Between Symbols and Particles: Investigating the Complexity of Learning Chemical Equations
Abstract
:1. Introduction
2. Theoretical Background
2.1. Chemical Equations as a Part of Chemical Education
2.2. Students’ Chemical Equations Abilities
3. Research Aim
4. Materials and Methods
4.1. Research Sample
4.2. Research Procedure
4.3. Equipment
4.4. Research Tools
4.5. Data Analysis
4.5.1. Tests
4.5.2. Eye-Tracking
4.5.3. Statistical Analysis
5. Results
5.1. Success Rate and Time to Balance Chemical Equations
5.2. Results in the Pre-Tests and Their Relation to the Overall Equations Balancing Result
- Group 1—successful students 1, 2, 3, 6, 10;
- Group 2—average students 4, 5, 7;
- Group 3—unsuccessful students 8, 9, 11.
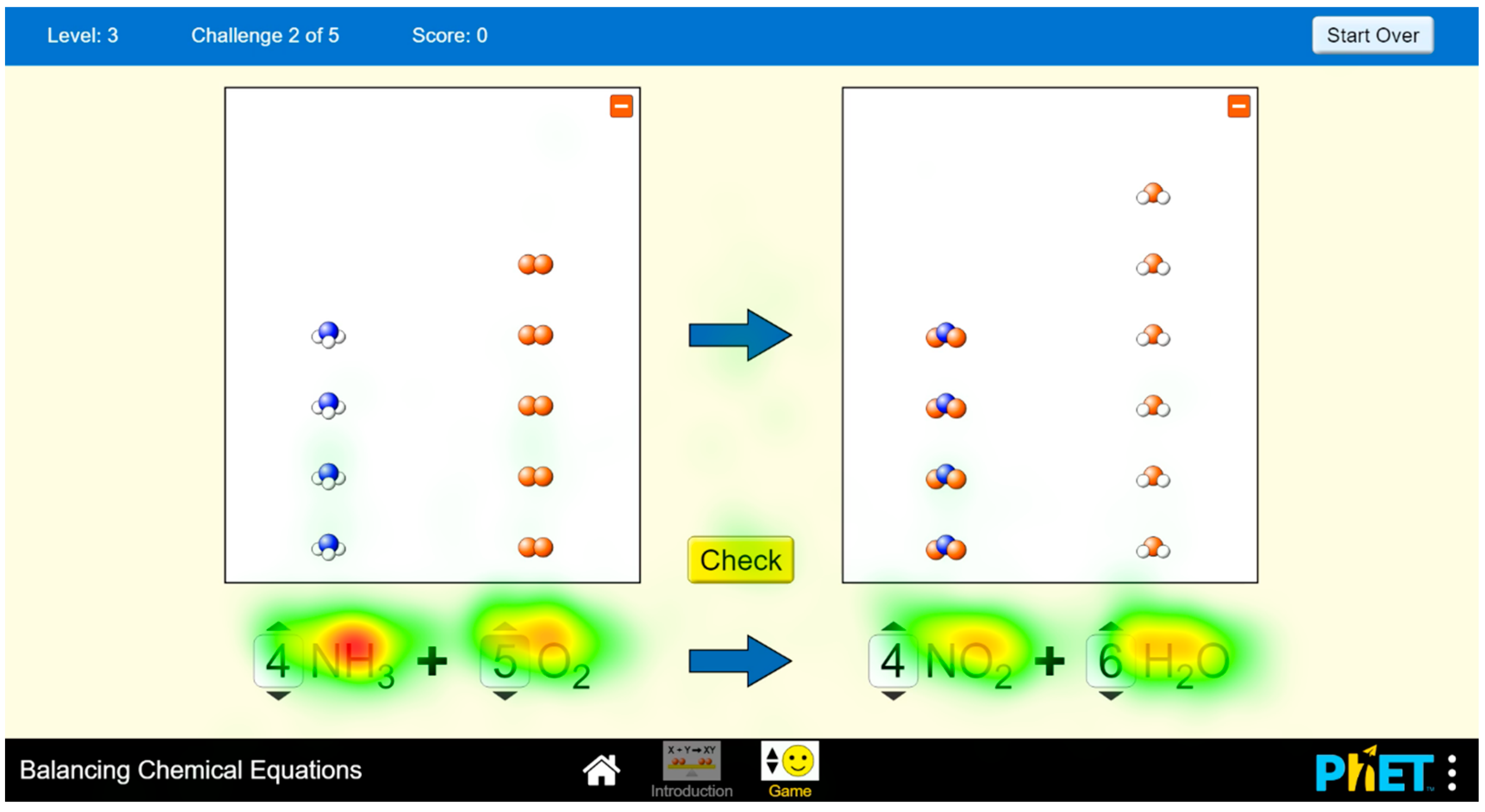
5.3. Use of the Applet Environment
“…, it always appeared there. I looked at it, saw it was there. Hmm, that’s the molecule.”(S2)
“I know I was surprised that something changed and something new appeared there, so I looked at it.”(S7)
“Hmm, I had to consciously look at them [note: models], but I didn’t deduce anything from it [note: models]. Just like: it doesn’t equal, well. But I see that here too. I calculated within those coefficients that they are not equal. … I really can’t say to what extent watching those balls, for instance in the case of this equation, was then a result of some frustration from it not working out.”(S5)
“Here, I was counting the balls when it didn’t add up. But I knew it wasn’t balanced. That I could see it from those numbers. … Based on the principle that a drowning man will clutch at straws, I similarly attempted to calculate the models.”(S8)
“I tried looking at the models to see if they would help me. But I concluded that I‘d rather go back to that equation.”(S7)
Reasons for Non-Use of Sub-Micro Representations
“I preferred to proceed through the numbers. Even though math isn’t exactly my strongest subject, I still preferred to work numerically rather than rely on pictures in this case... Looking back now, those pictures, the way those molecules are depicted, don’t look bad either. Maybe I could have focused more on them as well. That I might have managed to balance the previous equation quicker. But still, numbers and paper are my favorites.”(S3)
“We never had any models anywhere. So, a person primarily focuses on the equation.”(S5)
“I tried not to look at them [note: models] because I’m not used to it, so it didn’t seem all that important or helpful to me.”(S8)
“Now I realize that always, the things that are increasing, the balls that are added, are above it. It’s really silly, but I didn’t get it at the moment. That here oxygen is being added, so it’s added here. Here we have ethyne, so it’s added above it. At that moment, I didn’t assess it like this at all. Just left, right.”(S5)
“It rather distracts me, I don’t take it as help, that those balls or molecules are accumulating there.”(S8)
5.4. Students’ Approach When Balancing Chemical Equations
5.4.1. Group 1
5.4.2. Group 2
5.4.3. Group 3
6. Discussion
6.1. Chemical Equations as Chemical Concepts’ Intersection
6.2. Balancing Chemical Equations as a Problem-Solving Task
6.3. Balancing Chemical Equations in the PhET Applet Environment
7. Limitations
8. Conclusions
- Integrated Instructional Designs: To design instructional materials that seamlessly integrate macroscopic, sub-microscopic, and symbolic representations to help students make meaningful connections between different levels of understanding to chemical reactions.
- Curriculum Development: To develop curricular materials that emphasize the interconnectedness of different representations of chemical concepts and ensure that these materials foster a comprehensive understanding of chemical processes rather than treating skills like equation balancing as isolated tasks.
- Teacher Training: To provide professional development opportunities for teachers to help them understand and implement instructional strategies that integrate multiple representations of chemical concepts and to emphasize the importance of moving beyond algorithmic approaches to fostering deep conceptual understanding.
- Use of Digital Tools: To carefully integrate digital tools into the chemistry curriculum in a way that complements and enhances traditional teaching methods and to ensure that these tools are used to support students’ understanding across all representational levels without causing confusion or distraction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnstone, A.H. Macro-and micro-chemistry. Sch. Sci. Rev. 1982, 64, 377–379. [Google Scholar]
- Gilbert, J.K.; Treagust, D. Multiple Representations in Chemical Education; Gilbert, J.K., Treagust, D., Eds.; Models and Modeling in Science Education; Springer: Dordrecht, The Netherlands, 2009; Volume 4. [Google Scholar]
- Talanquer, V. Macro, Submicro, and Symbolic: The many faces of the chemistry “triplet”. Int. J. Sci. Educ. 2011, 33, 179–195. [Google Scholar] [CrossRef]
- Liaghatdar, M.J.; Soltani, A.; Abedi, A. A Validity Study of Attitudes toward Science Scale among Iranian Secondary School Students. Int. Educ. Stud. 2011, 4, 36–46. [Google Scholar] [CrossRef]
- Najdi, S. Students attitude toward learning chemistry. J. Al-Quds Open Univ. Educ. Psychol. Res. Stud. 2018, 1, 12. [Google Scholar] [CrossRef]
- Osborne, J.; Collins, S.; Ratcliffe, M.; Millar, R.; Duschl, R. What “ideas-about-science” should be taught in school science? A Delphi study of the expert community. J. Res. Sci. Teach. 2003, 40, 692–720. [Google Scholar] [CrossRef]
- Salta, K.; Tzougraki, C. Attitudes toward chemistry among 11th grade students in high schools in Greece. Sci. Educ. 2004, 88, 535–547. [Google Scholar] [CrossRef]
- Weinburgh, M. Gender differences in student attitudes toward science: A meta-analysis of the literature from 1970 to 1991. J. Res. Sci. Teach. 1995, 32, 387–398. [Google Scholar] [CrossRef]
- Cahill, M.J.; McDaniel, M.A.; Frey, R.F.; Hynes, K.M.; Repice, M.; Zhao, J.; Trousil, R. Understanding the relationship between student attitudes and student learning. Phys. Rev. Phys. Educ. Res. 2018, 14, 010107. [Google Scholar] [CrossRef]
- Solem, M.; Vaughan, P.W. Reducing Inequality in Student Outcomes in U.S. Geography Education: The Importance of Understanding Student Attitudes. Educ. Sci. 2024, 14, 9. [Google Scholar] [CrossRef]
- Flaherty, A.A. A review of affective chemistry education research and its implications for future research. Chem. Educ. Res. Pract. 2020, 21, 698–713. [Google Scholar] [CrossRef]
- Childs, P.E.; Sheehan, M. What’s difficult about chemistry? An Irish perspective. Chem. Educ. Res. Pract. 2009, 10, 204–218. [Google Scholar] [CrossRef]
- Johnstone, A.H. Topic Difficulties in Chemistry. Educ. Chem. 1971, 8, 212–218. [Google Scholar]
- Morabe, O.N. The Impact of the SEDIBA Project on the Attitude of Participating Educators towards Chemistry and Chemistry Teaching. Master’s Thesis, Northwest University, Kirkland, WA, USA, 2004. [Google Scholar]
- Moyo, C. Investigating the Areas of Student Difficulty in Chemistry Curriculum: A Case Study in Qatar. Texila Int. J. Acad. Res. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Rychtera, J.; Bilek, M. Kritická Místa Kurikula Chemie na 2. Stupni Základní Školy I.; Západočeská univerzita v Plzni: Pilsen, Poland, 2019. [Google Scholar]
- Ben-Zvi, N.; Genut, S. Uses and limitations of scientific models: The Periodic Table as an inductive tool. Int. J. Sci. Educ. 1998, 20, 351–360. [Google Scholar] [CrossRef]
- Yarroch, W.L. Student understanding of chemical equation balancing. J. Res. Sci. Teach. 1985, 22, 449–459. [Google Scholar] [CrossRef]
- Hinton, M.E.; Nakhleh, M.B. Students’ microscopic, macroscopic, and symbolic representations of chemical reactions. Chem. Educ. 1999, 4, 158–167. [Google Scholar] [CrossRef]
- Jaber, L.Z.; BouJaoude, S. A Macro–Micro–Symbolic Teaching to Promote Relational Understanding of Chemical Reactions. Int. J. Sci. Educ. 2012, 34, 973–998. [Google Scholar] [CrossRef]
- Chittleborough, G.D.; Treagust, D.F.; Mocerino, M. Constraints to the development of first year university chemistry students’ mental models of chemical phenomena. In Teaching and Learning Forum: Focusing on the Student; Edith Cowan University: Perth, WA, Australia, 2002. [Google Scholar]
- Marais, P.; Jordaan, F. Are We Taking Symbolic Language for Granted? J. Chem. Educ. 2000, 77, 1355. [Google Scholar] [CrossRef]
- Al-Kunifed, A.A. Investigation of High School Chemistry Students’ Concepts of Chemical Symbol, Formula, and Equation: Students’ Prescientific Conceptions. Ph.D. Thesis, The Department of Curriculum and Instruction, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 1993. [Google Scholar]
- Vojíř, K.; Rusek, M. Of teachers and textbooks: Lower secondary teachers’ perceived importance and use of chemistry textbook components. Chem. Educ. Res. Pract. 2022, 23, 786–798. [Google Scholar] [CrossRef]
- Chen, X.; de Goes, L.F.; Treagust, D.F.; Eilks, I. An analysis of the visual representation of redox reactions in secondary chemistry textbooks from different chinese communities. Educ. Sci. 2019, 9, 42. [Google Scholar] [CrossRef]
- Chlumecká, L. Analýza Vizuálních Reprezentací Zařazených v Tematickém Celku Organické Sloučeniny v Učebnicích Chemie pro Základní Školy [Analysis of Visual Representations Included in the Thematic Unit of Organic Compounds in Chemistry Textbooks for Elementary Schools]. Bachelor’s Thesis, Univerzita Karlova, Pedagogická Fakulta, Praha, Poland, 2021. [Google Scholar]
- Krumlová, E. Hodnocení Vizuálních Reprezentací Využitých v Učebnicích Chemie pro ZŠ v Tématech Kyselin, Zásad a Neutralizace [Evaluation of Visual Representations Used in Chemistry Textbooks for Elementary Schools in the Topics of Acids, Bases, and Neutralization]. Bachelor’s Thesis, Univerzita Karlova, Pedagogická Fakulta, Praha, Poland, 2022. [Google Scholar]
- Upahi, J.E.; Ramnarain, U. Representations of chemical phenomena in secondary school chemistry textbooks. Chem. Educ. Res. Pract. 2019, 20, 146–159. [Google Scholar] [CrossRef]
- Kelly, R.M.; Barrera, J.H.; Mohamed, S.C. An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions. J. Chem. Educ. 2010, 87, 113–118. [Google Scholar] [CrossRef]
- Kern, A.L.; Wood, N.B.; Roehrig, G.H.; Nyachwaya, J. A Qualitative Report of the Ways High School Chemistry Students Attempt to Represent a Chemical Reaction at the Atomic/Molecular Level. Chem. Educ. Res. Pract. 2010, 11, 165–172. [Google Scholar] [CrossRef]
- Méndez Gutiérrez, L.; Duarte, P.P.; Méndez, P.L.P. Balanceo de ecuaciones químicas usando propiedades de los vectores en el espacio tridimensional R3. Educ. Química 2024, 35, 127–134. [Google Scholar] [CrossRef]
- Hussien, N.M.; Mohialden, Y.M.; Mahmood, N.T. Intelligent Educational Software for Chemical Reaction Balance Problems. Int. J. Early Child. Spec. Educ. 2022, 14, 6509–6512. [Google Scholar]
- Tóth, Z.; Sebestyén, A. Relationship between Students’ Knowledge Structure and Problem-Solving Strategy in Stoichiometric Problems based on the Chemical Equation. Int. J. Phys. Chem. Educ. 2009, 1, 8–20. [Google Scholar]
- Rusek, M.; Vojíř, K.; Bártová, I.; Klečková, M.; Sirotek, V.; Štrofová, J. To what extent do freshmen university chemistry students master chemistry calculations? Acta Chim. Slov. 2022, 69, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Krajcik, J.S. Developing student’s understanding of chemical concepts. In The Psychology of Learning Science; Glynn, S.M., Yeany, R.H., Britton, B.K., Eds.; Routledge: Abingdon, UK, 2012; pp. 117–148. [Google Scholar]
- Hesse, J.J.; Anderson, C.W. Students’ Conceptions of Chemical Change. J. Res. Sci. Teach. 1992, 29, 277–299. [Google Scholar] [CrossRef]
- Eylon, B.S.; Ben-Zvi, R.; Silberstein, J. Hierarchical task analysis—An approach for diagnosing students’ conceptual difficulties. Int. J. Sci. Educ. 1987, 9, 187–196. [Google Scholar] [CrossRef]
- Osborne, R.J.; Cosgrove, M.M. Children’s conceptions of the changes of state of water. J. Res. Sci. Teach. 1983, 20, 825–838. [Google Scholar] [CrossRef]
- Cheng, M.M.W. Students’ visualisation of chemical reactions—Insights into the particle model and the atomic model. Chem. Educ. Res. Pract. 2018, 19, 227–239. [Google Scholar] [CrossRef]
- Ben-Zvi, R.; Silberstein, J.; Eylon, B.S. Students’ visualization of a chemical reaction. Educ. Chem. 1987, 24, 117–120. [Google Scholar]
- Musengimana, J.; Kampire, E.; Ntawiha, P. Factors Affecting Secondary Schools Students’ Attitudes toward Learning Chemistry: A Review of Literature. Eurasia J. Math. Sci. Technol. Educ. 2021, 17, em1931. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.H. You can’t get there from here. J. Chem. Educ. 2010, 87, 22–29. [Google Scholar] [CrossRef]
- Tóthová, M.; Rusek, M. “Do you just have to know that?” Novice and experts’ procedure when solving science problem tasks. Front. Educ. 2022, 7, 1051098. [Google Scholar] [CrossRef]
- Johnstone, A.H. Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn. 1991, 7, 75–83. [Google Scholar] [CrossRef]
- Piaget, J. The Psychology of Intelligence; Harcourt Brace: San Diego, CA, USA, 1950. [Google Scholar]
- Dale, L.G. The growth of systematic thinking: Replication and analysis of Piaget’s first chemical experiment. Aust. J. Psychol. 1970, 22, 277–286. [Google Scholar] [CrossRef]
- McKinnon, J.W.; Renner, J.W. Are Colleges Concerned with Intellectual Development? Am. J. Phys. 1971, 39, 1047–1052. [Google Scholar] [CrossRef]
- Devetak, I.; Glažar, S.A. The Influence of 16-year-old Students’ Gender, Mental Abilities, and Motivation on their Reading and Drawing Submicrorepresentations Achievements. Int. J. Sci. Educ. 2010, 32, 1561–1593. [Google Scholar] [CrossRef]
- Haidar, A.H.; Abraham, M.R. A comparison of applied and theoretical knowledge of concepts based on the particulate nature of matter. J. Res. Sci. Teach. 1991, 28, 919–938. [Google Scholar] [CrossRef]
- Treagust, D.; Chittleborough, G.; Mamiala, T. The role of submicroscopic and symbolic representations in chemical explanations. Int. J. Sci. Educ. 2003, 25, 1353–1368. [Google Scholar] [CrossRef]
- Ainsworth, S. The educational value of multiple-representations when learning complex scientific concepts. In Visualization: Theory and Practice in Science Education; Gilbert, J.K., Reiner, M., Nakhleh, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 191–208. [Google Scholar]
- Tóthová, M.; Rusek, M.; Chytrý, V. Students’ procedure when solving problem tasks based on the periodic table: An eye-tracking study. J. Chem. Educ. 2021, 98, 1831–1840. [Google Scholar] [CrossRef]
- Ahtee, M.; Varjola, I. Students’ understanding of chemical reaction. Int. J. Sci. Educ. 1998, 20, 305–316. [Google Scholar] [CrossRef]
- Sanger, M.J. Evaluating students’ conceptual understanding of balanced equations and stoichiometric ratios using a particulate drawing. J. Chem. Educ. 2005, 82, 131. [Google Scholar] [CrossRef]
- Nyachwaya, J.M.; Warfa, A.-R.M.; Roehrig, G.H.; Schneider, J.L. College chemistry students’ use of memorized algorithms in chemical reactions. Chem. Educ. Res. Pract. 2014, 15, 81–93. [Google Scholar] [CrossRef]
- Andraos, J. Using Balancing Chemical Equations as a Key Starting Point To Create Green Chemistry Exercises Based on Inorganic Syntheses Examples. J. Chem. Educ. 2016, 93, 1330–1334. [Google Scholar] [CrossRef]
- Jammeh, A.L.J.; Karegeya, C.; Ladage, S. Misconceptions on Basic Stoichiometry among the Selected Eleventh-grade Students in the Urban Regions of the Gambia. J. Balt. Sci. Educ. 2023, 22, 254–268. [Google Scholar] [CrossRef]
- Naah, B.M.; Sanger, M.J. Student misconceptions in writing balanced equations for dissolving ionic compounds in water. Chem. Educ. Res. Pract. 2012, 13, 186–194. [Google Scholar] [CrossRef]
- Hansen, S.J.R. Multimodal Study of Visual Problem Solving in Chemistry with Multiple Representations. Ph.D. Thesis, Columbia University, New York, NY, USA, 2014. [Google Scholar]
- Davidowitz, B.; Chittleborough, G.; Murray, E. Student-generated submicro diagrams: A useful tool for teaching and learning chemical equations and stoichiometry. Chem. Educ. Res. Pract. 2010, 11, 154–164. [Google Scholar] [CrossRef]
- Baluyut, J.Y.; Holme, T.A. Eye tracking student strategies for solving stoichiometry problems involving particulate nature of matter diagrams. Chem. Teach. Int. 2019, 1, 1. [Google Scholar] [CrossRef]
- Carpenter, Y.-Y.; Moore, E.B.; Perkins, K.K. ConfChem Conference on Interactive Visualizations for Chemistry Teaching and Learning: Using an Interactive Simulation to Support Development of Expert Practices for Balancing Chemical Equations. J. Chem. Educ. 2016, 93, 1150–1151. [Google Scholar] [CrossRef]
- Jung, Y.J.; Zimmerman, H.T.; Pérez-Edgar, K. Methodological Case Study with Mobile Eye-Tracking of Child Interaction in a Science Museum. TechTrends 2018, 62, 509–517. [Google Scholar] [CrossRef]
- Yun, E. Comparing the Reading Behaviours of Students with High- and Low-Level Comprehension of Scientific Terms by Eye Movement Analysis. Res. Sci. Educ. 2020, 51, 939–956. [Google Scholar] [CrossRef]
- Langner, A.; Graulich, N.; Nied, M. Eye-Tracking as a Promising Tool in Pre-Service Teacher Education—A New Approach to Promote Skills for Digital Multimedia Design. J. Chem. Educ. 2022, 99, 1651–1659. [Google Scholar] [CrossRef]
- van Gog, T.; Paas, F.; van Merriënboer, J.J.; Witte, P. Uncovering the problem-solving process: Cued retrospective reporting versus concurrent and retrospective reporting. J. Exp. Psychol. Appl. 2005, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Tiemann, R. Factors of problem-solving competency in a virtual chemistry environment: The role of metacognitive knowledge about strategies. Comput. Educ. 2012, 59, 1199–1214. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]
- van Aalderen-Smeets, S.; van der Molen, J.W. Measuring Primary Teachers’ Attitudes Toward Teaching Science: Development of the Dimensions of Attitude Toward Science (DAS) Instrument. Int. J. Sci. Educ. 2013, 35, 577–600. [Google Scholar] [CrossRef]
- Eisenmann, P.; Přibyl, J.; Novotná, J.; Břehovský, J.; Cihlář, J. Volba řešitelských strategií v závislosti na věku. Sci. Educ. 2017, 8, 21–38. [Google Scholar] [CrossRef]
- Hamerská, L. Schopnost Studentů Učitelství Chemie na Začátku Svého Studia Vyčíslovat Chemické Rovnice: Úspěšnost, Postup a Vliv Využití Appletu [Freshman Chemistry Student Teachers’ Ability to Balance Chemical Equations: Performance, Procedure and the Effect of Applet Use]. Master’s Thesis, Univerzita Karlova, Pedagogická Fakulta, Katedra Chemie a Didaktiky Chemie, Praha, Poland, 2023. [Google Scholar]
- Holme, T.A.; Luxford, C.J.; Brandriet, A. Defining Conceptual Understanding in General Chemistry. J. Chem. Educ. 2015, 92, 1477–1483. [Google Scholar] [CrossRef]
- Cracolice, M.S.; Deming, J.C.; Ehlert, B. Concept Learning versus Problem Solving: A Cognitive Difference. J. Chem. Educ. 2008, 85, 873–878. [Google Scholar] [CrossRef]
- Nurrenbern, S.C.; Pickering, M. Concept learning versus problem solving: Is there a difference? J. Chem. Educ. 1987, 64, 508–510. [Google Scholar] [CrossRef]
- Agung, S.; Schwartz, M.S. Students’ Understanding of Conservation of Matter, Stoichiometry and Balancing Equations in Indonesia. Int. J. Sci. Educ. 2007, 29, 1679–1702. [Google Scholar] [CrossRef]
- Chiu, M.-H. Algorithmic Problem Solving and Conceptual Understanding of Chemistry by Students at a Local High School in Taiwan. Proc. Natl. Sci. Counc. 2001, 11, 20–38. [Google Scholar]
- Davidowitz, B.; Chittleborough, G.D. Linking the Macroscopi cand Sub-microscopic Levels: Diagrams. In Multiple Representations in Chemical Education; Gilbert, J.K., Treagust, D.F., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Nakhleh, M.B. Are Our Students Conceptual Thinkers or Algorithmic Problem Solvers? Identifying Conceptual Students in General Chemistry. J. Chem. Educ. 1993, 70, 52. [Google Scholar] [CrossRef]
- Tsaparlis, G.; Zoller, U. Evaluation of higher vs. lower-order cognitive skills-type examinations in chemistry: Implications for university in-class assessment and examinations. Univ. Chem. Educ. 2003, 7, 50–57. [Google Scholar]
- Papaphotis, G.; Tsaparlis, G. Conceptual versus algorithmic learning in high school chemistry: The case of basic quantum chemical concepts. Part 1. Statistical analysis of a quantitative study. Chem. Educ. Res. Pract. 2008, 9, 323–331. [Google Scholar] [CrossRef]
- Goldhammer, F.; Naumann, J.; Stelter, A.; Tóth, K.; Rölke, H.; Klieme, E. The time on task effect in reading and problem solving is moderated by task difficulty and skill: Insights from a computer-based large-scale assessment. J. Educ. Psychol. 2014, 106, 608–626. [Google Scholar] [CrossRef]
- Dodonova, Y.A.; Dodonov, Y.S. Faster on easy items, more accurate on difficult ones: Cognitive ability and performance on a task of varying difficulty. Intelligence 2013, 41, 1–10. [Google Scholar] [CrossRef]
- Payne, S.J.; Duggan, G.B. Giving up problem solving. Mem. Cogn. 2011, 39, 902–913. [Google Scholar] [CrossRef]
- Niaz, M.; Lawson, A.E. Balancing chemical equations: The role of developmental level and mental capacity. J. Res. Sci. Teach. 1985, 22, 41–51. [Google Scholar] [CrossRef]
- Cranford, K.N.; Tiettmeyer, J.M.; Chuprinko, B.C.; Jordan, S.; Grove, N.P. Measuring Load on Working Memory: The Use of Heart Rate as a Means of Measuring Chemistry Students’ Cognitive Load. J. Chem. Educ. 2014, 91, 641–647. [Google Scholar] [CrossRef]
- Abdelrahman, Y.; Velloso, E.; Dingler, T.; Schmidt, A.; Vetere, F. Cognitive Heat: Exploring the Usage of Thermal Imaging to Unobtrusively Estimate Cognitive Load. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 33. [Google Scholar] [CrossRef]
- Ogilvie, C.A. Changes in students’ problem-solving strategies in a course that includes context-rich, multifaceted problems. Phys. Rev. ST Phys. Educ. Res. 2009, 5, 020102. [Google Scholar] [CrossRef]
- Taber, K.S. Learning at the symbolic level. In Multiple Representations in Chemical Education; Gilbert, J.K., Treagust, D.F., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 75–105. [Google Scholar]
- Lawry, J.A.; Welsh, M.C.; Jeffrey, W.E. Cognitive tempo and complex problem solving. Child Dev. 1983, 54, 912–920. [Google Scholar] [CrossRef]
- Kagan, J.; Rosman, B.L.; Day, D.; Albert, J.; Phillips, W. Information processing in the child: Significance of analytic and reflective attitudes. Psychol. Monogr.-Gen. Appl. 1964, 78, 1–37. [Google Scholar] [CrossRef]
- Zehavi, N.; Mann, G. Instrumented techniques and reflective thinking in analytic geometry. Math. Enthus. 2005, 2, 83–92. [Google Scholar] [CrossRef]
- Hrast, Š.; Savec, V.F. The Integration of Submicroscopic Representations Used in Chemistry Textbook Sets into Curriculum Topics. Acta Chim. Slov. 2017, 64, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Reys, B.J.; Reys, R.E.; Chavez, O. Why Mathematics Textbooks Matter. Educ. Leadersh. 2004, 61, 61–66. [Google Scholar]
- Vojíř, K.; Rusek, M. Preferred Chemistry Curriculum Perspective: Teachers’ Perception of Lower-Secondary School Textbooks. J. Balt. Sci. Educ. 2021, 20, 316–331. [Google Scholar] [CrossRef]
- Chittleborough, G.; Treagust, D.F. The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level. Chem. Educ. Res. Pract. 2007, 8, 274–292. [Google Scholar] [CrossRef]
- Park, C.-Y.; Won, J.; Kim, S.; Choi, H.; Paik, S.-H. Lack of Sub-microscopic Representation Ability of 12th Grade Science Students in Various Acid and Base Problem Solving Processes. J. Korean Chem. Soc. 2020, 64, 30–37. [Google Scholar]



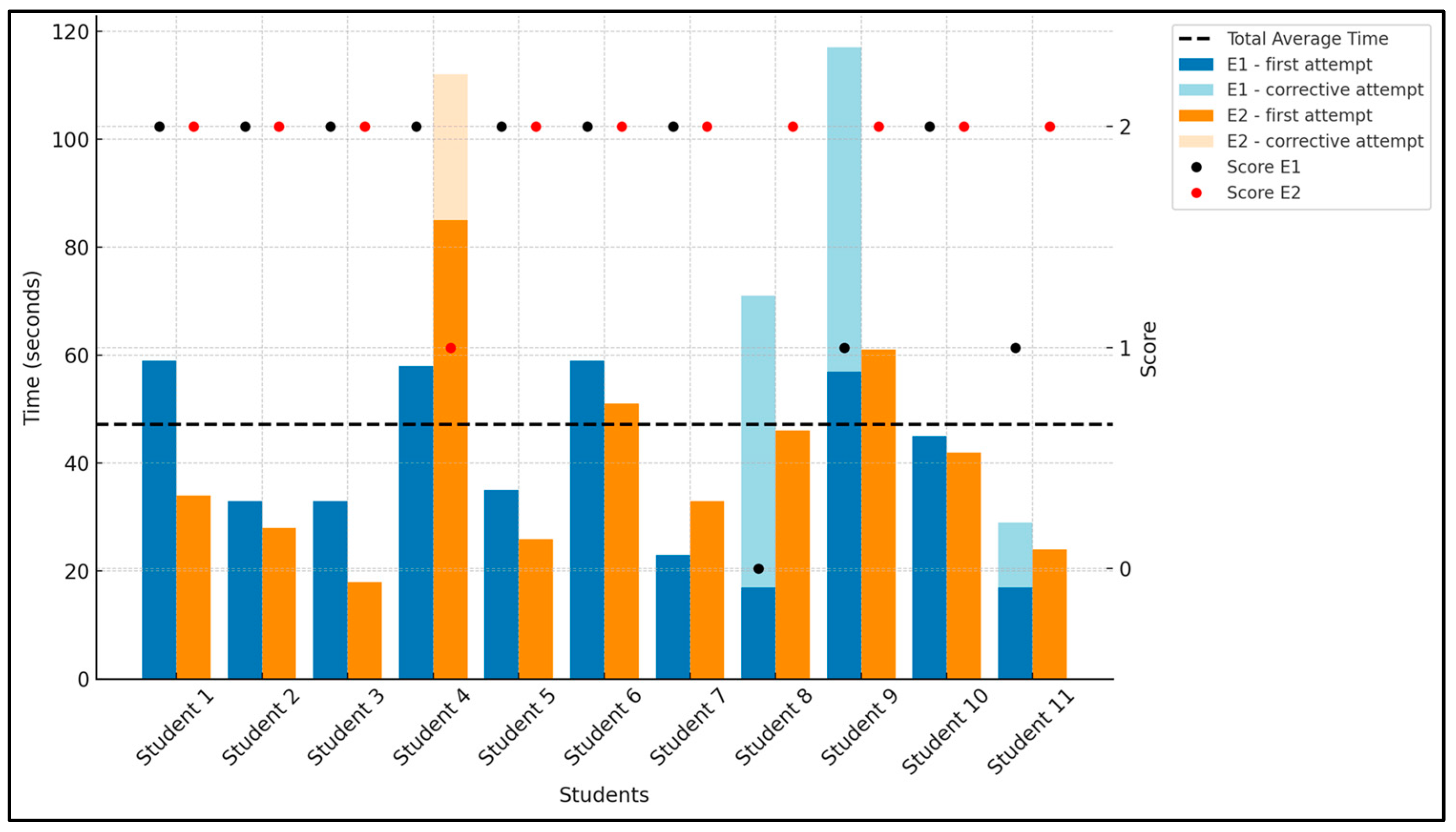
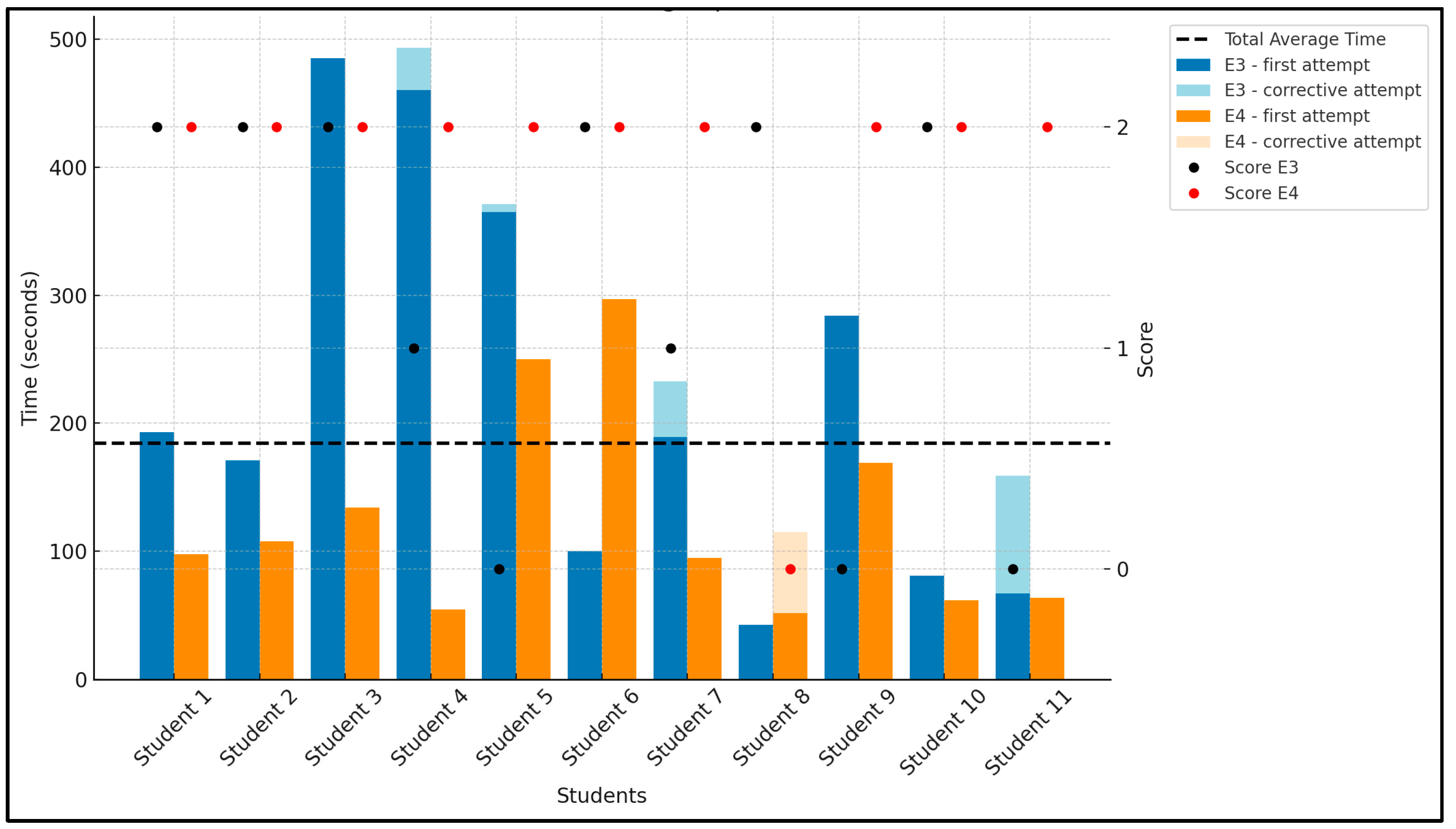

| E1 | E2 | E3 | E4 | Total Score | Overall Success Rate | |
|---|---|---|---|---|---|---|
| Student 1 | 2 | 2 | 2 | 2 | 8 | 100% |
| Student 2 | 2 | 2 | 2 | 2 | 8 | 100% |
| Student 3 | 2 | 2 | 2 | 2 | 8 | 100% |
| Student 4 | 2 | 1 | 1 | 2 | 6 | 75% |
| Student 5 | 2 | 2 | 0 | 2 | 6 | 75% |
| Student 6 | 2 | 2 | 2 | 2 | 8 | 100% |
| Student 7 | 2 | 2 | 1 | 2 | 7 | 88% |
| Student 8 | 0 | 2 | 2 | 0 | 4 | 50% |
| Student 9 | 1 | 2 | 0 | 2 | 5 | 63% |
| Student 10 | 2 | 2 | 2 | 2 | 8 | 100% |
| Student 11 | 1 | 2 | 0 | 2 | 5 | 63% |
| Law of Conservation of Mass | Understanding of Sub-Micro | Stoichiometry | PNM Test Sum Score | Balancing Equations | |
|---|---|---|---|---|---|
| Student 1 | 100% | 100% | 80% | 93% | 100% |
| Student 3 | 75% | 100% | 100% | 92% | 100% |
| Student 4 | 75% | 75% | 80% | 77% | 75% |
| Student 2 | 50% | 100% | 60% | 70% | 100% |
| Student 7 | 75% | 75% | 40% | 63% | 88% |
| Student 10 | 50% | 50% | 80% | 60% | 100% |
| Student 8 | 50% | 100% | 20% | 57% | 50% |
| Student 6 | 50% | 50% | 60% | 53% | 100% |
| Student 5 | 25% | 75% | 20% | 40% | 75% |
| Student 9 | 0% | 50% | 60% | 37% | 63% |
| Student 11 | 50% | 0% | 60% | 37% | 63% |
| E1 | E2 | E3 | E4 | Overall Ratio of TFD | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ratio of TFD | Score | Ratio of TFD | Score | Ratio of TFD | Score | Ratio of TFD | Score | ||
| Student 1 | 3.3% | 2 | 0.7% | 2 | 1.2% | 2 | 0.7% | 2 | 1.3% |
| Student 2 | 2.2% | 2 | 0% | 2 | 0.3% | 2 | 0.7% | 2 | 0.6% |
| Student 3 | 7.0% | 2 | 0% | 2 | 1.3% | 2 | 1.1% | 2 | 1.5% |
| Student 4 | 4.2% | 2 | 1.4% | 1 | 0.6% | 1 | 0.4% | 2 | 1.0% |
| Student 5 | 5.3% | 2 | 2.5% | 2 | 8.1% | 0 | 6.2% | 2 | 7.0% |
| Student 6 | 3.2% | 2 | 1.6% | 2 | 0.1% | 2 | 0.5% | 2 | 0.8% |
| Student 7 | 3.4% | 2 | 1.5% | 2 | 0.5% | 1 | 0.3% | 2 | 0.8% |
| Student 8 | 21.7% | 0 | 5.4% | 2 | 0.5% | 2 | 20.3% | 0 | 14.5% |
| Student 9 | 0.8% | 1 | 0.6% | 2 | 6.8% | 0 | 0.5% | 2 | 1.9% |
| Student 10 | 0.5% | 2 | 0.7% | 2 | 0.2% | 2 | 0% | 2 | 0.3% |
| Student 11 | 0.5% | 1 | 0.0% | 2 | 0.7% | 0 | 0% | 2 | 0.4% |
| Average ratio of TFD | 4.7% | 1.30% | 1.80% | 2.80% | 2.74% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamerská, L.; Matěcha, T.; Tóthová, M.; Rusek, M. Between Symbols and Particles: Investigating the Complexity of Learning Chemical Equations. Educ. Sci. 2024, 14, 570. https://doi.org/10.3390/educsci14060570
Hamerská L, Matěcha T, Tóthová M, Rusek M. Between Symbols and Particles: Investigating the Complexity of Learning Chemical Equations. Education Sciences. 2024; 14(6):570. https://doi.org/10.3390/educsci14060570
Chicago/Turabian StyleHamerská, Lucie, Tadeáš Matěcha, Martina Tóthová, and Martin Rusek. 2024. "Between Symbols and Particles: Investigating the Complexity of Learning Chemical Equations" Education Sciences 14, no. 6: 570. https://doi.org/10.3390/educsci14060570
APA StyleHamerská, L., Matěcha, T., Tóthová, M., & Rusek, M. (2024). Between Symbols and Particles: Investigating the Complexity of Learning Chemical Equations. Education Sciences, 14(6), 570. https://doi.org/10.3390/educsci14060570







