Building a Sensor Benchmark for E-Nose Based Lung Cancer Detection: Methodological Considerations
Abstract
1. Introduction
2. Literature Review
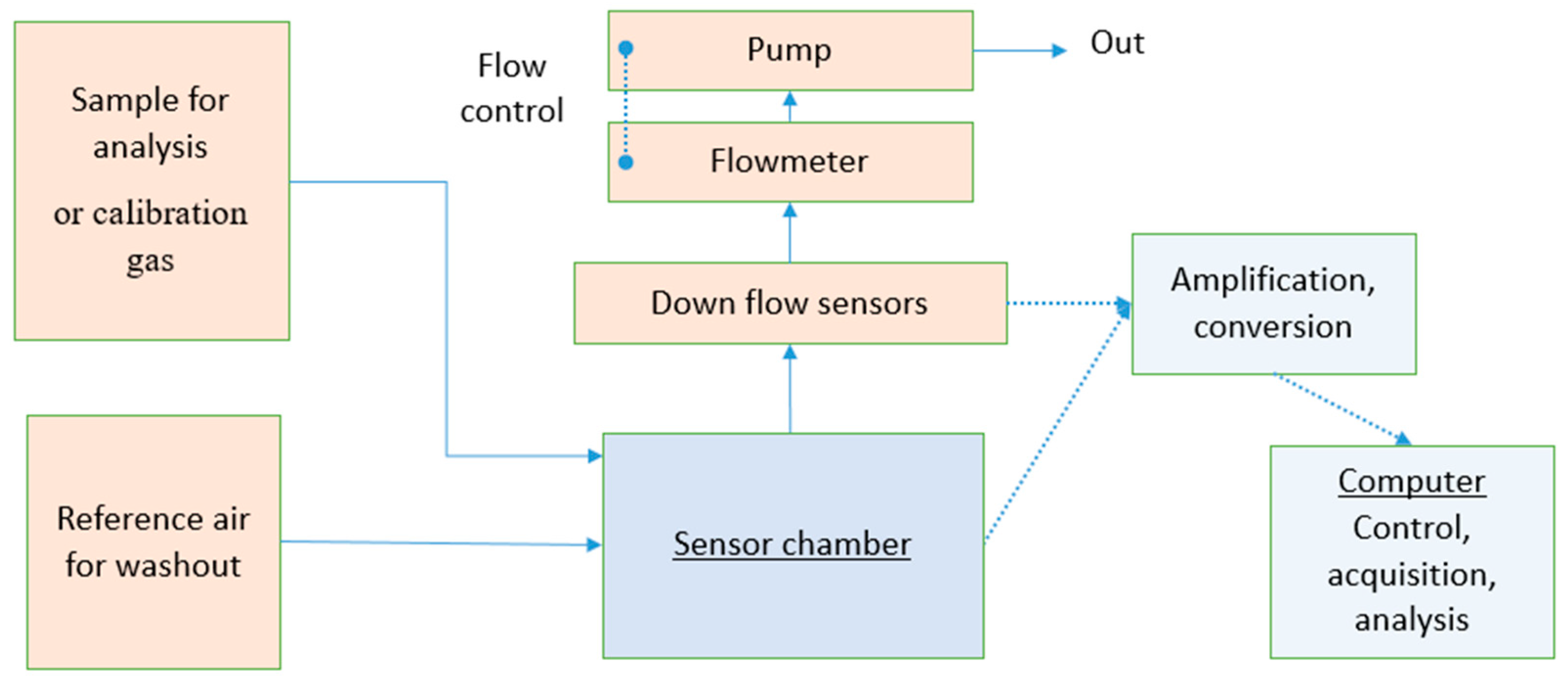
2.1. Instrumental Odour Monitoring System (IOMS)
- Sample storage, which includes piping and any form of sample containment before analysis. It can have a form of pre-concentration or pre-treatment against interfering compounds.
- The sensor array is usually composed of 4 to 32 sensors housed in one (or several) chamber(s). Sensors previously used in cancer breath detection are surface acoustic wave sensors (e.g., SAW, BAW, QMB/QCM) [13], polymer gas sensors [14,15] or carbon nanotube based sensors [16], but the most common are likely metal oxide semiconductor (MOS) sensors as the technology is well known and commercially available [17,18,19].
- A signal treatment system, which converts the analogic output of the sensors to a numeric output interpretable by the processing unit.
- A processing unit that will control the other units, collect and save the data from the sensors.
2.2. Establishing a Performance Metric for Gas Sensors
- Relevant (to the purpose of the device tested).
- Equitable (all sensors are tested and are compared on the same basis).
- Repeatable (results can be verified).
- As effective as possible in regard of cost and logistics.
- Should work for all kind of purposes and concentration ranges, in order to be usable. across devices with different purposes using the same concept.
- Transparent (metrics should be easy to understand).
2.3. On the Use of Breath-like Gaseous Samples
2.3.1. Composition of Breath
2.3.2. On the Selection of Target VOCs
- With 12 occurrences, 1-propanol is the most cited compound.
- With 11 occurrences, 2-butanone (or methyl-ethyl-ketone).
- Isoprene has been cited 10 times in the reviewed papers.
- Hexanal, ethylbenzene and 2-propanol have nine occurrences each.
- Acetone was cited eight times.
- Pentane, benzene, and styrene were cited seven times each.
- Hexane, toluene and decane were cited sox times each.
- Propanal, nonanal, heptanal, undecane, 2-methylpentane, pseudocumene and ethanol were cited five times each.
- Pentane, 1-propanol, ethanol, dodecane, hexanal (potential biomarkers or behavioral contaminants).
- Acetone (unavoidable metabolism by-product).
- Toluene, 2-propanol (potential biomarker or smoking marker).
2.3.3. Sampling and Sample Storage
Offline Sampling
Inline Sampling
2.4. Data Treatment
- The steady-state response (the mean, median or maximum value of the signal’s plateau), usually with baseline subtraction to enhance response reproducibility and comparability. The baseline is the steady-state response of the reference air.
- The area under the curve (AUC), with baseline subtraction as well.
- The greatest ascending or descending slope, to give a measurement of the transient response). If the sensor is well-behaved (such as a polymer sensor or quartz microbalance sensor), it is possible to use the transient response as a predictor of the steady state response to reduce measurement time.
3. Methodological Proposal
3.1. Choice of VOCs
3.1.1. Using Real Breath as Dilution Gas
3.1.2. Contaminants
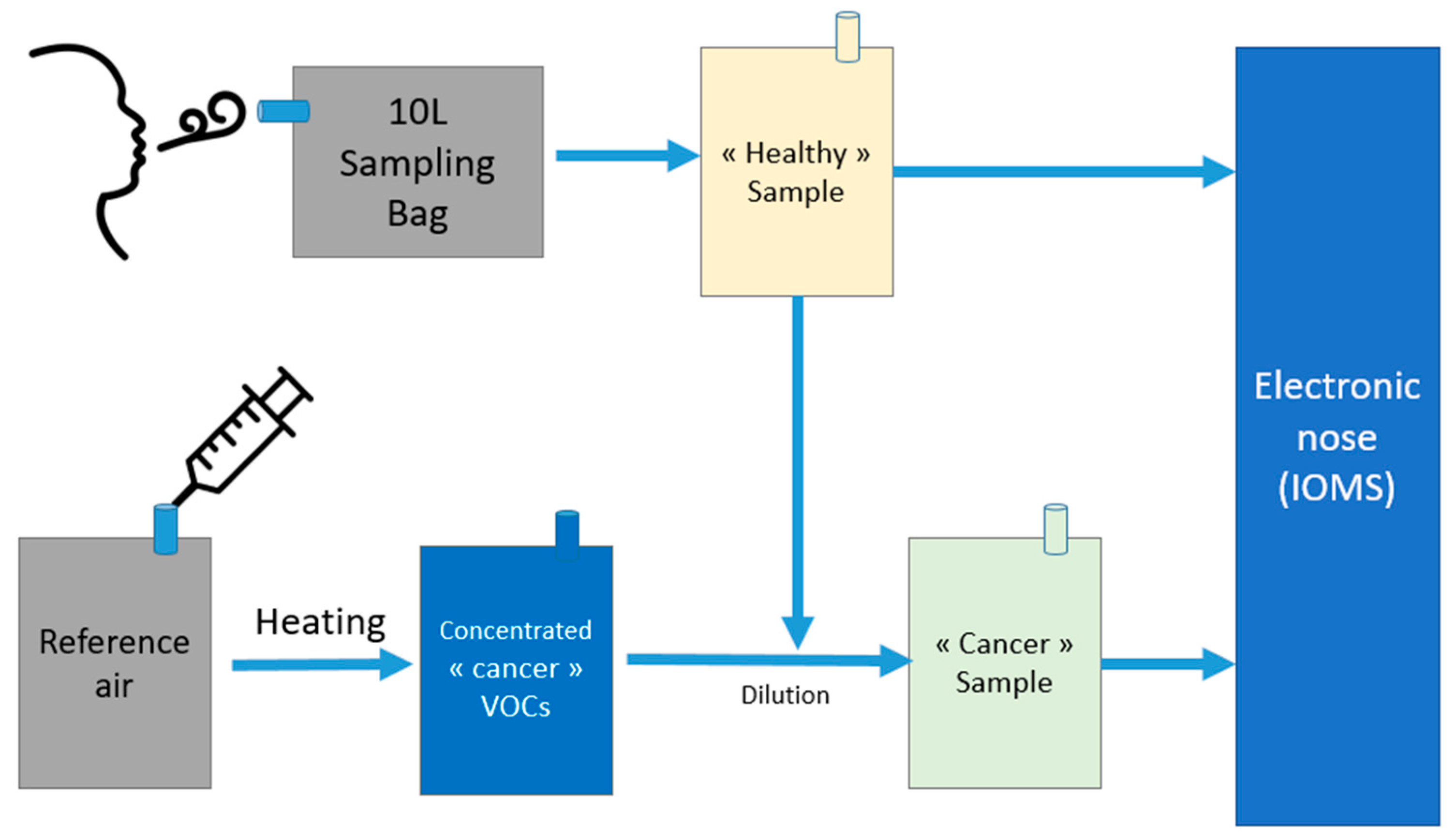
3.1.3. Sample Creation Procedure
- Flowmeter accuracy (and therefore dilution ratios and sample VOC concentrations).
- Sorption efficiency on sorption cartridges for TD–GC–MS.
3.1.4. Sampling and Sensor Testing
- Each bag contained a single VOC in a concentration well above those observed in real breath: sensors are rarely sensitive enough to sense a single compound at 1–100 ppb. The collective signal of all VOCs is what is measured. To find the sensitivity to a single compound, a range of concentrations of 500 ppb to 5 ppm are often needed. Four different concentrations were used to obtain a four-point calibration line. This enables the verification that sensor response is indeed linear over the range.
- In addition to VOCs, on different repetitions, humidity was set at 40% RH or 90% RH to check the cross influence of water vapor on the sensor readings.
- Usual confounders were added on different repetitions, at the highest concentration of the tested biomarker VOC for cross influence assessment. Therefore, for a 0.5 to 5 ppm series of samples, the confounder was set at 5 ppm in all samples.
- A mix using the concentrations found in the breath of cancer persons.
- A mix using the concentrations found in the breath of healthy persons.
- A mix using the concentrations found in the breath of healthy persons with some common smoking-related VOCs in usual concentrations.
- A mix using the concentrations found in non-cancerous persons having comorbidities frequently associated with lung cancer patients (such as Chronic Obstructive Pulmonary Disease, or COPD).
3.1.5. Gas Chromatography
3.2. Gas Sensor Benchmarking Apparatus
- Carbon dioxide measurement, by the mean of a CO2 sensor. Compact sensors using infrared in the 0–6% range are available on the market. This is an important parameter as most MOS sensors behave differently with differences in the oxygen content of the sample, and it serves as a simple way to ensure no leaks occurred in the system. O2 sensors can also be used alternatively, but often take more time than CO2 sensors to reach a stable signal. CO2 sensors and valves can also be used to select the most interesting part of each exhalation (alveolar air, for example).
- As breath is water-saturated at about 37 °C and sensors are influenced by both temperature and humidity, both parameters are crucial to monitor. A significant problem to consider is condensation. Liquid water in the system could remove some chemical species from the gas phase, which would alter responses from sensors. Accumulation of liquid water can also cause a range of problems (bacteria proliferation, VOC retention and contamination, material degradation, short circuits…). The whole system should therefore be heated to avoid condensation.
- The sensor chamber should be heated, and the temperature controlled with a feedback loop (i.e., Proportional-Integral-Derivative controller (PID)) in order to keep the ambient temperature around the sensors as stable as possible regardless of the device’s surroundings. As sensors are very sensitive to heat loss, this enables reproducible sensing and constant sensor properties [12]. To avoid contamination, the sensor chamber should always be upstream from everything else if possible.
- Flow speed is also an important parameter. Flow speed and sensor chamber design should be chosen to enable laminar flow [28]. A flow control device (e.g., rotameter, Mass Flow Meter, MFC) and pump were placed downstream from the sensor chamber to ensure constant and reproducible flow. Offline analysis of collected samples gave more stable sensor signals than having a patient directly blow into the sensor chamber. Sorbent cartridges instead of gas sampling bags, while manageable, have not been chosen for the testing device. The increased complexity would make the device harder to develop, and it was chosen to not use sorption for the first versions of the device.
- All materials in contact with the samples should be non-emissive and resistant to chemical alterations to avoid sample alteration, as mentioned before [47]. Stainless steel, glass or PTFE are the most appropriate materials in this regard. Other materials should be placed downflow of the sensor chamber. If this is not possible, rigorous testing of their influence on samples should be realized, preferably by using IOMS and a reference method such as GCMS.
- A small volume sensor chamber is preferred, as this avoids the dilution of samples and provides quicker signal stabilization. However, without enough dead volume the heat dissipation of the sensors is likely to be too low, and this might result in lower reproducibility and damage to the sensors if the heaters are under constant voltage. There is no straightforward way to dimension a sensor chamber to have an adequate inner dead volume. The sensor chamber currently being used for this project has an internal dead volume of 7.5 cubic centimeters, has shown excellent heat dissipation properties (40 °C interior temperature while in function without external heating, at room temperature), and the sensors used displayed quick reaction times (40 s mean reaction time). To complement the setup, each sensor heater is controlled by an individual PID to ensure optimum sensor temperature is maintained even under changing or uneven conditions.
- To enable experiment monitoring in real time, measurements can be displayed on a computer showing the evolution of sensor outputs. To ensure all variables are under control and experiments proceed as expected, this feature is invaluable.
- Sensors should be placed so that they are in the same conditions in regard to the flow. In this case, the sensors were placed radially so that they were all perpendicular to the flow in the same way, and therefore were evaluated in the exact same environment.
- TGS® 2603 (Figaro Engineering™, Osaka, Japan)
- G3530, G1430, G2530, G8530 (Umwelt Sensor Technik® GmbH, Thuringia, Germany)
- MP901 sensors (Winsen™ Electronics Technology Co., Ltd., Zhengzhou, China)
- BME680 (Bosch Sensortec™ GmbH, Reutlingen, Germany)
3.3. Statistical Aspects
3.4. Data Treatment
3.4.1. Pre-Treatment
3.4.2. Principal Component Analysis
- The quality of the separation between groups, by looking at the location and spread of clusters of samples. The further away groups are from each other, and the less overlapping there is, the better the classification by multivariate analysis will be, and therefore the higher the quality of the array is.
- The effect of the modification of one variable on the results, appearing as a shift in data (variation in VOC concentrations or humidity, for example).
- The contribution of each sensor to the separation of groups. Using the loading plot, it is possible to see if a feature is either redundant, not contributing to the separation, or important for the separation. One can therefore identify which sensors are worth keeping and which should be replaced or improved. It is however important to keep in mind that artificial mixtures are not breath, and that an apparently redundant sensor might become useful using real breath. The hypothesis that mixtures give a good enough representation of actual breath must be verified and will be exposed in future work. If it is indeed verified, it will be possible to discard or keep sensors based on the mixtures’ results alone, prior to any form of clinical trial.
- The effect of each feature (signal slopes, AUC, maximum value), the effect of each data treatment (e.g., with or without normalization, noise cancelling…).
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute SEER Explorer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 22 February 2021).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of Lung Cancer. Chest 2013, 143, e1S–e29S. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Pastis, N.J.; Tanner, N.T.; Jett, J.R. Clinical Aspects of Lung Cancer. In Murray and Nadel’s Textbook of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 940–964.e22. ISBN 978-1-4557-3383-5. [Google Scholar]
- Zhou, J.; Huang, Z.-A.; Kumar, U.; Chen, D.D.Y. Review of Recent Developments in Determining Volatile Organic Compounds in Exhaled Breath as Biomarkers for Lung Cancer Diagnosis. Anal. Chim. Acta 2017, 996, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive Detection of Lung Cancer by Analysis of Exhaled Breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of Volatile Lung Cancer Markers by Gas Chromatography–Mass Spectrometry: Comparison with Discrimination by Canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.H.; Goyette, J.; Thomas, P.S. Proteomics as a Method for Early Detection of Cancer: A Review of Proteomics, Exhaled Breath Condensate, and Lung Cancer Screening. J. Gen. Intern. Med. 2008, 23, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society Technical Standard: Exhaled Biomarkers in Lung Disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Romain, A.-C.; Nicolas, J.; Andre, P. Three Years Experiment with the Same Tin Oxide Sensor Arrays for the Identification of Malodorous Sources in the Environment. Sens. Actuators. B Chem. 2002, 84, 271–277. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W.; Bartlett, P.N. Electronic Noses, Principles and Applications; IOP Publishing Ltd.: New York, NY, USA, 1999. [Google Scholar]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile Signature for the Early Diagnosis of Lung Cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A Selective Chemiresistive Sensor for the Cancer-Related Volatile Organic Compound Hexanal by Using Molecularly Imprinted Polymers and Multiwalled Carbon Nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S. An Electronic Nose in Respiratory Disease. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chatterjee, S.; Castro, M.; Feller, J.F. An E-Nose Made of Carbon Nanotube Based Quantum Resistive Sensors for the Detection of Eighteen Polar/Nonpolar VOC Biomarkers of Lung Cancer. J. Mater. Chem. B 2013, 1, 4563–4575. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Lee, D.-S.; Ban, S.-W.; Oh, J.; Jung, M.Y.; Kim, S.-H.; Park, S.; Persaud, K.; Jheon, S. Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Diagnosis Using a Sensor System. Sens. Actuators B Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Jaeschke, C.; Padilla, M.; Turppa, E.; Polaka, I.; Gonzalez, O.; Mitrovics, J. Overview on Sniffphone: A Portable Device for Disease Diagnosis. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 298–299. [Google Scholar]
- Akamatsu, T.I.T.; Tsuruta, A.; Shin, W. Selective Detection of Target Volatile Organic Compounds in Contaminated Humid Air Using a Sensor Array with Principal Component Analysis. Sensors 2017, 17, 1662. [Google Scholar] [CrossRef] [PubMed]
- Shlomi, D.; Abud, M.; Liran, O.; Bar, J.; Gai-Mor, N.; Ilouze, M.; Onn, A.; Ben-Nun, A.; Haick, H.; Peled, N. Detection of Lung Cancer and EGFR Mutation by Electronic Nose System. J. Thorac. Oncol. 2017, 12, 1544–1551. [Google Scholar] [CrossRef]
- van de Goor, R.; van Hooren, M.; Dingemans, A.-M.; Kremer, B.; Kross, K. Training and Validating a Portable Electronic Nose for Lung Cancer Screening. J. Thorac. Oncol. 2018, 13, 676–681. [Google Scholar] [CrossRef]
- Kort, S.; Tiggeloven, M.M.; Brusse-Keizer, M.; Gerritsen, J.W.; Schouwink, J.H.; Citgez, E.; de Jongh, F.H.C.; Samii, S.; van der Maten, J.; van den Bogart, M.; et al. Multi-Centre Prospective Study on Diagnosing Subtypes of Lung Cancer by Exhaled-Breath Analysis. Lung Cancer 2018, 125, 223–229. [Google Scholar] [CrossRef]
- McWilliams, A.; Beigi, P.; Srinidhi, A.; Lam, S.; MacAulay, C.E. Sex and Smoking Status Effects on the Early Detection of Early Lung Cancer in High-Risk Smokers Using an Electronic Nose. IEEE Trans. Biomed. Eng. 2015, 62, 2044–2054. [Google Scholar] [CrossRef]
- Kou, L.; Zhang, D.; Liu, D. A Novel Medical E-Nose Signal Analysis System. Sensors 2017, 17, 402. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung Cancer Identification by the Analysis of Breath by Means of an Array of Non-Selective Gas Sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- de Vries, R.; Brinkman, P.; van der Schee, M.P.; Fens, N.; Dijkers, E.; Bootsma, S.K.; de Jongh, F.H.C.; Sterk, P.J. Integration of Electronic Nose Technology with Spirometry: Validation of a New Approach for Exhaled Breath Analysis. J. Breath Res. 2015, 9, 046001. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Berleant, D. Benchmarking Contemporary Deep Learning Hardware and Frameworks: A Survey of Qualitative Metrics. In Proceedings of the 2019 IEEE First International Conference on Cognitive Machine Intelligence (CogMI), Los Angeles, CA, USA,, 12–14 December 2019; pp. 148–155. [Google Scholar]
- Endres, H.-E.; Jander, H.D.; Göttler, W. A Test System for Gas Sensors. Sens. Actuators B Chem. 1995, 23, 163–172. [Google Scholar] [CrossRef]
- (PDF) Data Analysis for Electronic Nose Systems. Available online: https://www.researchgate.net/publication/226437091_Data_analysis_for_electronic_nose_systems (accessed on 14 February 2020).
- Johnson, K.J.; Rose-Pehrsson, S.L. Sensor Array Design for Complex Sensing Tasks. Annual Rev. Anal. Chem. 2015, 8, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, X.; Lu, S. Integrated Sensor Array Optimization with Statistical Evaluation. Sens. Actuators B Chem. 2010, 149, 239–244. [Google Scholar] [CrossRef]
- Gasparri, R.; Romano, R.; Sedda, G.; Borri, A.; Petrella, F.; Galetta, D.; Casiraghi, M.; Spaggiari, L. Diagnostic Biomarkers for Lung Cancer Prevention. J. Breath Res. 2018, 12, 027111. [Google Scholar] [CrossRef] [PubMed]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Kermani, B.G.; Schiffman, S.S.; Nagle, H.T. Performance of the Levenberg–Marquardt Neural Network Training Method in Electronic Nose Applications. Sens. Actuators B Chem. 2005, 110, 13–22. [Google Scholar] [CrossRef]
- Li, Y.; Täffner, T.; Bischoff, M.; Niemeyer, B. Test Gas Generation from Pure Liquids: An Application-Oriented Overview of Methods in a Nutshell. Int. J. Chem. Eng. 2012, 2012, e417029. [Google Scholar] [CrossRef]
- Namies´nik, J. Generation of Standard Gaseous Mixtures. J. Chromatogr. A 1984, 300, 79–108. [Google Scholar] [CrossRef]
- Walden, J.; Macé, T.; Haerri, H.-P.; Sutour, C.; Couette, J.; Niederhauser, B. Guide on Dynamic Dilution Methods for NO, NO2 and SO2 at Limit Values; European Metrology Research Program (EMRP): Braunschweig, Germany, 2014; p. 31. [Google Scholar]
- Helwig, N.; Schüler, M.; Bur, C.; Schütze, A.; Sauerwald, T. Gas Mixing Apparatus for Automated Gas Sensor Characterization. Meas. Sci. Technol. 2014, 25, 055903. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.B.; Bezverkhyy, I.; Guy, W.; Berger, F.; Fierro, V.; Bellat, J.P.; Celzard, A. Detection and Quantification of Lung Cancer Biomarkers by a Micro-Analytical Device Using a Single Metal Oxide-Based Gas Sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and in Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; Duan, Y. Breath Analysis: Technical Developments and Challenges in the Monitoring of Human Exposure to Volatile Organic Compounds. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1002, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M. Breathprinting Roadmap Based on Experts’ Opinions; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128145623. [Google Scholar]
- Herbig, J.; Beauchamp, J. Towards Standardization in the Analysis of Breath Gas Volatiles. J. Breath Res. 2014, 8, 037101. [Google Scholar] [CrossRef]
- Tan, J.-L.; Yong, Z.-X.; Liam, C.-K. Using a Chemiresistor-Based Alkane Sensor to Distinguish Exhaled Breaths of Lung Cancer Patients from Subjects with No Lung Cancer. J. Thorac. Dis. 2016, 8, 2772–2783. [Google Scholar] [CrossRef]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of Exhaled Breath Composition on Exogenous Factors, Smoking Habits and Exposure to Air Pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Martin, J.D.M.; Romain, A.-C. Experimental Evaluation of Gas Sensors Array for the Identification of Complex Voc Mixtures in Human Breath. Chem. Eng. Trans. 2021, 85, 199–204. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Pesesse, R. Contribution of Comprenhensive Two-Dimensional Gas Chromatography to Untargeted Volatilomics of Lung Cancer. Ph.D. Thesis, Université de Liège, Sart-Tilman, Liege, Belgium, 2019. [Google Scholar]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Barash, O.; Peled, N.; Hirsch, F.R.; Haick, H. Sniffing the Unique “Odor Print” of Non-Small-Cell Lung Cancer with Gold Nanoparticles. Small 2009, 5, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A Study of an Electronic Nose for Detection of Lung Cancer Based on a Virtual SAW Gas Sensors Array and Imaging Recognition Method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Biehl, W.; Hattesohl, A.; Jörres, R.A.; Duell, T.; Althöhn, U.; Koczulla, A.R.; Schmetzer, H. VOC Pattern Recognition of Lung Cancer: A Comparative Evaluation of Different Dog- and ENose-Based Strategies Using Different Sampling Materials. Acta Oncol. 2019, 58, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the Use of Tedlar® Bags for Breath-Gas Sampling and Analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Unterkofler, K.; Amann, A. Stability of Selected Volatile Breath Constituents in Tedlar, Kynar and Flexfilm Sampling Bags. Analyst 2013, 138, 1405. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Wzorek, B.; Śliwka, I.; Amann, A. Suitability of Different Polymer Bags for Storage of Volatile Sulphur Compounds Relevant to Breath Analysis. J. Chromatogr. B 2009, 877, 189–196. [Google Scholar] [CrossRef]
- Kononov, A.; Korotetsky, B.; Jahatspanian, I.; Gubal, A.; Vasiliev, A.; Arseniev, A.; Nefedov, A.; Barchuk, A.; Gorbunov, I.; Kozyrev, K.; et al. Online Breath Analysis Using Metal Oxide Semiconductor Sensors (Electronic Nose) for Diagnosis of Lung Cancer. J. Breath Res. 2019, 14, 016004. [Google Scholar] [CrossRef]
- Gregis, G. Étude et Réalisation d’un Système Miniaturisé Pour l’analyse de Composés Organiques Volatils Considérés Comme Des Marqueurs Chimiques Du Cancer Du Poumon. Ph.D. Thesis, Université de Bourgogne Franche-Comté, Franche-Comté, France, 2017. [Google Scholar]
- Strathmann, S. Sample Conditioning for Multi-Sensor Systems. Ph.D. Thesis, Der Fakultät für Chemie und Pharmazie der Eberhard-Karls-Universität Tübingen, Tübingen, Germany, 2001. [Google Scholar]
- Wilkinson, M.; White, I.R.; Goodacre, R.; Nijsen, T.; Fowler, S.J. Effects of High Relative Humidity and Dry Purging on VOCs Obtained during Breath Sampling on Common Sorbent Tubes. J. Breath Res. 2020, 14, 046006. [Google Scholar] [CrossRef]
- Harshman, S.W.; Mani, N.; Geier, B.A.; Kwak, J.; Shepard, P.; Fan, M.; Sudberry, G.L.; Mayes, R.S.; Ott, D.K.; Martin, J.A.; et al. Storage Stability of Exhaled Breath on Tenax TA. J. Breath Res. 2016, 10, 046008. [Google Scholar] [CrossRef] [PubMed]
- van der Schee, M.P.; Fens, N.; Brinkman, P.; Bos, L.D.J.; Angelo, M.D.; Nijsen, T.M.E.; Raabe, R.; Knobel, H.H.; Vink, T.J.; Sterk, P.J. Effect of Transportation and Storage Using Sorbent Tubes of Exhaled Breath Samples on Diagnostic Accuracy of Electronic Nose Analysis. J. Breath Res. 2012, 7, 016002. [Google Scholar] [CrossRef]
- Righettoni, M.; Ragnoni, A.; Güntner, A.; Loccioni, C.; Pratsinis, S.; Risby, T. Monitoring Breath Markers under Controlled Conditions. J. Breath Res. 2015, 9, 047101. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Xie, D.; He, Z.; Pi, X. Lung Cancer Screening Based on Type-Different Sensor Arrays. Sci. Rep. 2017, 7, 1969. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Fu, L.; Nie, B.; Peng, Z.; Liu, H. A Novel Framework with High Diagnostic Sensitivity for Lung Cancer Detection by Electronic Nose. Sensors 2019, 19, 5333. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.A.; Thomas, P.S.; Stone, E.; Lewis, C.; Yates, D.H. A Breath Test for Malignant Mesothelioma Using an Electronic Nose. Eur. Respir. J. 2012, 40, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Romain, A.C.; Nicolas, J. Long Term Stability of Metal Oxide-Based Gas Sensors for e-Nose Environmental Applications: An Overview. Sens. Actuators B Chem. 2010, 146, 502–506. [Google Scholar] [CrossRef]
- Burlachenko, J.; Kruglenko, I.; Snopok, B.; Persaud, K. Sample Handling for Electronic Nose Technology: State of the Art and Future Trends. TrAC Trends Anal. Chem. 2016, 82, 222–236. [Google Scholar] [CrossRef]
- Montuschi, P.; Mores, N.; Trové, A.; Mondino, C.; Barnes, P.J. The Electronic Nose in Respiratory Medicine. Respiration 2013, 85, 72–84. [Google Scholar] [CrossRef]
- Osorio-Arrieta, D.L.; Muñoz-Mata, J.L.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Mendoza-Barrera, C.O.; Altuzar-Aguilar, V.; Muñoz-Aguirre, S. Reduction of the Measurement Time by the Prediction of the Steady-State Response for Quartz Crystal Microbalance Gas Sensors. Sensors 2018, 18, 2475. [Google Scholar] [CrossRef]
- Marco, S.; Gutierrez-Galvez, A. Signal and Data Processing for Machine Olfaction and Chemical Sensing: A Review. IEEE Sens. J. 2012, 12, 3189–3214. [Google Scholar] [CrossRef]
- Zuppa, M.; Distante, C.; Siciliano, P.; Persaud, K. Drift Counteraction with Multiple Self-Organising Maps for an Electronic Nose. Sens. Actuators B Chem. 2004, 98, 305–317. [Google Scholar] [CrossRef]
- Liu, B.; Yu, H.; Zeng, X.; Zhang, D.; Gong, J.; Tian, L.; Qian, J.; Zhao, L.; Zhang, S.; Liu, R. Lung Cancer Detection via Breath by Electronic Nose Enhanced with a Sparse Group Feature Selection Approach. Sens. Actuators 2021, 339, 129896. [Google Scholar] [CrossRef]
- Fernandez, L.; Yan, J.; Fonollosa, J.; Burgués, J.; Gutierrez, A.; Marco, S. A Practical Method to Estimate the Resolving Power of a Chemical Sensor Array: Application to Feature Selection. Front. Chem. 2018, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How To? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Huang, C.-H.; Zeng, C.; Wang, Y.-C.; Peng, H.-Y.; Lin, C.-S.; Chang, C.-J.; Yang, H.-Y. A Study of Diagnostic Accuracy Using a Chemical Sensor Array and a Machine Learning Technique to Detect Lung Cancer. Sensors 2018, 18, 2845. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of Volatile Organic Compounds as Potential Markers of Lung Cancer by Gas Chromatography–Mass Spectrometry versus Trained Dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The Application of Statistical Methods Using VOCs to Identify Patients with Lung Cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef]
- Monedeiro, F.; Monedeiro-Milanowski, M.; Ratiu, I.-A.; Brożek, B.; Ligor, T.; Buszewski, B. Needle Trap Device-GC-MS for Characterization of Lung Diseases Based on Breath VOC Profiles. Molecules 2021, 26, 1789. [Google Scholar] [CrossRef]
- Ligor, T.; Pater, Ł.; Buszewski, B. Application of an Artificial Neural Network Model for Selection of Potential Lung Cancer Biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef]
- Amann, A.; Miekisch, W.; Pleil, J.D.; Risby, T.; Schubert, J. Methodological Issues of Sample Collection and Analysis of Exhaled Breath; Chapter 7; Maney Publishing: Leeds, UK, 2010; pp. 96–114. [Google Scholar]
- Beauchamp, J. Inhaled Today, Not Gone Tomorrow: Pharmacokinetics and Environmental Exposure of Volatiles in Exhaled Breath. J. Breath Res. 2011, 5, 037103. [Google Scholar] [CrossRef]
- Ge, D.; Zhou, J.; Chu, Y.; Lu, Y.; Zou, X.; Xia, L.; Liu, Y.; Huang, C.; Shen, C.; Zhang, L.; et al. Distinguish Oral-Source VOCs and Control Their Potential Impact on Breath Biomarkers. Anal. Bioanal. Chem. 2022, 414, 2275–2284. [Google Scholar] [CrossRef]
- Herbig, J.; Titzmann, T.; Beauchamp, J.; Kohl, I.; Hansel, A. Buffered End-Tidal (BET) Sampling—A Novel Method for Real-Time Breath-Gas Analysis. J. Breath Res. 2008, 2, 037008. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef]
- Bastuck, M. Improving the Performance of Gas Sensor Systems with Advanced Data Evaluation, Operation, and Calibration Methods; Linköping Studies in Science and Technology. Dissertations; Linköping University Electronic Press: Linköping, Sweden, 2019; Volume 2009, ISBN 978-91-7685-003-9. [Google Scholar]
- Diagnostic de Pathologies Humaines par Analyse de COV dans l’Air Expiré. Available online: https://pathacov-project.com/ (accessed on 6 July 2022).
- Fugit, J.L.; Dutaur, L.; Simon, V.; Riba, M.L.; Torres, L. Generation and Storage of Standard Gas Mixtures Containing Traces of Analytes. Fresenius Environ. Bull. 1996, 5, 682–687. [Google Scholar]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled Volatile Organic Compounds in Patients with Non-Small Cell Lung Cancer: Cross Sectional and Nested Short-Term Follow-up Study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of Volatile Organic Compounds from Lung Cancer Patients and Healthy Controls—Challenges and Limitations of an Observational Study. J. Breath Res. 2016, 10, 046007. [Google Scholar] [CrossRef]
- Hood, J.F.; Silvis, W.M. Predicting and Preventing Water Condensation in Sampled Vehicle Exhaust for Optimal CVS Dilution; SAE Technical Paper; SAE: Warrendale, PA, USA, 1998; p. 980404. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J. Clin. Diagn Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef]
- Alberg, A.J.; Brock, M.V.; Samet, J.M. Epidemiology of Lung Cancer. In Murray and Nadel’s Textbook of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 927–939.e5. ISBN 978-1-4557-3383-5. [Google Scholar]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Green, B.A.; Holemans, J.A.; Kavanagh, T.; Kerr, K.M.; et al. The UK Lung Cancer Screening Trial: A Pilot Randomised Controlled Trial of Low-Dose Computed Tomography Screening for the Early Detection of Lung Cancer. Health Technol. Assess. 2016, 20, 1–146. [Google Scholar] [CrossRef]
- van Klaveren, R.J.; Oudkerk, M.; Prokop, M.; Scholten, E.T.; Nackaerts, K.; Vernhout, R.; van Iersel, C.A.; van den Bergh, K.A.M.; van ’t Westeinde, S.; van der Aalst, C.; et al. Management of Lung Nodules Detected by Volume CT Scanning. N. Engl. J. Med. 2009, 361, 2221–2229. [Google Scholar] [CrossRef]
- Chudgar, N.P.; Bucciarelli, P.R.; Jeffries, E.M.; Rizk, N.P.; Park, B.J.; Adusumilli, P.S.; Jones, D.R. Results of the National Lung Cancer Screening Trial: Where Are We Now? Thorac. Surg. Clin. 2015, 25, 145–153. [Google Scholar] [CrossRef]

| Rank | Compound | Pool | Rank | Compound | Pool | Rank | Compound | Pool |
|---|---|---|---|---|---|---|---|---|
| 1 | 1-propanol | 2267 | 13 | 2-propanol | 905 | 25 | Cyclohexane | 424 |
| 2 | Isoprene | 1840 | 14 | 2-pentanone | 897 | 26 | Hexane | 408 |
| 3 | 2-butanone | 1559 | 15 | Benzaldehyde | 861 | 27 | Methyl-cyclopentane | 408 |
| 4 | Acetone | 1488 | 16 | Pentane | 832 | 28 | 1,2,4-trimethylbenzene | 403 |
| 5 | 3-hydroxy-2-butanone | 1285 | 17 | Ethanol | 669 | 29 | Ethylacetate | 370 |
| 6 | Pentanal | 1151 | 18 | Dimethylsulfide | 668 | 30 | Nonanal | 339 |
| 7 | Methanol | 1023 | 19 | Benzene | 542 | 31 | Octanal | 316 |
| 8 | Hexanal | 1019 | 20 | Styrene | 540 | 32 | Butanal | 244 |
| 9 | Propanal | 999 | 21 | Toluene | 512 | 33 | N-dodecane | 241 |
| 10 | Butane | 980 | 22 | Decane | 456 | 34 | Eicosane | 233 |
| 11 | Undecane | 973 | 23 | Heptanal | 455 | 35 | 2-propenal | 125 |
| 12 | Ethylbenzene | 962 | 24 | 2-methylpentane | 455 | 36 | Hexadecane | 117 |
| VOC | Healthy Average (ppb) | Smoker Average (ppb) | COPD Average (ppb) | Cancer Average (ppb) | |||
|---|---|---|---|---|---|---|---|
| [78] | [77] 1 | [78] | [79] | [77] 1 | [78] | [77] 1 | |
| 2-butanone | 5.1 | 7 | 10.6 | 1.45 | 6 | 8.8 | 9 |
| Decane | - | 11 | - | 0.23 | 7 | - | 9 |
| Pentane | 104.9 | 111 | 108.4 | 1.87 | - | 40 | 11 |
| 1-propanol | 6.6 | 61 | 17 | 28.15 | 28 | 54.8 | 99 |
| 2-propanol | 13.3 | 169 | 320.7 | 258.37 | 92 | 149.5 | 398 |
| Ethanol | 188.5 | 193 | 286.4 | 218.64 | 523 | 467 | 1203 |
| 2-pentanone | 5 | 6 | 5.3 | - | 492 | 7.5 | 9 |
| Acetone | 226 | 580 | 330.2 | - | 19 | 359 | 1000 |
| Hexanal | 0 | 3 | 0 | - | 719 | 4.5 | 4 |
| Toluene | 30.9 | 13 | 46.8 | 0.63 | 4 | 12.9 | 7 |
| Benzene | 6.3 | 7 | 9.2 | 0.57 | 7 | 5.4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, J.D.M.; Romain, A.-C. Building a Sensor Benchmark for E-Nose Based Lung Cancer Detection: Methodological Considerations. Chemosensors 2022, 10, 444. https://doi.org/10.3390/chemosensors10110444
Martin JDM, Romain A-C. Building a Sensor Benchmark for E-Nose Based Lung Cancer Detection: Methodological Considerations. Chemosensors. 2022; 10(11):444. https://doi.org/10.3390/chemosensors10110444
Chicago/Turabian StyleMartin, Justin D. M., and Anne-Claude Romain. 2022. "Building a Sensor Benchmark for E-Nose Based Lung Cancer Detection: Methodological Considerations" Chemosensors 10, no. 11: 444. https://doi.org/10.3390/chemosensors10110444
APA StyleMartin, J. D. M., & Romain, A.-C. (2022). Building a Sensor Benchmark for E-Nose Based Lung Cancer Detection: Methodological Considerations. Chemosensors, 10(11), 444. https://doi.org/10.3390/chemosensors10110444







