Abstract
The technology for manufacturing a film membrane of the metamizole-selective electrode containing ion associate metamizole-octadecylammonium ODAHMT as an electrode active component (EAC) has been proposed. The main potentiometric characteristics of the metamizole-selective electrode have been determined. The expediency of the proposed design of the metamizole selective electrode for the determination of metamizole in dosage forms has been substantiated. The best composition of the membrane (wt.%) of the metamizole-selective electrode has corresponded to: ODAHMT—5.3; 2-nitrophenyloctylether—63.1; poly(vinyl chloride)—31.6. Electrode-active component in the membrane phase functions as an ion associate ODAHMT. Potentiometric characteristics of metamizole-selective electrode have been determined, which corresponded to: linear range 1 × 10–1 × 10 with limit of detection 4.58 × 10 M, electrode function slope −48.5 mV/dec., working interval pH 4.5–7.3, response time 60 s. The potentiometric coefficients of selectivity of the metamizole-selective electrode with respect to various ions have been determined. The possibility of determining metamizole in a medicinal product has been tested. The results of the analyses show good agreement between the two methods (relative error less than 7.0%) with coefficients of variation less than 5% for MT-SE and iodometric methods.
Keywords:
metamizole; dipyrone; sensor; ISE; membrane; analysis; ion-selective electrodes; potentiometry; metamizole-ion sensor 1. Introduction
With the development of pharmaceutical science, 30–40 new drugs appear annually. Currently, more than 10,000 medicinal substances and over 100,000 dosage forms are registered in the world [1,2,3,4]. Because of the ever-increasing number of new medicinal substances, the problem of their identification and determination both in individual samples and in living organisms is relevant and timely. The need for the accumulation, generalization and systematization of data on the methods of control and analysis of medicinal substances has led to the emergence of specialized scientific publications [5,6,7,8,9,10,11,12,13]. Judging by the methods of analysis of medicinal substances based on the materials of the original articles, we can say that chromatographic methods of analysis are still in the lead. Electrochemical methods for the analysis of medicinal substances by the number of publications rank third, behind photometry and spectrophotometry, with ∼20% of works devoted to the electrochemical determination of medicinal substances [14,15,16,17,18,19,20,21,22,23,24,25].
Equilibrium electrochemical methods for the analysis of medicinal substances, including direct potentiometry (ionometry) and potentiometric titration, are extremely convenient, simple and accessible for the determination of those medicinal substances that can be converted into the corresponding ionic form [26,27]. They, as a rule, combine sufficient selectivity and determination accuracy, which can be significantly increased even with direct determination due to the functioning of the sensors according to the mechanism of super-Nernst response and increasing linear working range [25,28,29,30,31,32,33,34,35]. In addition, potentiometric sensors can be suitable for creating electronic tongue used for the analysis of urine, blood and other human fluids, as well as for determining the authenticity of drugs [36,37].
It is known that in the arsenal of medications, the leading substances are those containing heterocyclic fragments in their structure, including nitrogen-containing ones. A special place among them is occupied by pyrazole derivatives due to their good preparative availability. Pyrazole derivatives possess analgesic, antipyretic, anti-rheumatic [38,39], antimicrobial [40], anti-proliferative [41] and anti-inflammatory [42,43,44] activities. So, these are considered as persuasive pharmacophores in medicinal chemistry. The pyrazolone molecules with tautomeric hydrogen are known to be good free radical scavengers [15]. At the same time, many medications, in particular pyrazole derivatives, exhibit some toxicity and have a number of side effects, which hinder their widespread use in medical practice [45,46].
The aim of this work to propose a simple and effective method for rapid analysis of the qualitative and quantitative content of metamizole in drugs based on it.
2. Materials and Methods
2.1. Reagents’
The following compounds were used in the work: metamizole (MT) ((1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) (methyl) amino] sodium methanesulfonate, monohydrate, or dipyrone, or used medically in Germany under the brandname “Novalgin”, in Russian “Analgin”), and octadecylamine (ODA). Analgin solution 50% ampule 2 mL № 10 from Dal’khimfarm (Khabarovsk, Russia). Baralgin M solution 500 mg/mL ampule 5 mL and Baralgin in tablet from Sanofi Aventis Pharma (Paris, France). Baralgin M tablet from Aventis (Mumbai, India). Cation exchanger potassium tetrakis-p-Cl-phenylborate (KClTPB), plasticizers bis(2-butylpentyl)adipate (BBPA), bis(2-ethylhexyl)phthalate (DOP), and 2-nitrophenyloctylether (o-NPOE), and high molecular weight poly(vinyl chloride) (PVC) were Selectophore-grade reagents from Merck (Darmstadt, Germany). Anion exchanger ODA, CPC, DAPPM was from Vekton (St. Petersburg, Russia). Volatile solvents extra pure cyclohexanone (CH) and HPLC grade tetrahydrofuran (THF), as well as acetonitrile, were from Vekton (St. Petersburg, Russia). Inorganic salts were from Reaktiv (Moscow, Russia). All aqueous solutions were prepared with bidistilled water (Bidistiller BS, Himlaborpribor, Russia).
2.2. Apparatus
Zero current potentiometric measurements were performed with Ecotest-120 8-channel potentiometric station Econics (Moscow, Russia) and EMF 6 6-channel potentiometric station (Lawson Labs, Inc., Malvern, PA, USA). The reference electrode was a single junction Ag/AgCl electrode in 3.5 M KCl, with a salt bridge with a limited leak of KCl (Izmeritel, Belarus). Calibrations were carried out from 0.1 M down to 10 M respective solution MTNa, using sequential dilution. The pH measurements were carried out using a pH-15MI pH meter (Izmeritel’naya Tekhnika, Moscow, Russia) with a combined pH glass electrode ESK-10603/7 (Izmeritel’naya Tekhnika, Moscow, Russia). Ultraviolet visible (UV–Vis) spectroscopy was performed with Double-beam spectrophotometer Specord 210 plus (Analytik Jena, Germany) in Quartz Cells length 10 mm, with scan step 1 nm and scan speed 10 nm/s.
All the measurements were performed at room temperature: 19–21 C.
2.3. Sensor Fabrication
The membrane cocktails were prepared by dissolving appropriate amounts of PVC, plasticizer, ionophore, and ion exchanger in THF. The percentage of “dry” content in the cocktails was 15% [47]. To obtain the membranes, the cocktails were stirred for 30 min using Mixing device LOIP LS-220 shaker from “Laboratory Equipment and Instruments”, (St. Petersburg, Russia) and then cast on glass Petri dishes. The dishes were closed with filter paper to slow down the evaporation of THF. The complete evaporation of THF took 1 day, and after that, master membranes with a thickness of about 0.4 mm were obtained.
2.4. Semi-Empirical Calculations
We used the software package ACD Labs Chem Sketch 6.0 with addons: pKa, LogP, LogD, SolDB for semi-empirical calculations of the basicity and lipophilicity constants, LogD and LogS of organic compounds. This software package gives an error less than 10–15%.
2.5. Software
To collect potentiometric data from the Ecotest-120 + KM-8 system, we used the native Ecotest-120 software (Econics, Moscow, Russia), and for EMF 6 we used LL_USB Graphics Version 10.22.15 (Lawson Labs Inc., Malvern, PA, USA). To record the spectra in the UV/Vis region on the Specord 210 Plus, we used the native WinASPECT PLUS Version: 4.2.0.0 software (AnalytikJena AG, Thuringia, Germany). To write chemical formulas, optimize the 3D model, find the basicity and lipophilicity constants, LogD and LogS of organic compounds, we used the software package ACD Labs Chem Sketch 6.0 (Advanced Chemistry Development, Inc., Toronto, ON, Canada).
3. Results
3.1. UV-Visible Absorption Spectrum
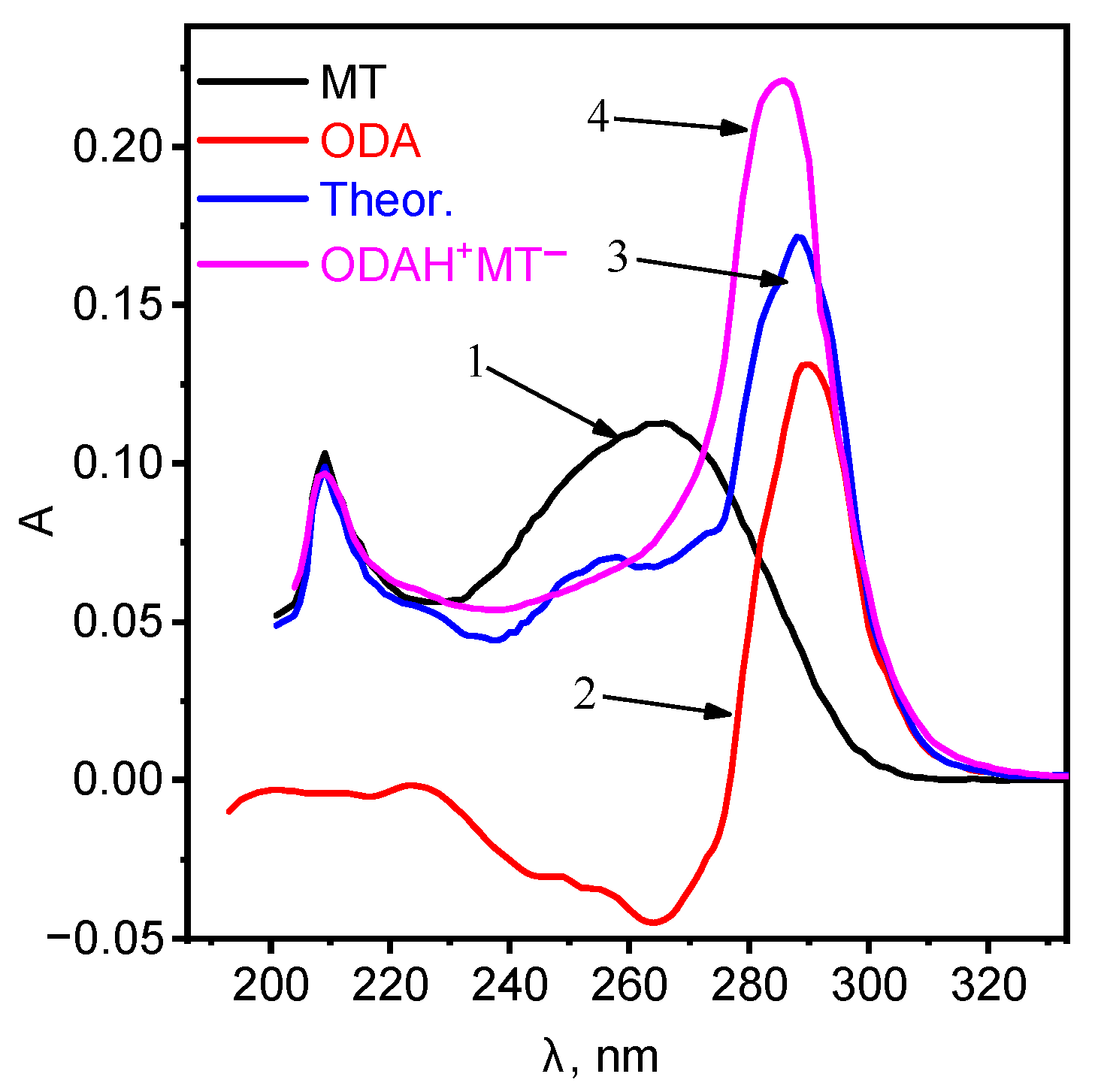
In order to elucidate the formation of a new compound, the electronic spectra of ion associates ODA with MT have been studied. The shift of the absorption spectra for octadecylamine was observed (Figure 1).
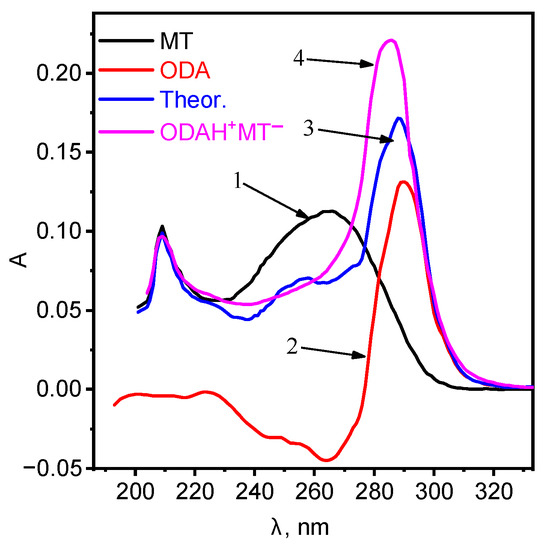
Figure 1.
Absorption spectra of: 1—MT, 2—ODA, 3—summation of curves 1 and 2, 4—ionic associate ODAHMT.
The absorption spectra of the ionic associate are bathochromically shifted relative to ODA and MT with a good hyperchromic effect, which indicates the formation of a new compound. Further studies were carried out using the ionic associate ODAHMT as EAC. The ionic associate ODAHMT was formed according to the scheme: Octadecylamine was dissolved in an equivalent amount in a flask with a capacity of 50.00 mL in hydrochloric acid with a concentration of 1 × 10 M:
Chloroform was added in small portions and mixed with an aqueous solution of MT (C = 1 × 10 M). The solution was transferred to a separatory funnel, the mixture was shaken for two hours, the formed chloroform layer was separated from the aqueous phase into a pre-weighed weighing bottle. Chloroform was evaporated in a water bath at the temperature of 50–60 C in order to avoid decomposition of the electrode active component (EAC). o-NPOE was used as a plasticizer, and tetrahydrofuran (THF) was used as a solvent. Ion exchange equation:
3.2. Optimization of the Preparation Conditions
It is well known that not only the nature of the ionophore, but also the membrane composition has a significant effect on the sensitivity, as well as the selectivity and linearity of the ISE. Four membranes were prepared; for the membrane compositions, see Table 1. In order to obtain a membrane with the optimal composition of the metamizole-selective electrode (MT-SE), the amounts of polymer, ionophore and plasticizer were varied. The composition of the membranes is shown in Table 1.

Table 1.
Composition of MT-SE membranes based on ODAH+MT−.
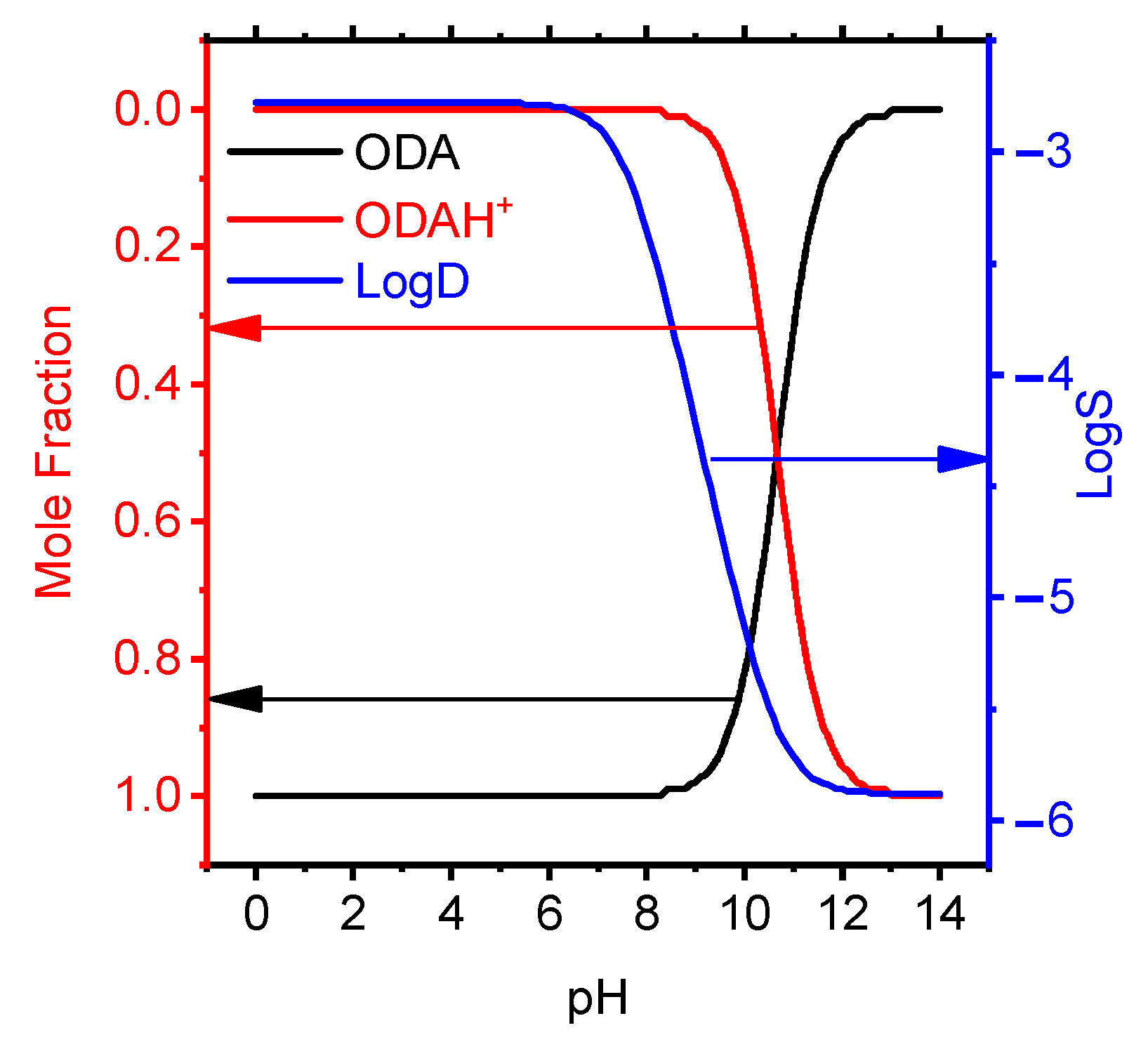
The optimized membrane had the composition wt.%: ODAHMT (5.3), o-NPOE (63.1), PVC (31.6) and the slope of the electrode function, as close as possible to the theoretical value for a singly charged ion S −48.5 mV/dec. As known, the high lipophilicity of the ionophore of the PVC-plasticized membrane limits its release into the solution, which contributes to the completeness of the electrode function, as well as the long lifespan of the ISE. The lipophilicity value was calculated using the ACD/ChemSketch program, which corresponds from 6.5 to 10.6 for octadecylamine. The dependency diagrams of octadecylamine mole fraction have been studied vs. pH in aqueous solution (Figure 2).
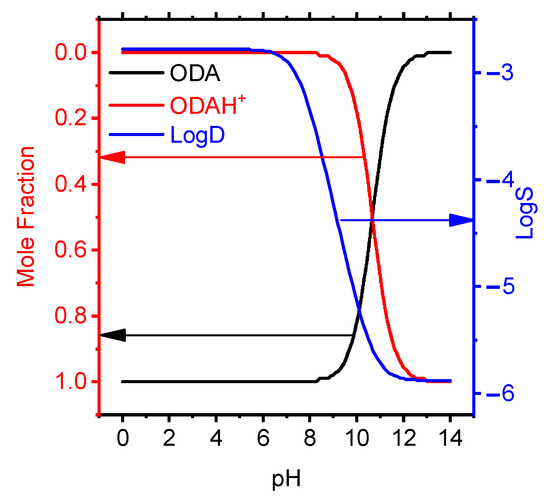
Figure 2.
Effect of pH on the molar fraction of protonated ODA particles in aqueous solution and solubility.
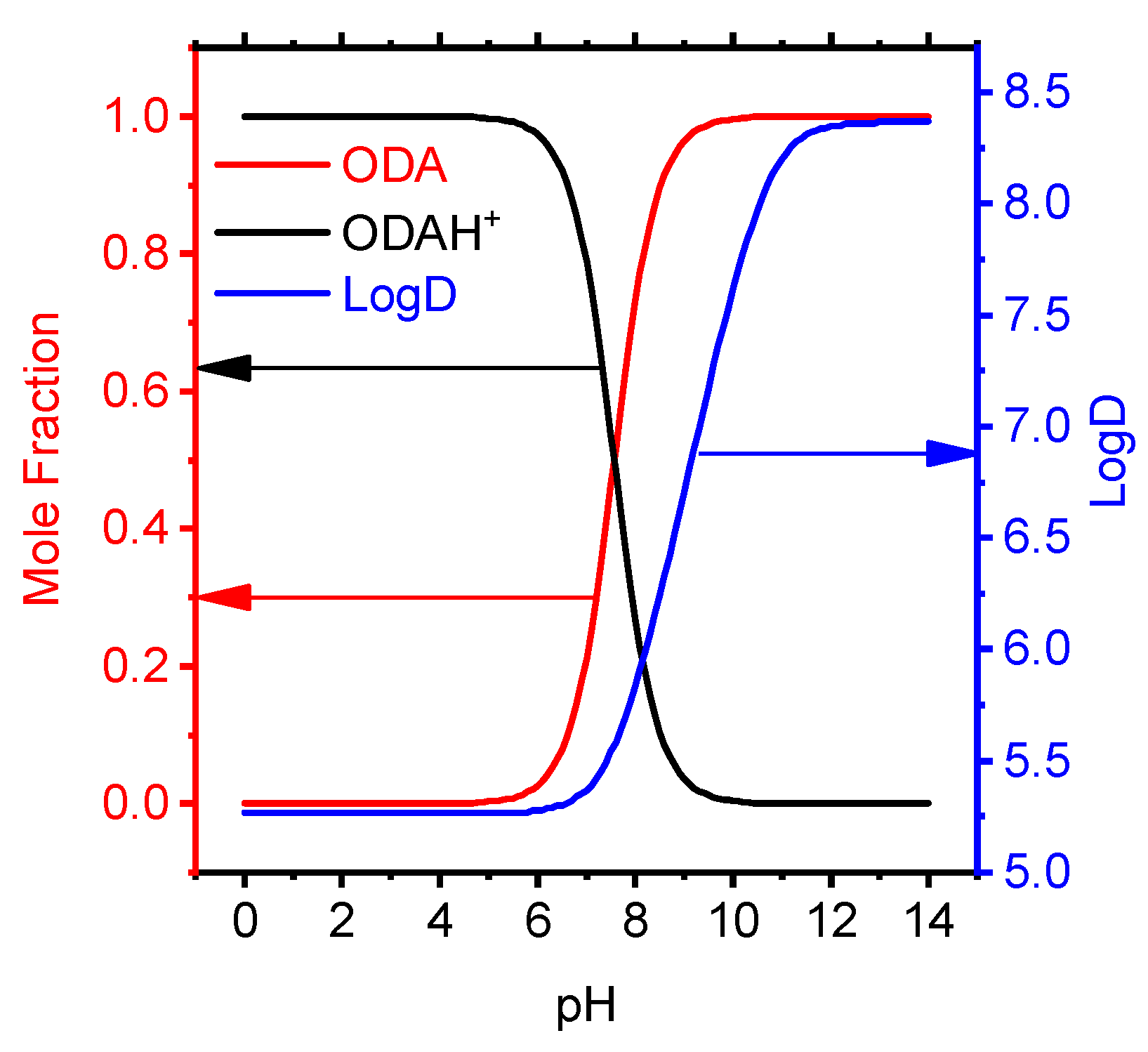
In the pH range from 0 to 8 in aqueous solution, octadecylamine exists in the form of an ionic particle ODAH+. Octadecylamine mole fraction and LogD from pH in organic/water mixture have been studied the dependency graph (Figure 3) with semiempirical method on ACDChem software package.
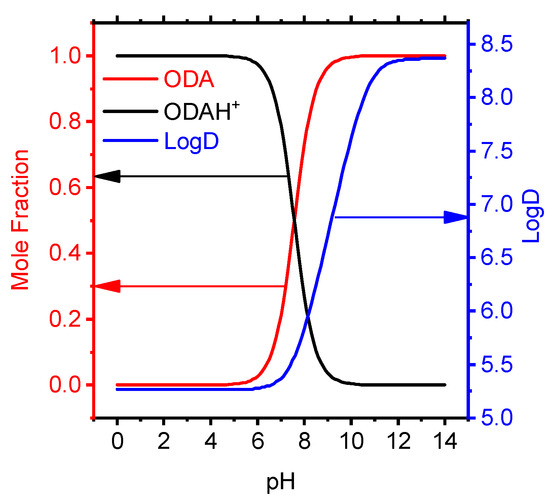
Figure 3.
Effect of pH on the molar fraction and distribution coefficient of octadecylamine particles.
Taking into account ion pairs, the separation factor provides information that the ODAH+ cation in the organic phase dominates at pH < 7 (Figure 3).
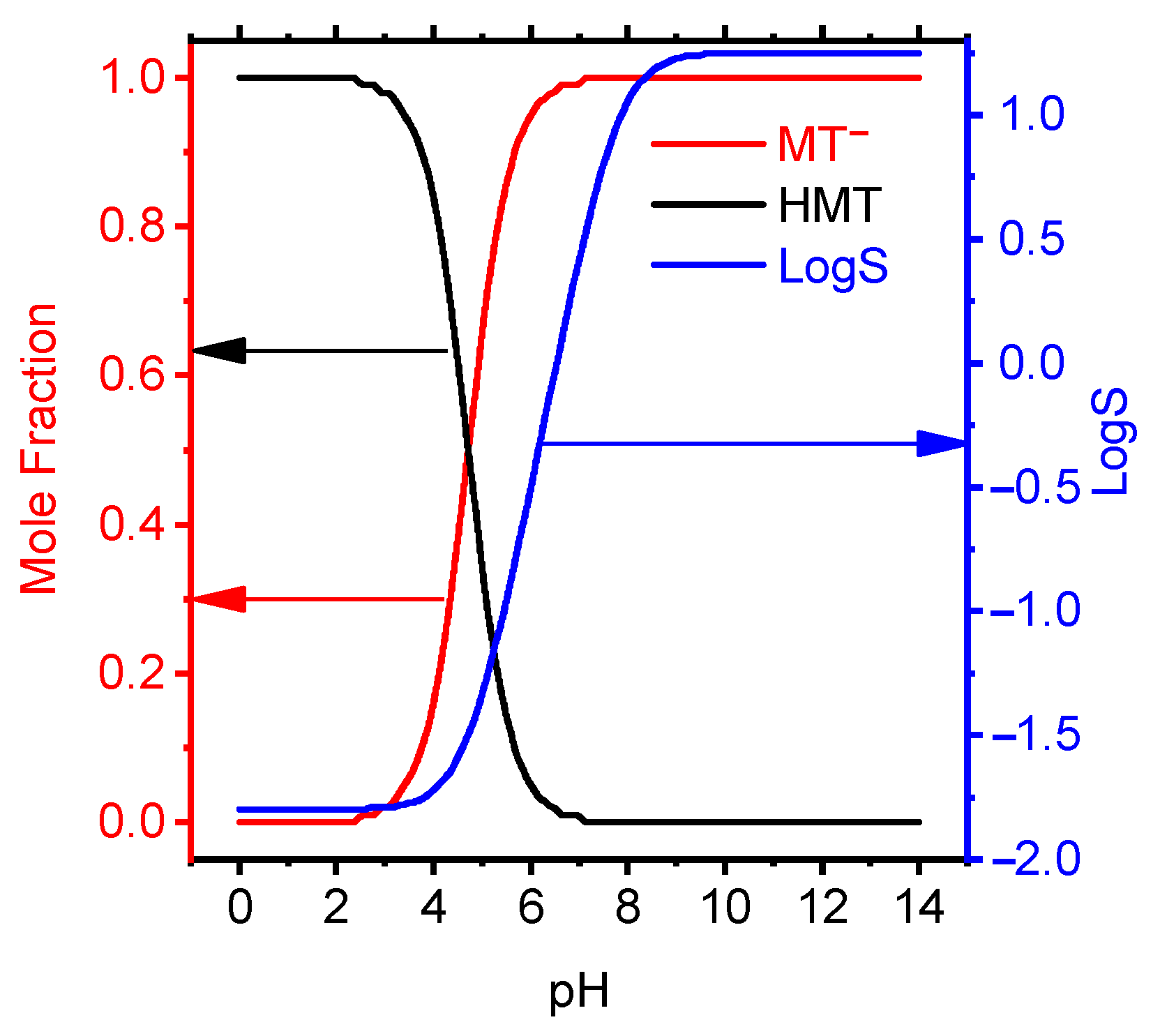
The ionic forms of MT in aqueous solution depending on the acidity of the medium have been studied (Figure 4).
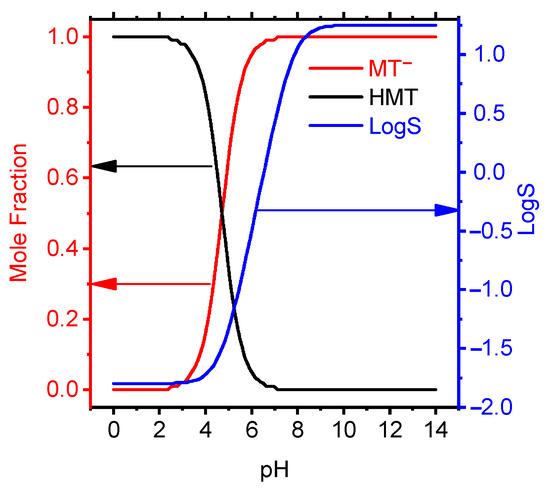
Figure 4.
Mole fraction and LogS data of MT depending on pH by the calculated semiempirical method using the ACDChem software package.
At pH from 0 to 3.5, MT exists in the cationic form, at pH > 7, in the anionic form, and in the acidity range of 3.5–7.0, tautomerism of the forms was observed. The dependence of the membrane solubility on the acidity of the solution is shown in Figure 4. It follows from the graph that at pH ≥ 7.3 MT has the maximum solubility and this interval is taken as the theoretical pH. functioning of the electrode.
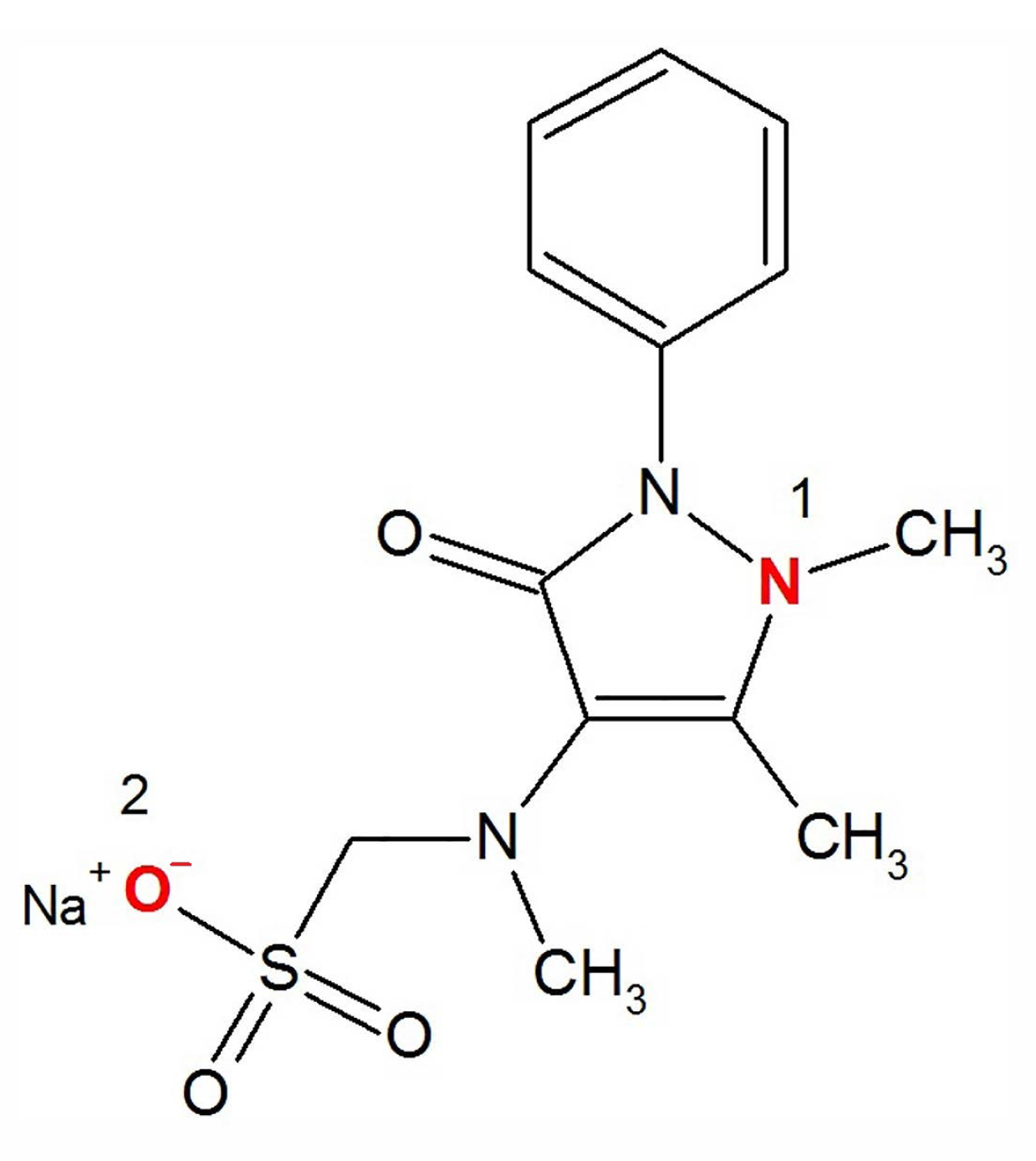
Metamizole begins to protonate in acid medium: (1) the nitrogen in the pyrazole ring (near the methyl) begins to protonate () (2) sodium is replaced by hydrogen near the sulfo group () and the particle takes on a positive charge Figure 5. In an acidic medium, metamizole is rather unstable; therefore, we did not use this pH range.
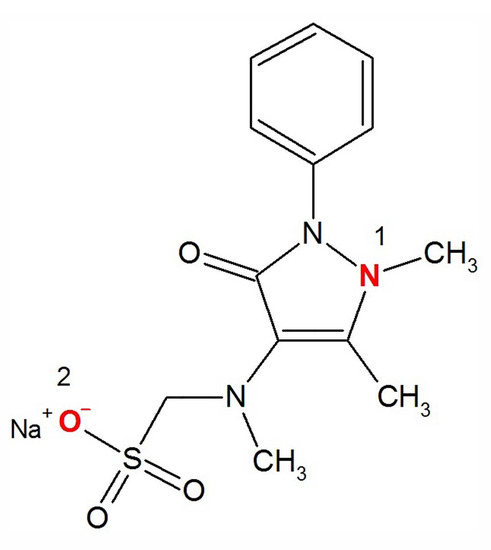
Figure 5.
The structural formula of sodium metamizole.
3.3. Dependence from pH
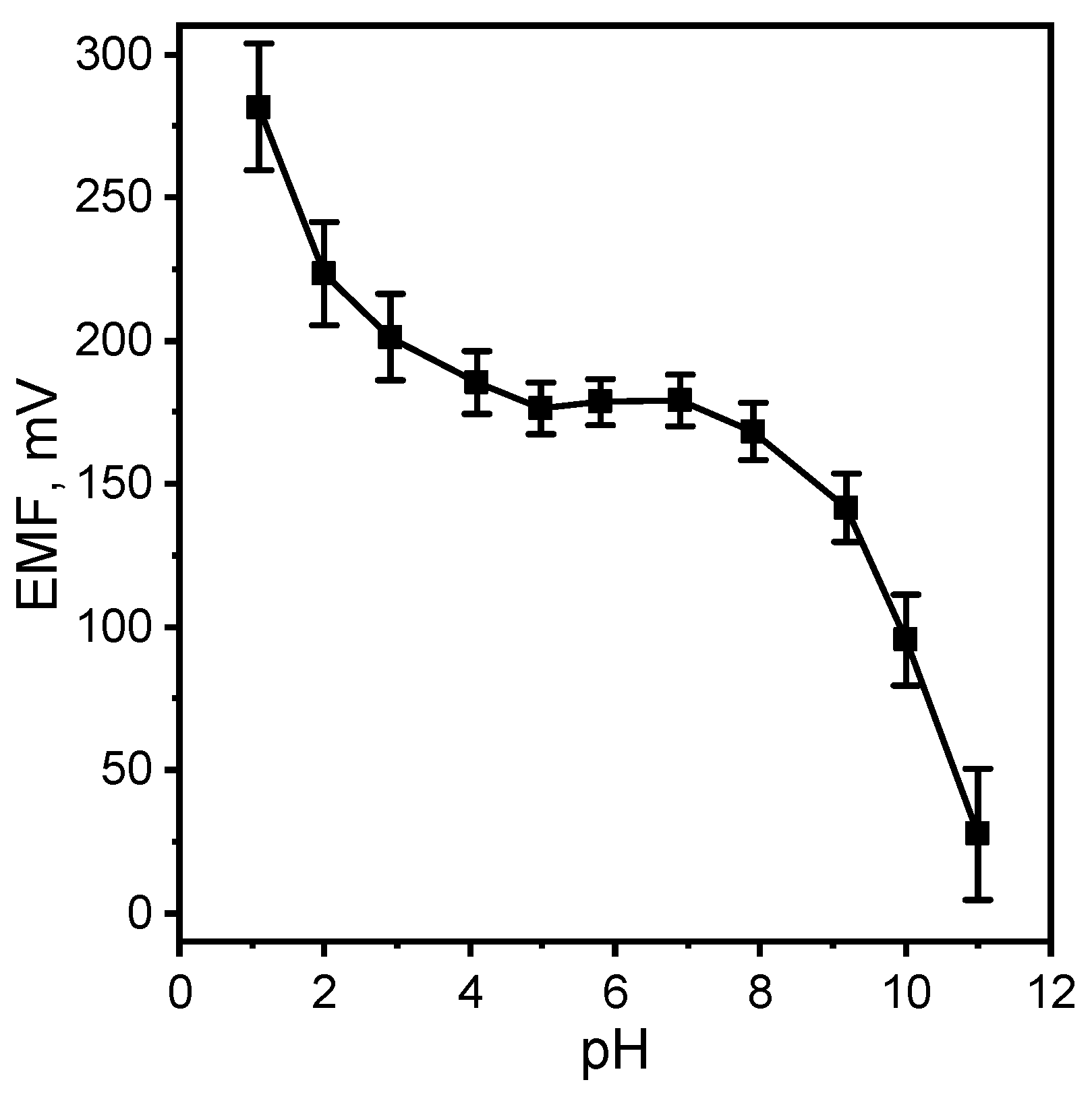
In order to study the pH-dependence of the electrode potential, the graph of emf versus pH was constructed. The pH of the initial solutions was changed by the addition of very small volumes of 1.0 M NaOH and HCl solution. Figure 6 shows a typical profile for a M MT concentration. As seen, the potential is rather independent of pH in the range 4.5–8.0. However, at very high acidic solution, a cationic response is observed which is due to Donnan failure. Above pH 6, the potential response decreases drastically because of the decreasing concentration of the cationic form of MT and an increase in hydroxide ions (which also have an effect on pH). In an acidic media, there is a problem that MT begins to degrade over time.
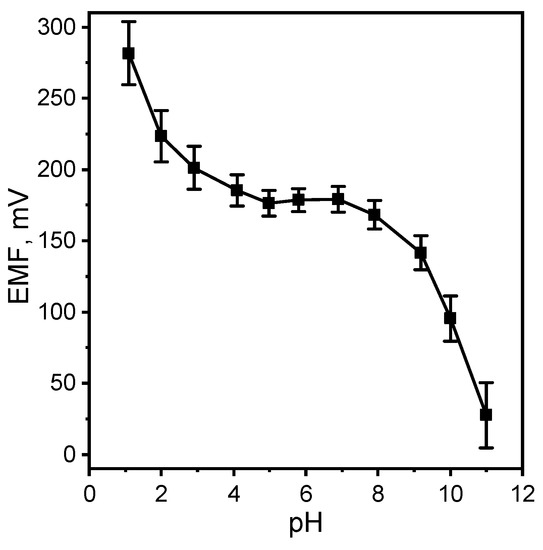
Figure 6.
Effect of pH of test solution on the potential response of the MT-SE.
3.4. Analytical Performance
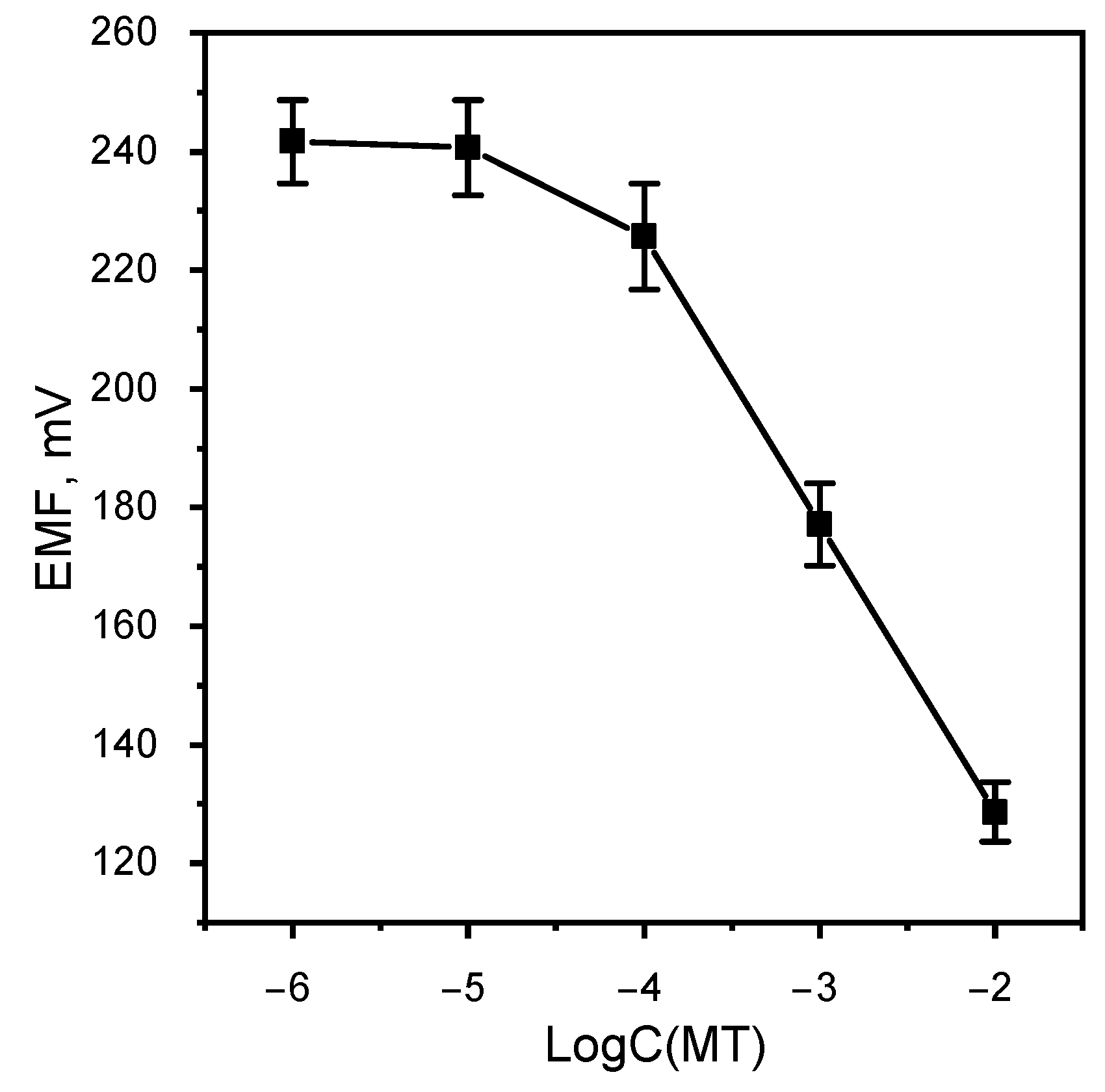
The Nernst region of functioning of the metamizole-selective electrode was determined (Figure 7). The linear range of functioning of the MT-SE is 1 × 10–1 × 10 M with limit of detection 4.58 × 10 M. The slope of the electrode function is −48.5 mV/dec. This result is better than similar work [24]. The response time is 60 s.
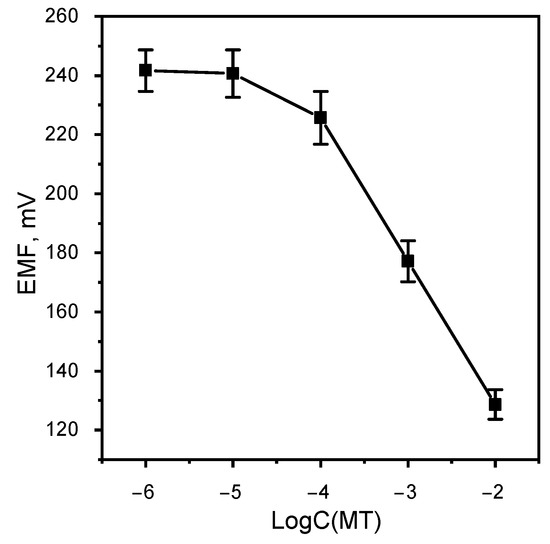
Figure 7.
Calibration graphic MT-SE.
3.5. Selectivity of the Sensor
The selectivity of the electrode with respect to some cations and anions has been studied. The potentiometric coefficients of selectivity of MT-SE were determined by the separate solutions method (sometimes called the bi-ionic potentials method) for concentration M (Table 2).

Table 2.
Potentiometric selectivity coefficients () for concentration M.
The study of the selectivity of MT-SE based on an ionic associate to the most important inorganic cations—K+, Na+, Ca2+ showed that these cations do not interfere with the determination of metamizole.
3.6. Application to Real Samples
Preparations containing metamizole sodium—baralgin, tempalgin, spazgan, spazmalgon, revalgin, andipal. The object of the study: baralgin is a combined medicinal product that prevents the development of spasms, which consists of several active ingredients—metamizole sodium, pitofenone hydrochloride, fenpiverinium bromide. It is produced in various dosage forms—in the form of tablets, suppositories, injection solution. The composition of the pharmaceutical product is presented in Table 3.

Table 3.
Active ingredients in a 5 ml baralgin ampoule.
The possibility of determining MT in a medicinal product has been tested. The samples of different commercial product, including tablets and injectables were analyzed with the MT-SE. Table 4 gives the obtained values, and compares them with the results provided from an analysis of the same products by the classical iodometric method. This was based on the addition of 5.0 mL of 0.02 mol/L hydrochloric acid to the samples, followed by titration at a temperature lower than 20 C, with a 0.1 mol/L iodine solution.

Table 4.
Determination of dipyrone in pharmaceutical preparations using the proposed MT-SE.
An analysis of the results concludes a good agreement between the two methods (relative error lower than 7.0%) with the coefficients of variation being lower than 5%, for the MT-SE and idometric methods. The iodometric method for the determination of sodium metamizole was carried out according to the pharmacopeial analysis, and the ionometric method using the MT-SE based on the membrane of the selected composition.
Thus, the electrode with a membrane based on the ODAHMT ion associate can be used to determine sodium metamizole in medicinal preparations.
4. Conclusions
- In order to select the electrode-active component, the acid-base properties of substances have been studied: CPC, DAPPM, ODA. The analysis of the spectra has made it possible to use ODA as the ionophore for further research.
- The composition of the membrane (wt.%) of the metamizole-selective electrode has corresponded to: EAC—5.3; o-NPOE—63.1; PVC—31.6.
- EAC in the membrane phase functions as an ion associate ODAHMT.
- Potentiometric characteristics of metamizole-selective electrode have been determined, which corresponded to: linear range 1 × 10–1 × 10 with limit of detection 4.58 × 10 M, electrode function slope −48.5 mV/dec., working interval pH 4.5–8.0. Response time t = 60 s. The time may vary in depending on the measurement conditions (from 45 s to 1 min). Equation: y(x) = 31.7(±2.4) − 48.5(±8.7) · x in brackets is the error for calibrations between three or more electrodes. For one electrode, the error did not exceed 1 mV; accordingly, the error in the analysis results with a drift of 1 mV does not exceed 5%.
- The potentiometric coefficients of selectivity of the MT-SE electrode with respect to various ions have been determined. The most important inorganic cations do not interfere with the determination of metamizole, and among the anions.
- The possibility of determining metamizole in the baralgin ampoule has been tested.
Author Contributions
Conceptualization, S.D.T., K.E.M. and R.Z.Z.; Data curation, N.D.B.; Formal analysis, V.S.M.; Investigation, N.D.B., A.A.R. and V.S.M.; Methodology, F.F.O. and K.E.M.; Validation, S.D.T. and R.Z.Z.; Visualization, A.A.R., R.Z.Z. and F.F.O.; LaTeX preparation, K.E.M.; Writing—original draft, S.D.T., K.E.M. and F.F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the State Assignment Russian Federation FZNZ-2020-0002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data availability.
Acknowledgments
We would like to express our gratitude to the Research and Education Center “Chemistry and Chemical Technology” and the Center for Collective Use “Analytical Spectroscopy” of the Dagestan State University for access to the equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olsson, S. Recent Developments in Pharmacovigilance at UMC. Pharm. Med. Transl. Clin. Res. 2018, 435–442. [Google Scholar] [CrossRef]
- Testa, C.J.; Hu, C.; Shvedova, K.; Wu, W.; Sayin, R.; Casati, F.; Halkude, B.S.; Hermant, P.; Shen, D.E.; Ramnath, A.; et al. Design and Commercialization of an End-to-End Continuous Pharmaceutical Production Process: A Pilot Plant Case Study. Org. Process Res. Dev. 2020, 24, 2874–2889. [Google Scholar] [CrossRef]
- Sinha, S.; Vohora, D. Drug Discovery and Development: An Overview. Pharm. Med. Transl. Clin. Res. 2018, 19–32. [Google Scholar] [CrossRef]
- Singh, H.; Khurana, L.K.; Singh, R. Pharmaceutical Development. Pharm. Med. Transl. Clin. Res. 2018, 33–46. [Google Scholar] [CrossRef]
- Miossec, C.; Mille, T.; Lanceleur, L.; Monperrus, M. Simultaneous determination of 42 pharmaceuticals in seafood samples by solvent extraction coupled to liquid chromatography–tandem mass spectrometry. Food Chem. 2020, 322, 126765. [Google Scholar] [CrossRef] [PubMed]
- El-Yazbi, A.F.; Elashkar, N.E.; Ahmed, H.M.; Talaat, W.; Abdel-Hay, K.M. Cost-effective green chromatographic method for the simultaneous determination of four commonly used direct-acting antiviral drugs in plasma and various pharmaceutical formulations. Microchem. J. 2021, 168, 106512. [Google Scholar] [CrossRef]
- Hu, J.; Christison, T.; Rohrer, J. Determination of dimethylamine and nitrite in pharmaceuticals by ion chromatography to assess the likelihood of nitrosamine formation. Heliyon 2021, 7, e06179. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Olemy, A.; Ramzy, S.; Abdelazim, A.H.; Omar, M.K.M.; Shahin, M. Spectrophotometric determination of lesinurad and allopurinol in recently approved FDA pharmaceutical preparation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119106. [Google Scholar] [CrossRef]
- Talib Humeidy, I. Spectrophotometric determination of cefotaxime sodium in pharmaceutical formulations. Mater. Today Proc. 2021, 47, 6043–6049. [Google Scholar] [CrossRef]
- Habib, A.; Mabrouk, M.M.; Hamed, N.A.; Mansour, F.R. An innovative spectrophotometric method for determination of polidocanol in pharmaceutical ampoules using phase equilibrium measurements. Microchem. J. 2020, 158, 105141. [Google Scholar] [CrossRef]
- El-Olemy, A.; Abdelazim, A.H.; Ramzy, S.; Hasan, M.A.; Madkour, A.W.; Almrasy, A.A.; Shahin, M. Application of different spectrofluorimetric approaches for quantitative determination of acetylsalicylic acid and omeprazole in recently approved pharmaceutical preparation and human plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120116. [Google Scholar] [CrossRef]
- Essam, H.M.; Bassuoni, Y.F.; Elzanfaly, E.S.; Zaazaa, H.E.S.; Kelani, K.M. Potentiometric sensing platform for selective determination and monitoring of codeine phosphate in presence of ibuprofen in pharmaceutical and biological matrices. Microchem. J. 2020, 159, 105286. [Google Scholar] [CrossRef]
- Safaei, M.; Shishehbore, M.R. A review on analytical methods with special reference to electroanalytical methods for the determination of some anticancer drugs in pharmaceutical and biological samples. Talanta 2021, 229, 122247. [Google Scholar] [CrossRef]
- Marra, M.C.; Cunha, R.R.; Vidal, D.T.R.; Munoz, R.A.A.; do Lago, C.L.; Richter, E.M. Ultra-fast determination of caffeine, dipyrone, and acetylsalicylic acid by capillary electrophoresis with capacitively coupled contactless conductivity detection and identification of degradation products. J. Chromatogr. A 2014, 1327, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sarojini, B.K.; Vidyagayatri, M.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. DPPH Scavenging Assay of Novel 1,3-disubstituted-1H-pyrazol-5-ols and their in silico Studies on Some Proteins Involved in Alzheimers Disease Signaling Cascade. Lett. Drug Des. Discov. 2010, 7, 214–224. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Analysis of anti-inflammatory, analgesic, stimulant and antidepressant drugs in purified water from wastewater treatment plants using SPE-LC tandem mass spectrometry. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Senyuva, H.Z.; Aksahin, I.; Ozcan, S.; Kabasakal, B.V. Rapid, simple and accurate liquid chromatography-diode array detection validated method for the determination of dipyrone in solid and liquid dosage forms. Anal. Chim. Acta 2005, 547, 73–77. [Google Scholar] [CrossRef]
- Gyenge-Szabó, Z.; Szoboszlai, N.; Frigyes, D.; Záray, G.; Mihucz, V. Monitoring of four dipyrone metabolites in communal wastewater by solid phase extraction liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 58–63. [Google Scholar] [CrossRef]
- Altun, M. HPLC method for the analysis of paracetamol, caffeine and dipyrone. Turk. J. Chem. 2002, 26, 521–528. [Google Scholar]
- Golubitskii, G.B.; Budko, E.V.; Ivanov, V.M. Quantitative analysis of pentalgin N tablets by gradient and isocratic high-performance liquid chromatography. J. Anal. Chem. 2006, 61, 74–79. [Google Scholar] [CrossRef]
- Vieira, J.C.; Sversut, R.A.; Maciel, I.T.; Carollo, A.R.H.; Serrou do Amaral, M.; Kassab, N.M. HPLC–DAD Method for Simultaneous Determination of Dipyrone (Metamizole) and Caffeine in Tablets and Identification of Major Degradation Product by Direct Infusion ESI–MS. Chromatographia 2017, 80, 489–495. [Google Scholar] [CrossRef]
- Marcolino-Júnior, L.H.; Bergamini, M.F.; Teixeira, M.F.; Cavalheiro, É.T.; Fatibello-Filho, O. Flow injection amperometric determination of dipyrone in pharmaceutical formulations using a carbon paste electrode. Il Farmaco 2003, 58, 999–1004. [Google Scholar] [CrossRef]
- Pérez, J.A.P.; Alegría, J.S.D.; Hernando, P.F.; Sierra, A.N. Determination of dipyrone in pharmaceutical preparations based on the chemiluminescent reaction of the quinolinic hydrazide—H2O2—Vanadium(IV) system and flow-injection analysis. Luminescence 2012, 27, 45–50. [Google Scholar] [CrossRef][Green Version]
- Bindewald, E.H.; Bergamini, M.F.; Marcolino, L.H., Jr. Disposable Solid-State Sensor Based on Polypyrrole Films Doped for Potentiometric Determination of Dipyrone in Human Urine and Pharmaceuticals Products. Electroanalysis 2013, 25, 1535–1540. [Google Scholar] [CrossRef]
- Albuquerque, J.S.; Silva, V.L.; Lima, F.; Araújo, A.N.; Montenegro, M.C.B. Determination of Dipyrone in Pharmaceutical Products by Flow Injection Analysis with Potentiometric Detection. Anal. Sci. 2003, 19, 691–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zolotov, Y.A. Evolution of Chemical Analysis Methods. Herald Russ. Acad. Sci. 2020, 90, 56–62. [Google Scholar] [CrossRef]
- Zolotov, Y.A. On some trends. Talanta 2011, 85, 2249–2250. [Google Scholar] [CrossRef] [PubMed]
- Mikhelson, K.N.; Peshkova, M.A. Advances and trends in ionophore-based chemical sensors. Russ. Chem. Rev. 2015, 84, 555–578. [Google Scholar] [CrossRef]
- Peshkova, M.A.; Mikhelson, K.N. Solvent polymeric membrane ion-selective electrodes under galvanostatic control: Powerful tool for analysis of extremely diluted samples. Electrochim. Acta 2013, 110, 829–835. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Koltashova, E.S.; Solovyeva, E.V.; Peshkova, M.A.; Mikhelson, K.N. Impact of the Electrolyte Co-Extraction to the Response of the Ionophore-based Ion-Selective Electrodes. Electrochim. Acta 2016, 213, 439–446. [Google Scholar] [CrossRef]
- Lisak, G.; Sokalski, T.; Bobacka, J.; Harju, L.; Mikhelson, K.; Lewenstam, A. Tuned galvanostatic polarization of solid-state lead-selective electrodes for lowering of the detection limit. Anal. Chim. Acta 2011, 707, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, M.A.; Korobeynikov, A.I.; Mikhelson, K.N. Estimation of ion-site association constants in ion-selective electrode membranes by modified segmented sandwich membrane method. Electrochim. Acta 2008, 53, 5819–5826. [Google Scholar] [CrossRef]
- Peshkova, M.A.; Koltashova, E.S.; Khripoun, G.A.; Mikhelson, K.N. Improvement of the upper limit of the ISE Nernstian response by tuned galvanostatic polarization. Electrochim. Acta 2015, 167, 187–193. [Google Scholar] [CrossRef]
- Cuartero, M.; Bakker, E. Environmental water analysis with membrane electrodes. Curr. Opin. Electrochem. 2017, 3, 97–105. [Google Scholar] [CrossRef]
- Bakker, E.; Bleiner, D.; Groh, K.; Bakker, E.; Bleiner, D. Perspectives and Future Directions of the Division of Analytical Sciences of the Swiss Chemical Society. Chimia 2021, 75, 455–456. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: Quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef]
- Ei ashry, E.S.H.; Awad, L.F.; Ibrahim, E.I.; Bdeewy, O.K. Synthesis of Antipyrine Derivatives Derived from Dimedone. Chin. J. Chem. 2007, 25, 570–573. [Google Scholar] [CrossRef]
- Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. Design, Synthesis, and Pharmacological Activity of Nonallergenic Pyrazolone-Type Antipyretic Analgesics. J. Med. Chem. 2010, 53, 8727–8733. [Google Scholar] [CrossRef]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Khan, M.T.H.; Yathirajan, H.S.; Raj, C.G.D.; Raghavendra, R. Antimicrobial, analgesic, DPPH scavenging activities and molecular docking study of some 1,3,5-triaryl-2-pyrazolines. Med. Chem. Res. 2012, 21, 2012–2022. [Google Scholar] [CrossRef]
- Rao, B.S.; Akberali, P.M.; Holla, B.S.; Sarojini, B.K. Synthesis and studies on some new fluorine containing hydroxypyrazolines and 1H pyrazoles-as possible antiproliferative agents. J. Pharmacol. Toxicol. 2008, 3, 102–110. [Google Scholar] [CrossRef]
- Bansal, E.; Srivastava, V.K.; Kumar, A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substitute daryl-3-(β-aminonaphthyl)-2-pyrazolines and β-(substitute daminoethyl) amidonaphthalenes. Eur. J. Med. Chem. 2001, 36, 81–92. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorganic Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Neeland, E.G.; Villanueva, E.B.; White, M.S.; El-Ashmawy, I.M.; Patrick, B.; Klegeris, A.; Abd-El-Aziz, A.S. Synthesis and biological evaluation of novel pyrazole compounds. Bioorganic Med. Chem. 2010, 18, 5685–5696. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Malato, S.; Agüera, A.; Fernández-Alba, A.R. Degradation of dipyrone and its main intermediates by solar AOPs: Identification of intermediate products and toxicity assessment. Catal. Today 2007, 129, 207–214. [Google Scholar] [CrossRef]
- Pamplona, J.H.; Oba, E.T.; da Silva, T.A.; Ramos, L.P.; Ramsdorf, W.A.; Cestari, M.M.; Oliveira Ribeiro, C.A.; Zampronio, A.R.; Silva de Assis, H.C. Subchronic effects of dipyrone on the fish species Rhamdia quelen. Ecotoxicol. Environ. Saf. 2011, 74, 342–349. [Google Scholar] [CrossRef]
- Tataeva, S.D.; Magomedova, V.S.; Magomedov, K.E. Determination of lead ions using an diantipyrylmethane-based electrode. J. Anal. Chem. 2016, 71, 1115–1119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).