Integrating PhET Simulations into Elementary Science Education: A Qualitative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Context and Participants
2.1.1. Remembering
- -
- The student will develop the capability to articulate the sequence of events occurring during the transformation of a specific substance from one state to another.
- -
- The student will acquire the knowledge that the application of heat to a particular substance can instigate a change in its state.
- -
- The student will possess the competence to establish connections between alterations in the state of a substance and observable occurrences in everyday life.
2.1.2. Understanding
- -
- The student will attain an understanding of the mechanism involved in the dissolution of salt within water.
- -
- The student will grasp the concept of saturation as it pertains to the point at which salt can no longer dissolve effectively in water.
2.1.3. Applying
2.1.4. Analyzing
2.1.5. Evaluation
- -
- By the end of this process, the student should have the capability to design and suggest an experimental procedure aimed at demonstrating the correlation between the quantity of solvent and solute within a specified solution.
- -
- Furthermore, the student should possess the competence to assess the effectiveness of the experiment they devised and executed, determining whether it can yield a precise and reliable outcome regarding the interplay between the amounts of solvent and solute.
2.1.6. Creation
- -
- The student will be able to illustrate the distribution of the salt particles within the water particles in solutions with a variety of salt concentrations.
- -
- The student role: The student drew two illustrations showing the distribution of salt particles within water particles in two solutions, one diluted and the other saturated.
2.2. Data Collecting and Analysis Tools
2.3. Validity and Reliability of the Analysis
3. Results
3.1. Remembering Phase
3.1.1. Description and Transcript
- 5
- Teacher: What will we do to convert a substance from the solid state to the liquid state?
- 6
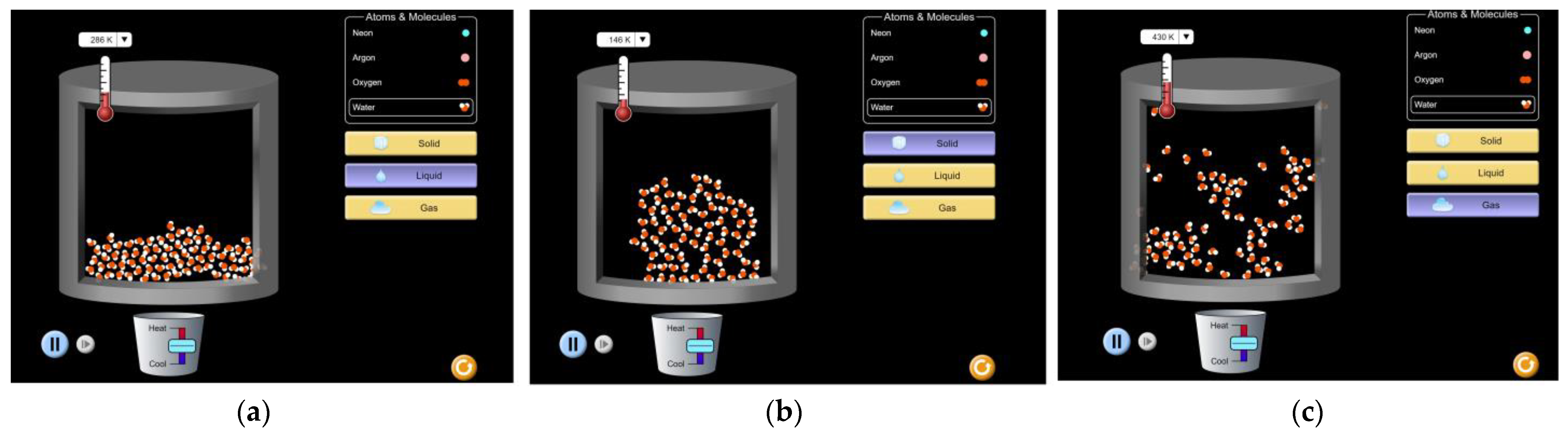
- Student 1: We are in the solid state, and I remember when we conducted the melting experiment in the laboratory. We lit a candle under the beaker containing ice. Now, I will do the same thing; I will ignite the fire under the beaker. Look how the particles move away from each other, and the substance takes the shape of the beaker. This was not visible in the previous experiment; it’s wonderful. I want to heat the substance a little, not too much, because if the heating process continues for a long time, the particles will move far apart and scatter. So, for now, this is sufficient; I will reduce the fire, as the substance has taken the shape of the container (See Figure 1a above).
- 7
- Student 2: And now we have the substance in the liquid state. I will ignite the fire again to convert the substance into the gaseous state (See Figure 1b above). It reminded me of an incident with my mother where she forgot the water on the stove, and when I returned, there was no water left. Hahaha, I will go and tell my mother that she should not leave the water on the stove for a long time because it will evaporate if it continues to boil for a long time, and there will be no water left for tea.
- 8
- Student 3: I remember when we learned about this topic in class, and you gave us an example of making an ice cream and how we can convert it from the liquid state to the solid state. Now, I want to do the same thing; I will place the ice under the container for it to solidify, and the particles come closer to each other (See Figure 1c above).
- 9
- Student 2: We can also create any shape we want using liquid dough. We made ice cream in different shapes like squares and circles when we learned about the topic, using suitable molds.
3.1.2. Analysis of Students’ Learning at the Remembering Phase
3.2. Understanding Phase
3.2.1. Description and Transcript
- 12
- Teacher: What happens when you place a quantity of salt in a water container?
- 13
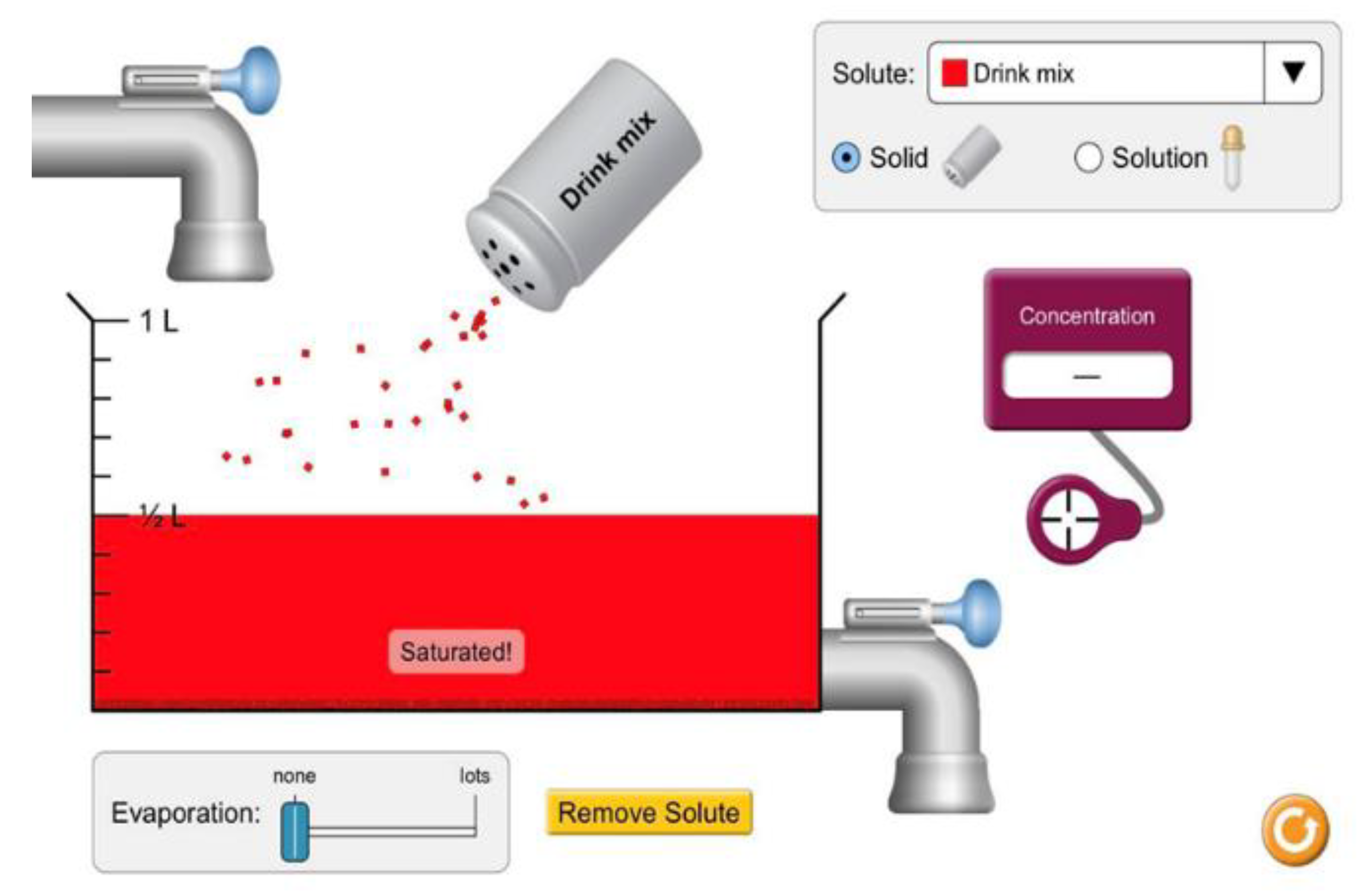
- Student 1: When I added some salt, it dissolved in the water, but when I added a lot of salt, the word “saturation” appeared, and some salt settled at the bottom of the container (See Figure 2 above).
- 14
- Students: (The other students started pressing the saltshaker to release the salt, and after adding a large amount of salt, they observed the word “saturation”).
- 15
- Student 2: This is indeed what happens because we added a large amount of salt that the water could no longer dissolve, so the salt precipitated at the bottom of the container.
3.2.2. Analysis of Students’ Learning at the Understanding Phase
3.3. Application Phase
3.3.1. Description and Transcript
- 16
- Teacher: Can you describe how to separate salt from water?
- 17
- Student 1: If we ignite the fire, the water will evaporate and only the salt will remain.
- 18
- Student 2: We evaporate the water to separate it from the salt.
- 19
- Student 3: During the experiment that we performed, we evaporated the water, causing it to turn into a gaseous state and disperse. As a result, only the salt remained. It became evident that the water evaporated while the salt was left behind (See Figure 3 above).
3.3.2. Analysis of Students’ Learning at the Application Phase
3.4. Analysis Phase
3.4.1. Description and Transcript
- 19
- Teacher: Can the same substance exist in three states? Please provide an example.
- 20
- Student 3: Yes, a substance that can exist in three states is water. It takes the form of solid as ice, then transforms into a liquid as water, and changes into a gas as vapor.
- 21
- Student 1: (Ignited the fire under the ice till it melted into water. She kept heating the water until it evaporated (See Figure 4 above)).
- 22
- Student 3: If we examine the three microscopic levels of water and compare them, we can categorize them as three states of the same substance.
3.4.2. Analysis of Students’ Learning at the Analysis Phase
3.5. Evaluation Phase
3.5.1. Description and Transcript
- 27
- Teacher: Now, I would like you to plan an experiment that demonstrates the relationship between the volume of the solvent and the quantity of the solute. (The students restarted the simulation by pressing the reset button).
- 28
- Student 1: Currently, we only have water as the solvent. We will add a small amount of salt and observe its dissolution. (The students observed that the salt particles separated from each other).
- 29
- Student 3: Let’s continue adding salt until the word “saturation” appears on the screen. Look, the word “saturation” has appeared, indicating that the water is saturated. If we add more salt now, it will precipitate to the bottom. (The students observed that the salt no longer dissolves in water due to reaching the saturation point (see Figure 5a above)).
- 30
- Student 2: What happens when we add water to the precipitated salt? (The students added water to the container).
- 31
- Student 1: Oh, the salt particles have separated again because we added water, and the salt dissolved in the added water (see Figure 5b above).
- 32
- Teacher: So, what is the relationship between the solvent and the solute?
- 33
- Student 3: Whenever the quantity of the solute (salt) increases, we must add more solvent (water). We also observed that the ion concentration of the salt increased when it dissolved, reaching a value of 271 (see Figure 5b above). On the other hand, when a portion of the salt precipitated, the ion concentration was 180. This indicates that the quantity of dissolved salt increases when water is added.
- 34
- Teacher: Based on the experiment you performed in the simulation system, is it possible to accurately determine the relationship between the amount of solvent and the amount of solute?
- 35
- Student 1: In the experiments we have carried out so far in the laboratory, we have come to the point where it is impossible to make a conclusion based on the results of one experiment. Therefore, we must have more measurements related to the volume of the solvent and the amount of the solute.
- 36
- Teacher: What additional measurements are we required to carry out in order to accurately determine the relationship?
- 37
- Student 2: We need to perform a number of experiments in which we maintain a constant volume of solvent (water) and add a different amount of salt each time and see if the salt dissolves or not. We then can observe when the salt begins to sink to the bottom of the container.
3.5.2. Analysis of Students’ Learning at the Evaluation Phase
3.6. Creation Phase
3.6.1. Description and Transcript
- 38
- Teacher: Please discuss the question and draw the required distribution.
- 39
- Students: (The students in the group discussed the questions and each student drew a drawing depicting the containers).
- 40
- Student 3: In the first container, the salt particles will be homogeneously distributed throughout the solvent particles, while in the second container, more salt particles will be in the cold lower part.
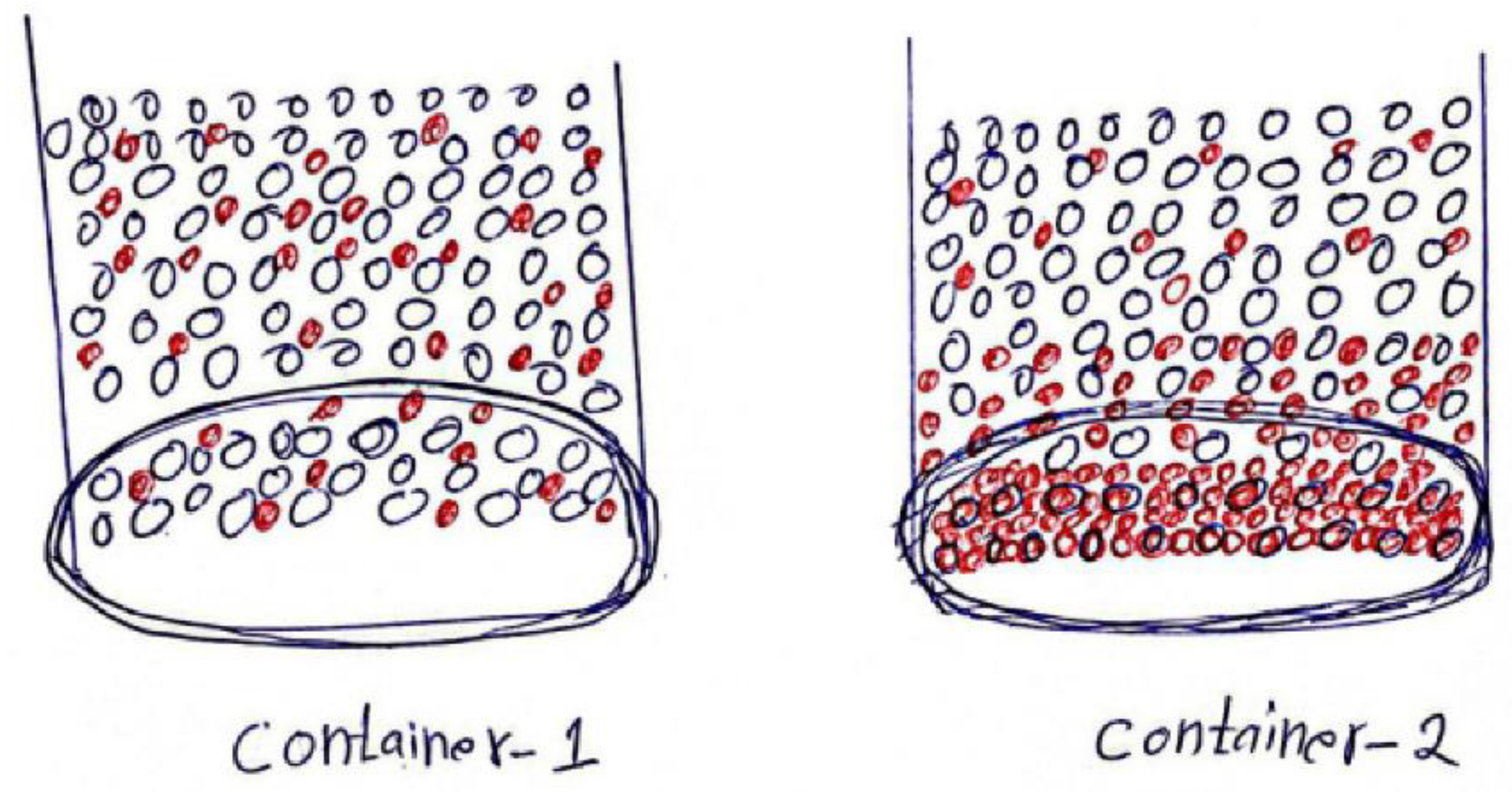
- 41
- Student 2: In both containers, the salt particles will be distributed fairly in the water, except that in the second container, there will be more salt particles in the water since we added more salt to the container.
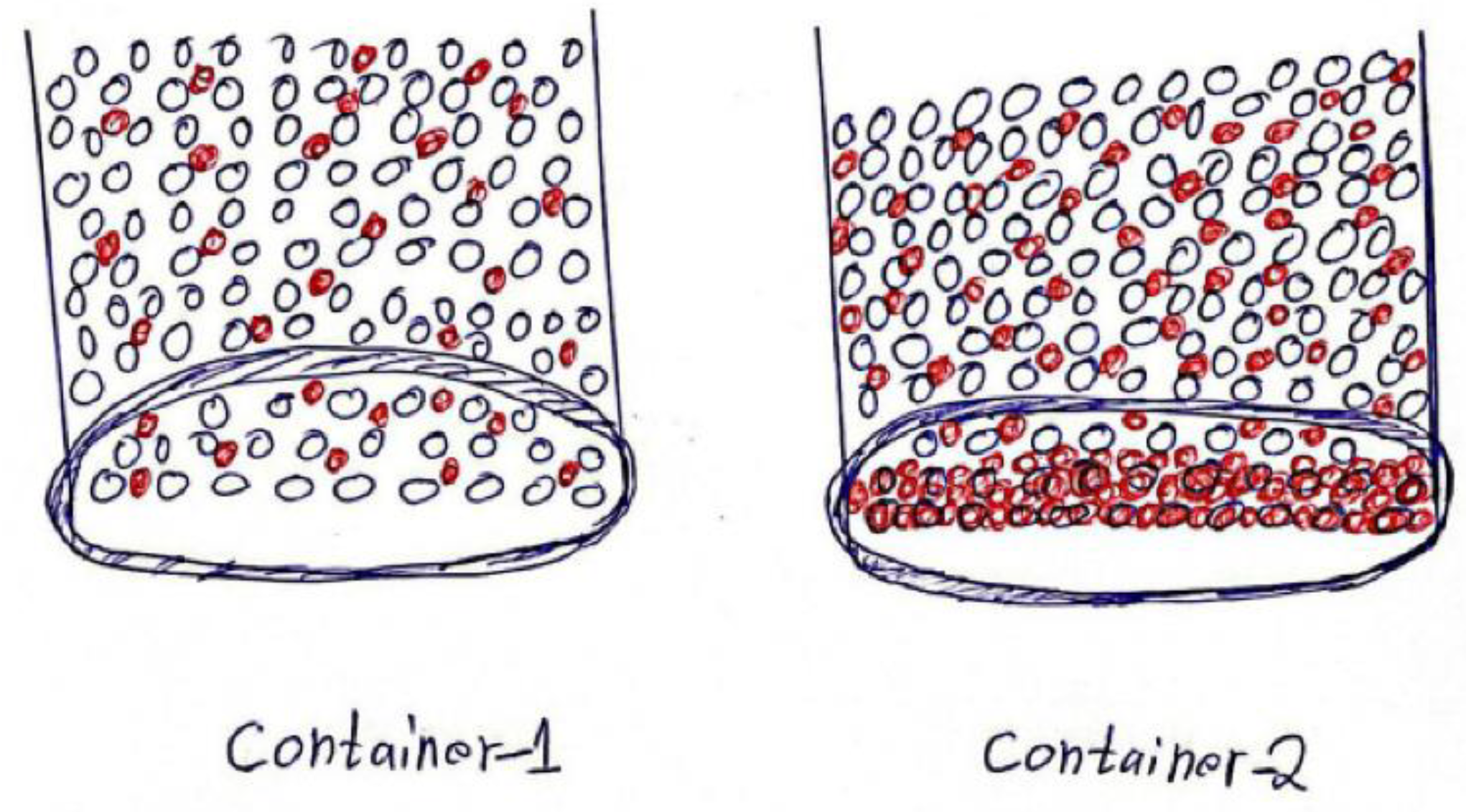
3.6.2. Analysis of Students’ Learning at the Creation Phase
4. Discussion
4.1. Remembering Knowledge
4.2. Understanding Knowledge
4.3. Application Knowledge
4.4. Analysis Knowledge
4.5. Evaluation Knowledge
4.6. The Creation Phase
5. Conclusions and Future Practice
6. Limitations and Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brigas, C.J. Modeling and simulation in an educational context: Teaching and learning sciences. Res. Soc. Sci. Technol. 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Lee, I.; Martin, F.; Apone, K. Integrating computational thinking across the K--8 curriculum. ACM Inroads 2014, 5, 64–71. [Google Scholar] [CrossRef]
- Shamir, G.; Tsybulsky, D.; Levin, I. Introducing Computational Thinking Practices in Learning Science of Elementary Schools. In Proceedings of the InSITE 2019: Informing Science+ IT Education Conferences, Jerusalem, Israel, 30 June–4 July 2019; pp. 187–205. [Google Scholar]
- Leutner, D. Guided discovery learning with computer-based simulation games: Effects of adaptive and non-adaptive instructional support. Learn. Instr. 1993, 3, 113–132. [Google Scholar] [CrossRef]
- Hallinger, P.; Wang, R. The Evolution of Simulation-Based Learning Across the Disciplines, 1965–2018: A Science Map of the Literature. Simul. Gaming 2019, 51, 32–39. [Google Scholar] [CrossRef]
- Jong, T.D.; Lazonder, A.W.; Pedaste, M.; Zacharia, Z.C. Simulations, Games, and Modeling Tools for Learning; Routledge: London, UK, 2018. [Google Scholar]
- de Jong, T.; van Joolingen, W. Model-facilitated learning. In Handbook of Research on Educational Communication and Technology, 3rd ed.; Spector, J.M., Merrill, M.D., Elen, J., Bishop, M.J., Eds.; Lawrence Erlbaum: Mahwah, NJ, USA, 2008; pp. 457–468. [Google Scholar]
- Crookall, D.; Saunders, D. Towards an integration of communication and simulation. In Communication and Simulation: From Two fields to One Theme; Crookall, D., Saunders, D., Eds.; Avon: London, UK; Multilingual Matters: Bristol, UK, 1989; pp. 3–19. [Google Scholar]
- Maran, N.J.; Glavin, R.J. Low- to high-fidelity simulation—A continuum of medical education? Med. Educ. 2003, 37 (Suppl. S1), 22–28. [Google Scholar] [CrossRef] [PubMed]
- Issenberg, S.B.; McGaghie, W.C.; Hart, I.R.; Mayer, J.W.; Felner, J.M.; Petrusa, E.R.; Waugh, R.A.; Brown, D.D.; Safford, R.R.; Gessner, I.H.; et al. Simulation technology for health care professional skills training and assessment. JAMA 1999, 282, 861–866. [Google Scholar] [CrossRef]
- Lu, J.-F.; Hallinger, P.; Showanasai, P. Simulation-based learning in management education: A longitudinal quasi-experimental evaluation of instructional effectiveness. J. Manag. Dev. 2014, 33, 218–244. [Google Scholar] [CrossRef]
- Jong, T.; Linn, M.; Zacharia, Z. Physical and Virtual Laboratories in Science and Engineering Education. Science 2013, 340, 305–308. [Google Scholar] [CrossRef]
- Keys, J.B.; Wolfe, J. The Role of Management Games and Simulation in Education and Research. J. Manag. 1990, 16, 307–336. [Google Scholar] [CrossRef]
- Garris, R.; Ahlers, R.; Driskell, J. Games, Motivation, and Learning: A Research and Practice Model; SAGE Publications: Singapore, 2017; pp. 475–501. [Google Scholar]
- Cayvaz, A.; Akcay, H.; Kapici, H.O. Comparison of simulation-based and textbook-based instructions on middle school students’ achievement, inquiry skills and attitude. Int. J. Educ. Math. Sci. Technol. 2020, 8, 34–43. [Google Scholar] [CrossRef]
- Learning Science through Computer Games and Simulations; The National Academies Press: Washington, DC, USA, 2011.
- Tsai, F.-H.; Hsu, I.Y. Exploring The Effects of Guidance in a Computer Detective Game for Science Education. J. Balt. Sci. Educ. 2020, 19, 647–658. [Google Scholar] [CrossRef]
- Land, S.; Hannafin, M. A conceptual framework for the development of theories-in-action with open learning environments. Educ. Technol. Res. Dev. 1996, 44, 37–53. [Google Scholar] [CrossRef]
- Talan, T. The effect of simulation technique on academic achievement: A meta-analysis study. Int. J. Technol. Educ. Sci. 2021, 5, 17–36. [Google Scholar] [CrossRef]
- Lindgren, R.; Schwartz, D. Spatial Learning and Computer Simulations in Science. Int. J. Sci. Educ. 2009, 31, 419–438. [Google Scholar] [CrossRef]
- Clark, D.; Nelson, B.; Sengupta, P.; D’Angelo, C. Rethinking Science Learning through Digital Games and 1 Simulations: Genres, Examples, and Evidence; ResearchGate: Berlin, Germany, 2009. [Google Scholar]
- Faria, A. The Changing Nature of Business Simulation/Gaming Research: A Brief History. Simul. Gaming 2001, 32, 97–110. [Google Scholar] [CrossRef]
- Thisgaard, M.W.; Makransky, G. Virtual Learning Simulations in High School: Effects on Cognitive and Non-cognitive Outcomes and Implications on the Development of STEM Academic and Career Choice. Front. Psychol. 2017, 8, 805. [Google Scholar] [CrossRef]
- Kember, D. Misconceptions about the Learning Approaches, Motivation and Study Practices of Asian Students. High. Educ. 2000, 40, 99–121. [Google Scholar] [CrossRef]
- Collins, A.; Duguid, P. Situated Cognition and Culture of Learning. Educ. Res. 1989, 18, 32–42. [Google Scholar]
- Moore, E.B.; Perkins, K.K. Advances in PhET interactive simulations: Interoperable and accessible. In Cyber-Physical Laboratories in Engineering and Science Education; Auer, M.E., Azad, A.K.M., Edwards, A., de Jong, T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 141–162. [Google Scholar]
- Anderson, L.W.; Krathwohl, D.R. A Taxonomy for Learning, Teaching, and Assessing, Abridged Edition; Allyn and Bacon: Boston, MA, USA, 2001; Available online: https://www.astate.edu/dotAsset/11ca93f7-da45-4fe3-821b-b82a20cbc017.pdf (accessed on 9 August 2023).
- Daher, W. Discursive positionings and emotions in modelling activities. Int. J. Math. Educ. Sci. Technol. 2015, 46, 1149–1164. [Google Scholar] [CrossRef]
- Daher, W. Saturation in Qualitative Educational Technology Research. Educ. Sci. 2023, 13, 98. [Google Scholar] [CrossRef]
- Lincoln, Y.S.; Guba, E.G. Naturalistic Inquiry; Sage Publications: Newbury Park, CA, USA, 1985. [Google Scholar]
- Hernández-Ramos, J.; Cáceres-Jensen, L.; Rodríguez-Becerra, J. Educational Computational Chemistry for In-Service Chemistry Teachers: A Data Mining Approach to E-Learning Environment Redesign. Educ. Sci. 2023, 13, 796. [Google Scholar] [CrossRef]
- Yusa, N.; Hamada, R. Board Game Design to Understand the National Power Mix. Educ. Sci. 2023, 13, 793. [Google Scholar] [CrossRef]
- Janeš, A.; Madsen, S.S.; Saure, H.I.; Lie, M.H.; Gjesdal, B.; Thorvaldsen, S.; Brito, R.; Krasin, S.; Jwaifell, M.; Konca, A.S.; et al. Preliminary Results from Norway, Slovenia, Portugal, Turkey, Ukraine, and Jordan: Investigating Pre-Service Teachers’ Expected Use of Digital Technology When Becoming Teachers. Educ. Sci. 2023, 13, 783. [Google Scholar] [CrossRef]
- Thyssen, C.; Huwer, J.; Irion, T.; Schaal, S. From TPACK to DPACK: The “Digitality-Related Pedagogical and Content Knowledge”-Model in STEM-Education. Educ. Sci. 2023, 13, 769. [Google Scholar] [CrossRef]
- Rayment, S.; Evans, J.R.; Coffey, M.; Kirk, S.; Sivasubramaniam, S.D.; Moss, K. The Role of Technology in Undergraduate Bioscience Laboratory Learning: Bridging the Gap between Theory and Practice. Educ. Sci. 2023, 13, 766. [Google Scholar] [CrossRef]
- Daher, W.; Swidan, O. Positioning–Emotions Association of Young Students Using Digital Technology. Mathematics 2021, 9, 1617. [Google Scholar] [CrossRef]
- Daher, W.; Shayeb, S.; Jaber, R.; Dawood, I.; Abo Mokh, A.; Saqer, K.; Bsharat, M.; Rabbaa, M. Task design for online learning: The case of middle school mathematics and science teachers. Front. Educ. 2023, 8, 1161112. [Google Scholar] [CrossRef]
- Luo, R.; Song, L. The unique and compensatory effects of home and classroom learning activities on Migrant and Seasonal Head Start children’s Spanish and English emergent literacy skills. Front. Psychol. 2022, 13, 1016492. [Google Scholar] [CrossRef] [PubMed]
- Daher, W. Students’ adoption of social networks as environments for learning and teaching: The case of the Facebook. Int. J. Emerg. Technol. Learn. 2014, 9, 16–24. [Google Scholar] [CrossRef]
- Mengistu, A.; Kahsay, G. The effect of computer simulation used as a teaching aid in students’ understanding in learning the concepts of electric fields and electric forces. Lat. Am. J. Phys. Educ. 2015, 9, 3. [Google Scholar]
- Nafidi, Y.; Alami, A.; Moncef, Z.A.K.I.; El Batri, B.; Afkar, H. Impacts of the use of a digital simulation in learning earth sciences (the case of relative dating in high school). J. Turk. Sci. Educ. 2018, 15, 89–108. [Google Scholar]
- Daher, W.; Sleem, H. Middle school students’ learning of social studies in the video and 360-degree videos contexts. IEEE Access 2021, 9, 78774–78783. [Google Scholar] [CrossRef]
- Daher, W.; Baya’a, N. Characteristics of middle school students learning actions in outdoor mathematical activities with the cellular phone. Teach. Math. Its Appl. Int. J. IMA 2012, 31, 133–152. [Google Scholar] [CrossRef]
- Jing, D. The Study on Educational Technology Abilities Evaluation Method. Phys. Procedia 2012, 24, 2111–2116. [Google Scholar] [CrossRef]
- Daher, W.; Awawdeh Shahbari, J. Secondary students’ identities in the virtual classroom. Sustainability 2020, 12, 4407. [Google Scholar] [CrossRef]
- Wigfield, A. A Questionnaire Measure of Children’s Motivations for Reading; National Reading Research Center: College Park, MD, USA, 1996. [Google Scholar]
- Kohake, K.; Heemsoth, T. Need support, need satisfaction and types of motivation in Physical Education for children aged 8 to 13. Development and preliminary validation of the German SMoPE-instrument. Curr. Issues Sport Sci. 2021, 6, 5. [Google Scholar] [CrossRef]



| Category | Themes |
|---|---|
| Remembering | Recall, tell, what, when, list, find |
| Understanding | Explain, extend, classify, relate, rephrase |
| Application | Apply, choose, select, utilize, use |
| Analysis | Compare, classify, categorize, contrast, infer |
| Evaluation | Agree, assess, appraise, criticize, estimate |
| Creating | Build, change, combine, create, elaborate |
| Raw | Participant | Action/Interaction | Analysis |
|---|---|---|---|
| 5 | Teacher: | What will we do to convert a substance from the solid state to the liquid state | |
| 6 | Student 1 | We are in the solid state, and I remember when we conducted the melting experiment in the laboratory. We lit a candle under the beaker containing ice. Now, I will do the same thing; I will ignite the fire under the beaker. Look how the particles move away from each other, and the substance takes the shape of the beaker. This was not visible in the previous experiment; it’s wonderful. | Remembering: The students remembered what they did in the previous experiment. |
| 7 | Student 2 | Now we have the substance in the liquid state. I will ignite the fire again to convert the substance into the gaseous state. It reminded me of an incident with my mother where she forgot the water on the stove, and when I returned, there was no water left. | Remembering: The student remembered a real-life incident related to the topic of the lesson that happened at home. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayan, B.; Daher, W.; Diab, H.; Issa, N. Integrating PhET Simulations into Elementary Science Education: A Qualitative Analysis. Educ. Sci. 2023, 13, 884. https://doi.org/10.3390/educsci13090884
Rayan B, Daher W, Diab H, Issa N. Integrating PhET Simulations into Elementary Science Education: A Qualitative Analysis. Education Sciences. 2023; 13(9):884. https://doi.org/10.3390/educsci13090884
Chicago/Turabian StyleRayan, Baraa, Wajeeh Daher, Hussam Diab, and Nael Issa. 2023. "Integrating PhET Simulations into Elementary Science Education: A Qualitative Analysis" Education Sciences 13, no. 9: 884. https://doi.org/10.3390/educsci13090884
APA StyleRayan, B., Daher, W., Diab, H., & Issa, N. (2023). Integrating PhET Simulations into Elementary Science Education: A Qualitative Analysis. Education Sciences, 13(9), 884. https://doi.org/10.3390/educsci13090884







