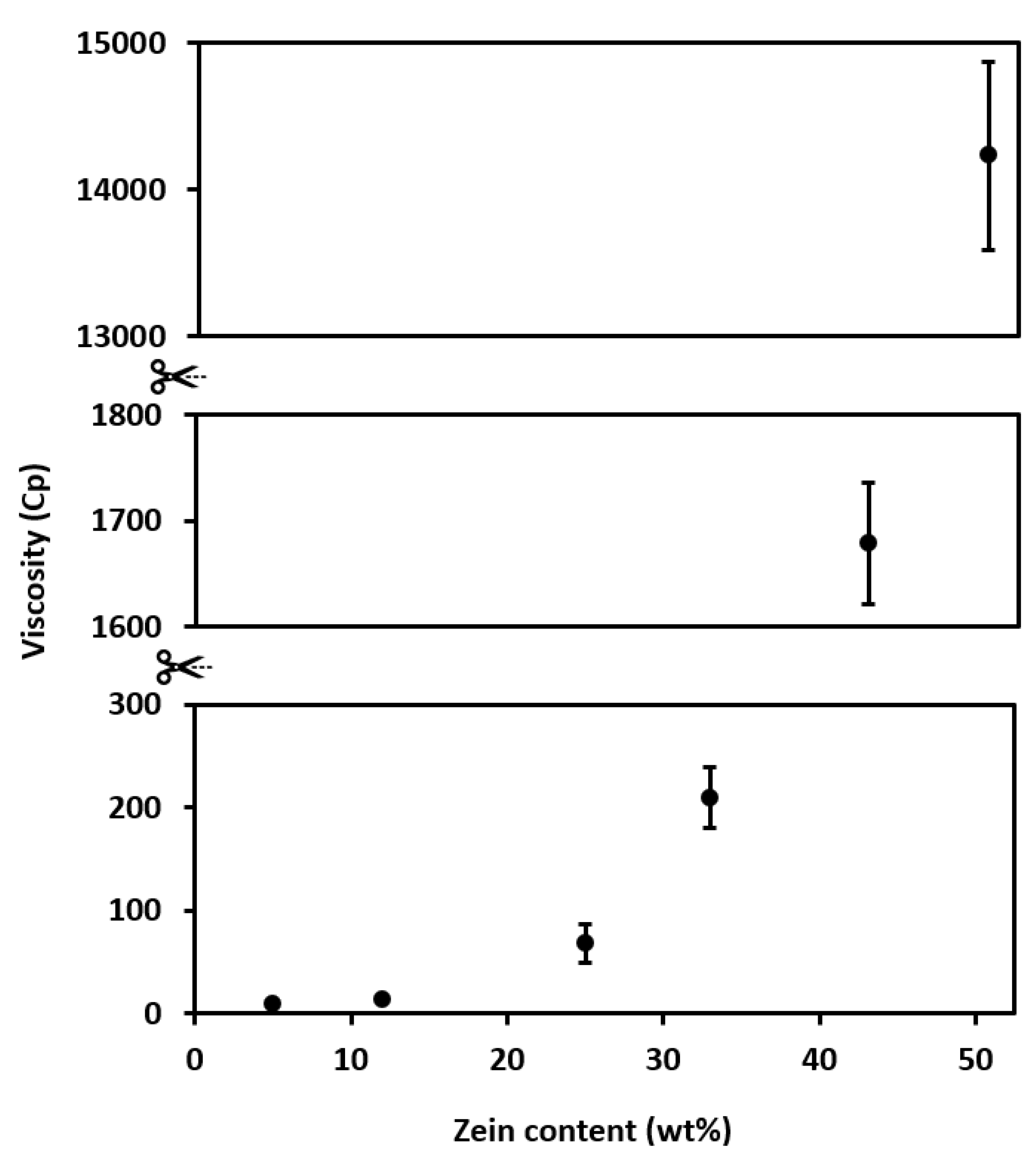
3.3.2. Viscosity
- The appropriate needle was selected according to the expected viscosity of the fluid to be studied. The higher the viscosity, the higher the gauge number of the needle.
- The studied solution was poured into a 250-mL beaker up to the needle mark, avoiding the protector to hit the bottom of the beaker.
- The viscometer was turned on with the start selector and a suitable rotation speed was selected to obtain a proper reading: 6, 12, 30, and 60 rpm.
- Once stabilized, the rear selector was pressed and held to fix the reading value.
- Without releasing the selector, the equipment was stopped by pressing the start selector to proceed with the dial reading.
- The viscosity was determined from the speed and needle selected as shown in Table 2.
- Finally, from the dial reading and the constant (k), the viscosity value of each solution was estimated by means of Equation (2).
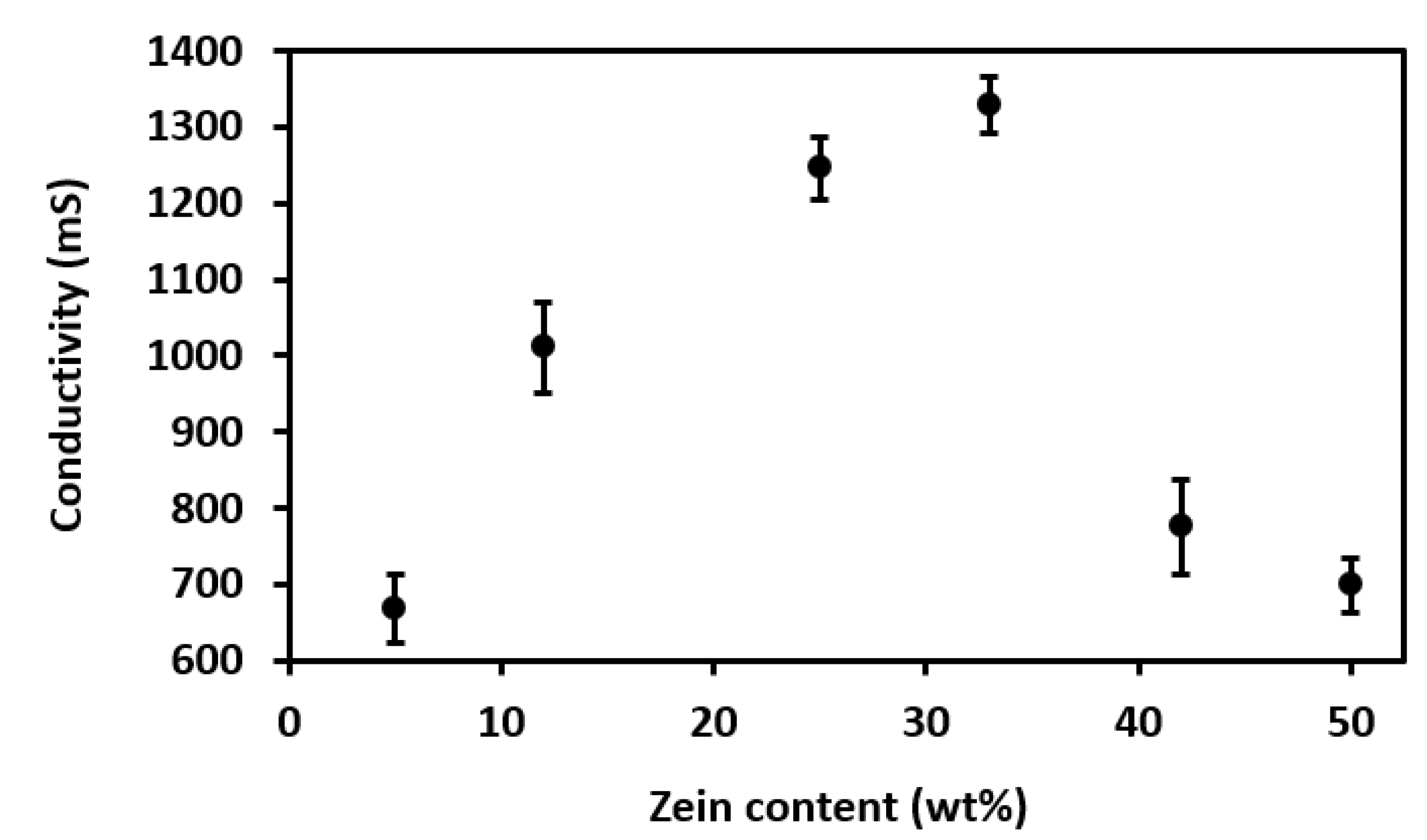
3.3.3. Conductivity
- The conductivity meter was connected in its “Conductivity” mode.
- The conductivity meter was calibrated with a standard solution of 0.01 M KCl (according to the instructions in the manual, specific conductivity KCl = 1.41 mS/cm at 25 °C) and the “CAL” key was pressed.
- The sensor was placed in a beaker with distilled water. Before measuring, the sensor was gently dried with paper.
- The sensor was introduced in the studied solution, preventing contact with the magnet during stirring.
- The value was read when the measurement stabilized. As the ranges in aqueous solutions are usually small, the basic units of measurements were expressed in milliSiemens/cm (mS/cm) or microSiemens/cm (µS/cm).
- The sensor was washed with distilled water and stored with its protector at the end of the test.
3.3.4. Surface Tension
- The platform was moved down.
- The RI 21 ring was suspended from the hook located in the force sensor.
- The SV20 vessel was placed on the platform with the solution to be studied.
- In the main menu, the “Du Noüg Ring” method was selected.
- The density of the sample to be measured was added in the menu.
- The platform with the sample was moved upwards until the ring was positioned just above the surface of the liquid, waiting until the ring remained stable and the liquid was at rest.
- The “tare” button was pressed to tare the weight of the ring in air, waiting until a weight of “0.0000 g” was displayed in the upper right-hand corner of the display.
- The sample platform finally moved upwards and the ring was immersed in the liquid to a depth of about 1 mm.
- The “start” button was pressed to measure the surface tension of the liquid.
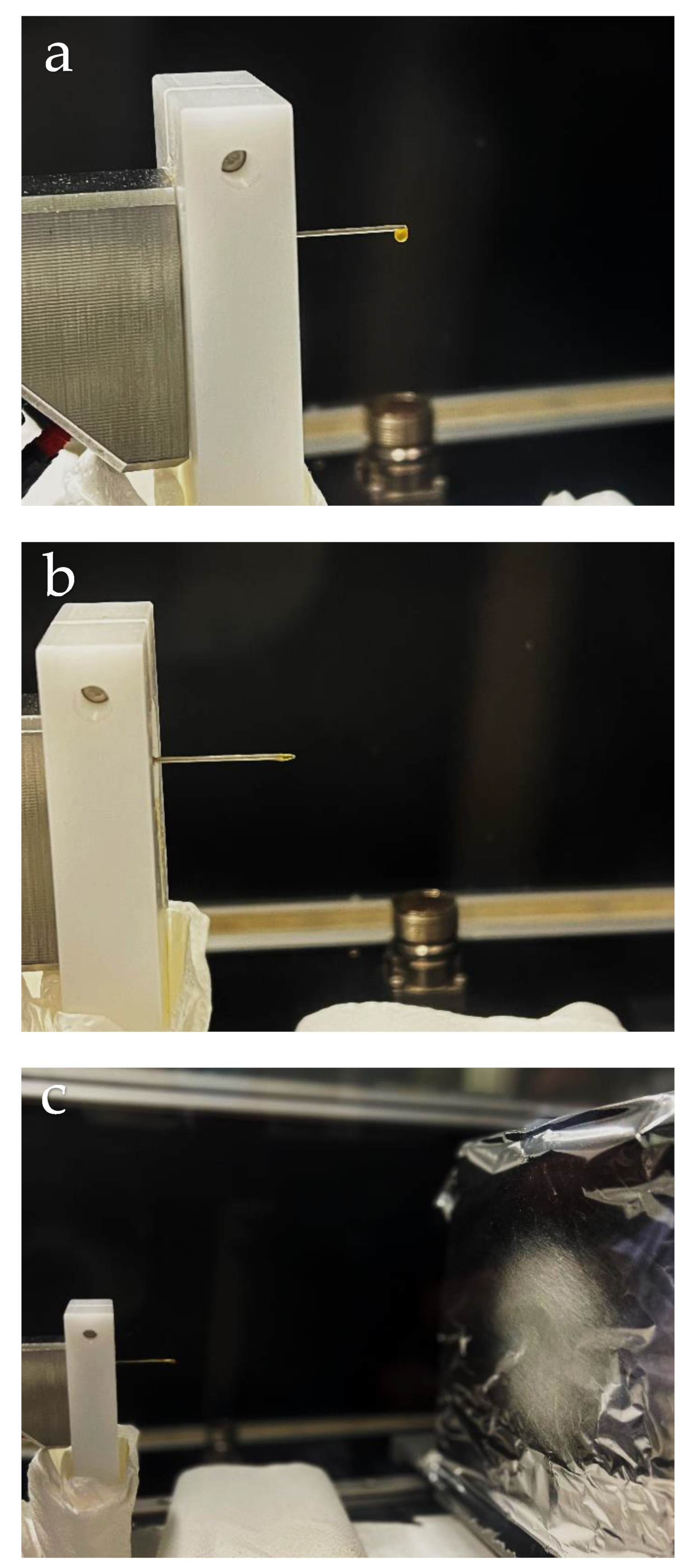
3.4. EHDP of the Zein Solutions
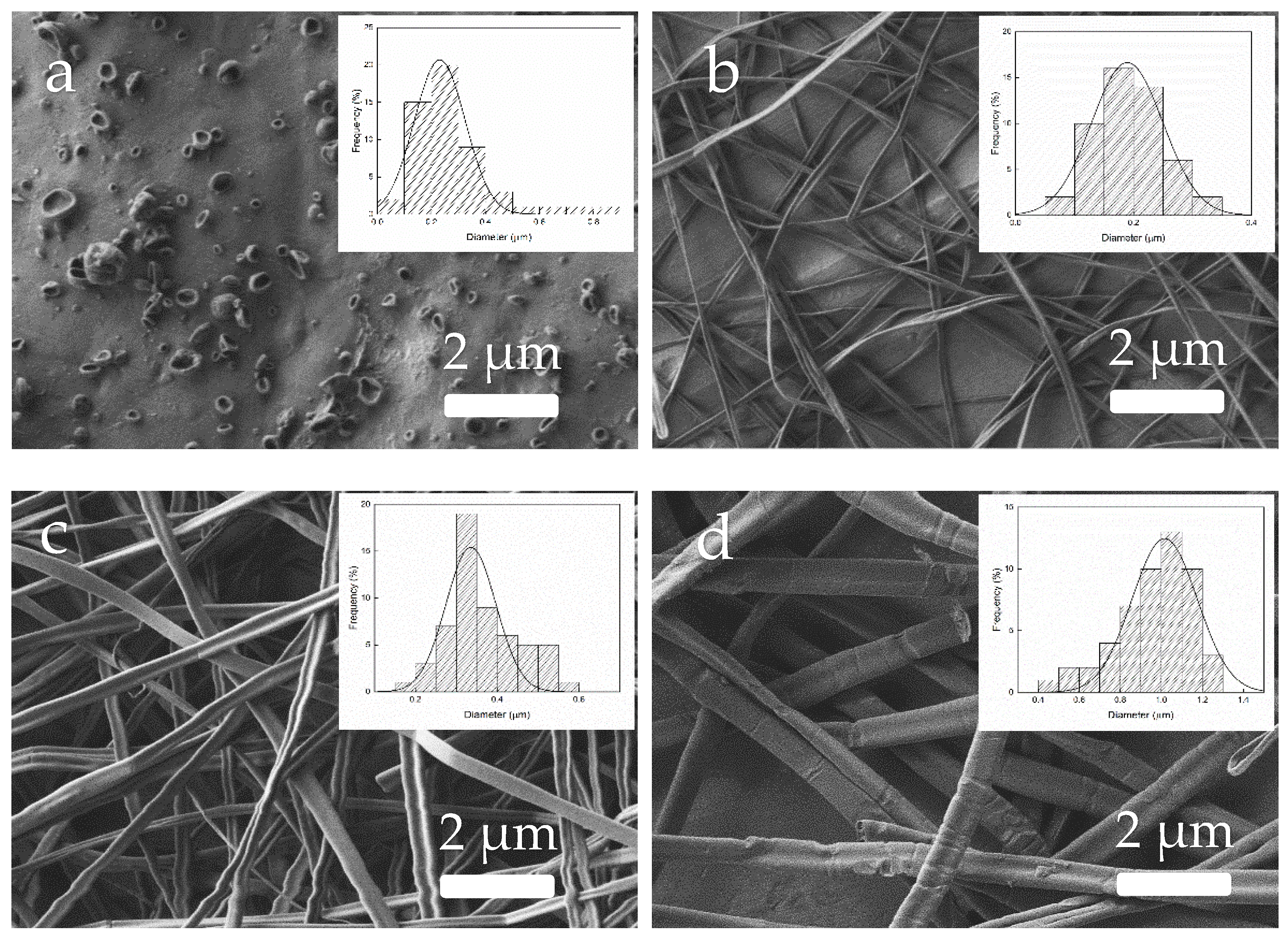
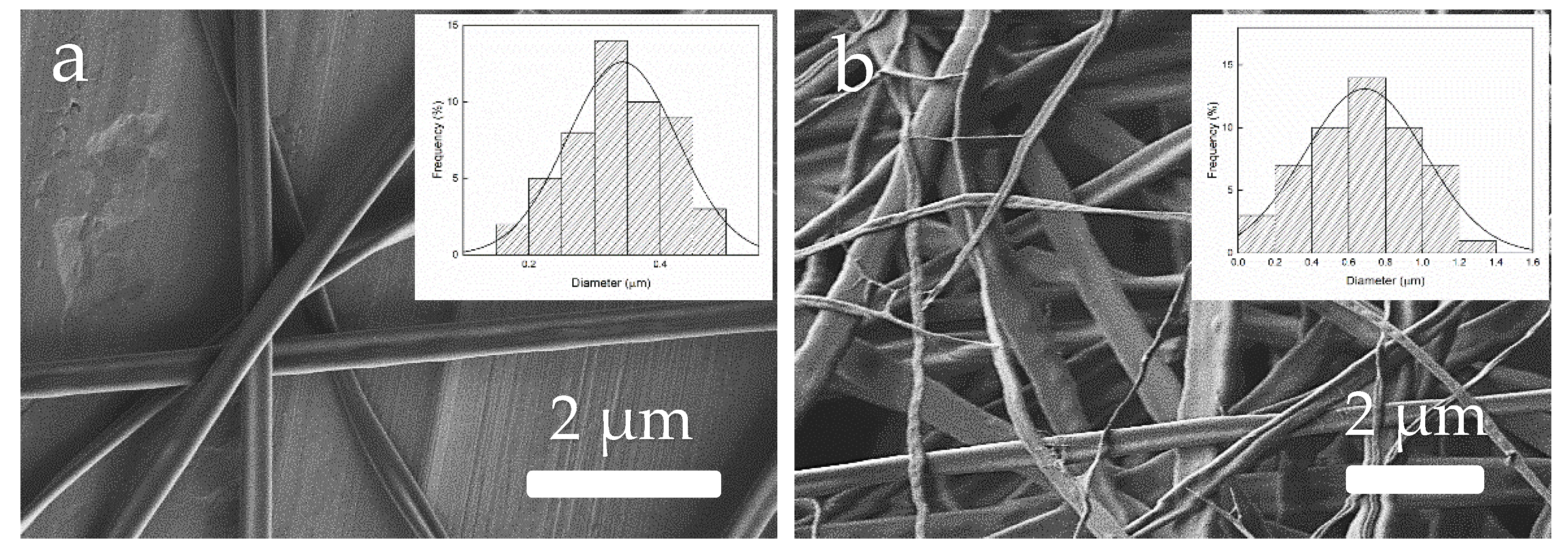
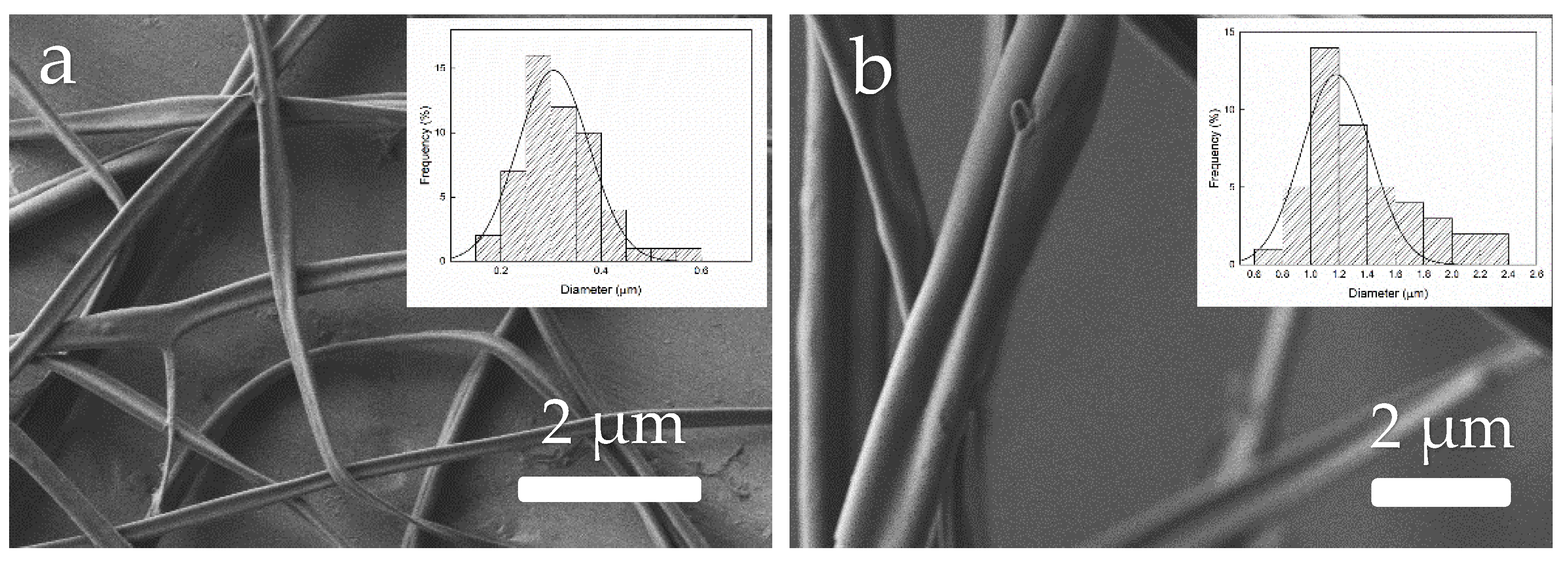
3.5. Morphology of Zein Materials Processed by EHDP
3.6. Student Survey
4. Results and Discussion
4.1. Properties of Zein Solutions
4.2. Characterization of Zein Materials Processed by EHDP
4.3. Student Feedback
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Dhahebi, A.M.; Ling, J.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Saheed, M.S.M.; Ramakrishna, S.; Jose, R. Electrospinning research and products: The road and the way forward. Appl. Phys. Rev. 2022, 9, 011319. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Coppola, S.; Nasti, G.; Vespini, V.; Mecozzi, L.; Castaldo, R.; Gentile, G.; Ventre, M.; Netti, P.A.; Ferraro, P. Quick liquid packaging: Encasing water silhouettes by three-dimensional polymer membranes. Sci. Adv. 2019, 5, 5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, N.; Yousif, M.; Mehdi, M.; Ali, H.F.; Hussain, T.; Hashemikia, S.; Van Langenhove, L.; Kim, I.S. Electroless Deposition: A Superficial Route to Synthesis of Highly Conductive Electrospun Nylon 6 Nanofibers. Fibers Polym. 2022, 23, 680–689. [Google Scholar] [CrossRef]
- Hussain, N.; Yousif, M.; Ali, A.; Mehdi, M.; Ullah, S.; Ullah, A.; Mahar, F.K.; Kim, I.S. A facile approach to synthesize highly conductive electrospun aramid nanofibers via electroless deposition. Mater. Chem. Phys. 2020, 255, 123614. [Google Scholar] [CrossRef]
- Hussain, N.; Mehdi, M.; Yousif, M.; Ali, A.; Ullah, S.; Hussain Siyal, S.; Hussain, T.; Kim, I.S. Synthesis of Highly Conductive Electrospun Recycled Polyethylene Terephthalate Nanofibers Using the Electroless Deposition Method. Nanomaterials 2021, 11, 531. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Gennari, O.; Battista, L.; Silva, B.; Grilli, S.; Miccio, L.; Vespini, V.; Coppola, S.; Orlando, P.; Aprin, L.; Slangen, P.; et al. Investigation on cone jetting regimes of liquid droplets subjected to pyroelectric fields induced by laser blasts. Appl. Phys. Lett. 2015, 106, 054103. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A History of Processing and Use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimenez, E.; Lagaron, J.M. Characterization of the morphology and thermal properties of Zein Prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Khatri, M.; Khatri, Z.; El-Ghazali, S.; Hussain, N.; Qureshi, U.A.; Kobayashi, S.; Ahmed, F.; Kim, I.S. Zein nanofibers via deep eutectic solvent electrospinning: Tunable morphology with super hydrophilic properties. Sci. Rep. 2020, 10, 15307. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Isobe, S.; Maekawa, T. Influence of preparation conditions on the physical properties of zein films. J. Am. Oil Chem. Soc. 2002, 79, 345–349. [Google Scholar] [CrossRef]
- Samuel, J.; Spackman, C.; Ruff, L.; Crucetti, J.J.; Chiappone, S.; Schadler, L. A Research University and Community College Collaboration Model to Promote Micro-manufacturing Education: Preliminary Findings. Procedia Manuf. 2016, 5, 1168–1182. [Google Scholar] [CrossRef] [Green Version]
- Nuryantini, A.Y.; Rajak, A.; Ekaputra, M.P.; Rahma, A.; Munir, M.M.; Khairurrijal. Introducing Electrospinning Technique for Producing Nanofibers to Improve Scientific Literacy of Senior High School Students. In Proceedings of the International Conference on Advances in Education Technology (ICAET-14), Bandung, Indonesia, 16 October 2014; pp. 129–132.
- Xinrui, Z. Zein as a structural protein in gluten-free systems: An overview. Food Sci. Hum. Wellness 2021, 10, 270–277. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of active antimicrobial fiber based chitosan polysaccharide nanostructures using electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Incorporation of zein nanofibers produced by needle-less electrospinning within the casted gelatin film for improvement of its physical properties. Food Bioprod. Process. 2020, 122, 193–204. [Google Scholar] [CrossRef]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Electrohydrodynamic processing for the production of zein-based microstructures and nanostructures. Curr. Opin. Colloid Interface Sci. 2021, 56, 101504. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J. Appl. Polym. Sci. 2014, 131, 9270–9276. [Google Scholar] [CrossRef]
- Correia, D.M.; Gonçalves, R.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S. Electrosprayed poly(vinylidene fluoride) microparticles for tissue engineering applications. RSC Adv. 2014, 4, 33013–33021. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Management of Operational Parameters and Novel Spinneret Configurations for the Electrohydrodynamic Processing of Functional Polymers. Macromol. Mater. Eng. 2022, 307, 2100858. [Google Scholar] [CrossRef]
- Jain, E.; Scott, K.M.; Zustiak, S.P.; Sell, S.A. Fabrication of Polyethylene Glycol-Based Hydrogel Microspheres Through Electrospraying. Macromol. Mater. Eng. 2015, 300, 823–835. [Google Scholar] [CrossRef]








| Solution | Zein (wt%) | Zein (g) | Ethanol (g) | Water (g) |
|---|---|---|---|---|
| A | 5 | 10.0 | 152.0 | 38.0 |
| B | 12 | 24.0 | 140.8 | 35.2 |
| C | 25 | 50.0 | 120.0 | 30.0 |
| D | 33 | 66.0 | 107.2 | 26.8 |
| E | 42 | 84.0 | 92.8 | 23.2 |
| F | 50 | 100.0 | 80.0 | 20.0 |
| Value of the Constant (k) for Each Needle as a Function of Velocity | ||||
|---|---|---|---|---|
| Speed (rpm) | Needle 1 | Needle 2 | Needle 3 | Needle 4 |
| 6 | 10 | 50 | 200 | 1000 |
| 12 | 5 | 25 | 100 | 500 |
| 30 | 2 | 10 | 40 | 200 |
| 60 | 1 | 5 | 20 | 100 |
| Number | Statement |
| 1 | Use was relevant to my academic field. |
| 2 | My knowledge in the field of knowledge has increased as a result of use. |
| 3 | My confidence in the field of knowledge has increased because of use. |
| 4 | Use of the materials and equipment motivated me to learn the content. |
| 5 | The activity provided opportunities to relate knowledge to technology. |
| 6 | The activity reflected classroom course content. |
| 7 | Use of the equipment reflected real-world/industrial practice. |
| 8 | The time of the experimental activity was adequate. |
| 9 | Use of the materials and equipment suited my learning needs. |
| 10 | The hands-on approach was important in my preparation as an engineer. |
| Statement Number | Score * |
| 1 | 4.4 ± 1.0 |
| 2 | 5.8 ± 0.4 |
| 3 | 5.3 ± 0.7 |
| 4 | 5.6 ± 0.7 |
| 5 | 5.8 ± 0.4 |
| 6 | 5.6 ± 0.5 |
| 7 | 5.8 ± 0.4 |
| 8 | 4.7 ± 0.8 |
| 9 | 5.6 ± 0.7 |
| 10 | 5.6 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Giner, S.; Yuste, A.; González-Martínez, C. Improving the Scientific Literacy of Food Engineering Students in Electrohydrodynamic Processing by Means of Zein Solutions. Educ. Sci. 2022, 12, 503. https://doi.org/10.3390/educsci12080503
Torres-Giner S, Yuste A, González-Martínez C. Improving the Scientific Literacy of Food Engineering Students in Electrohydrodynamic Processing by Means of Zein Solutions. Education Sciences. 2022; 12(8):503. https://doi.org/10.3390/educsci12080503
Chicago/Turabian StyleTorres-Giner, Sergio, Alberto Yuste, and Chelo González-Martínez. 2022. "Improving the Scientific Literacy of Food Engineering Students in Electrohydrodynamic Processing by Means of Zein Solutions" Education Sciences 12, no. 8: 503. https://doi.org/10.3390/educsci12080503







