Electroanalytical Detection of Indigo Carmine in Presence of Tartrazine Using a Poly(dl-phenylalanine) Modified Carbon Nanotube Paste Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Preparation of BCNTPE
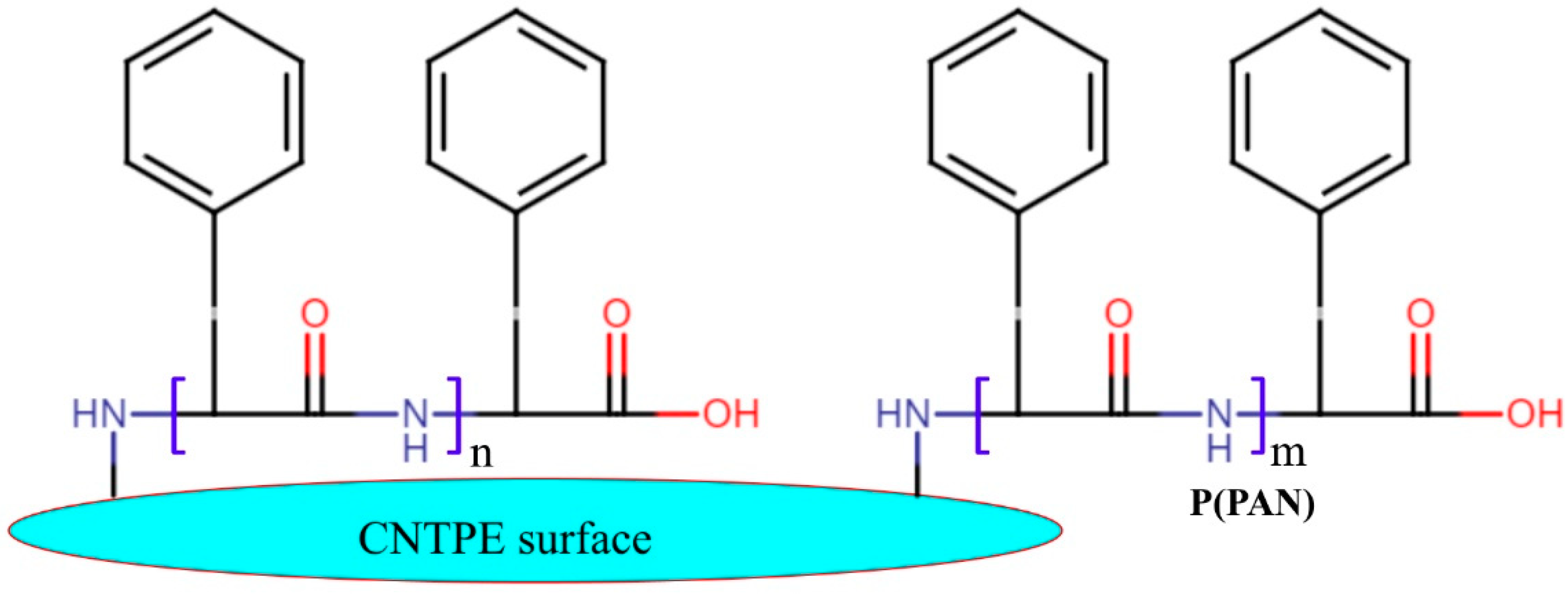
2.4. Preparation of P(PAN)LCNTPE
3. Results and Discussions
3.1. FE-SEM and EDX Analysis of BCNTPE and P(PAN)LCNTPE
3.2. EIS Study of BCNTPE and P(PAN)LCNTPE
3.3. Active Surface Area of BCNTPE and P(PAN)LCNTPE
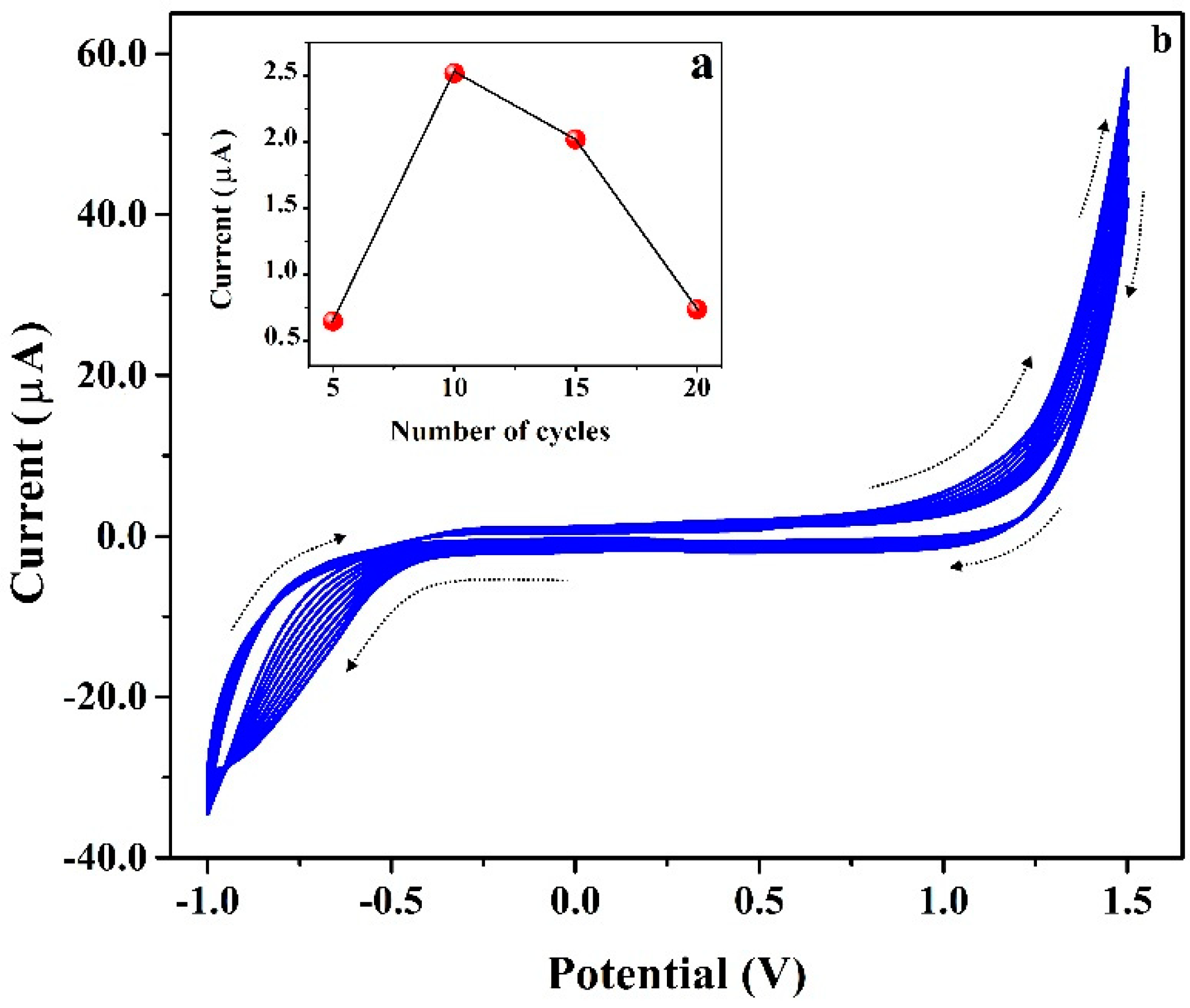
3.4. Electrochemical Polymerization of PAN on CNTPE Surface
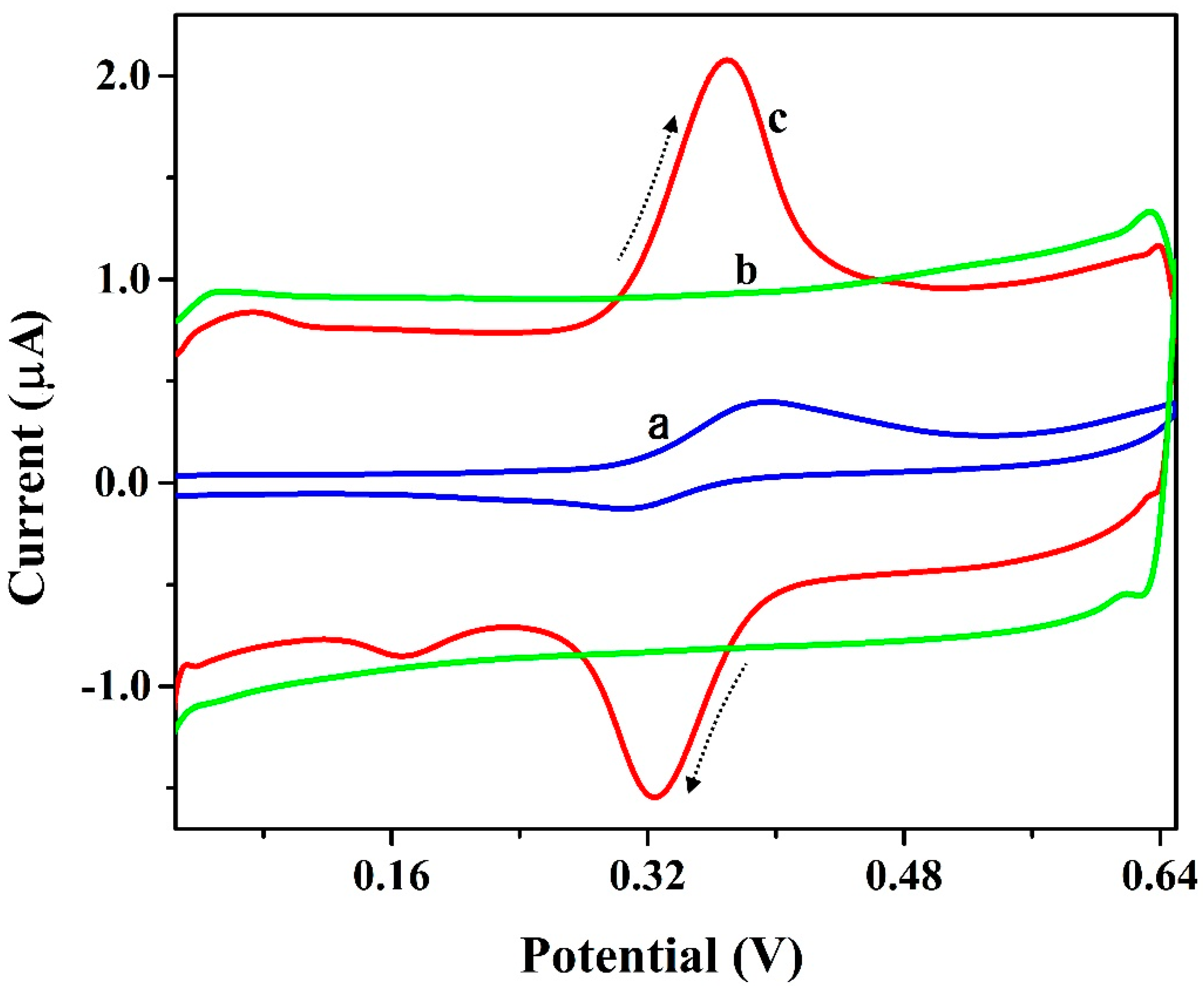
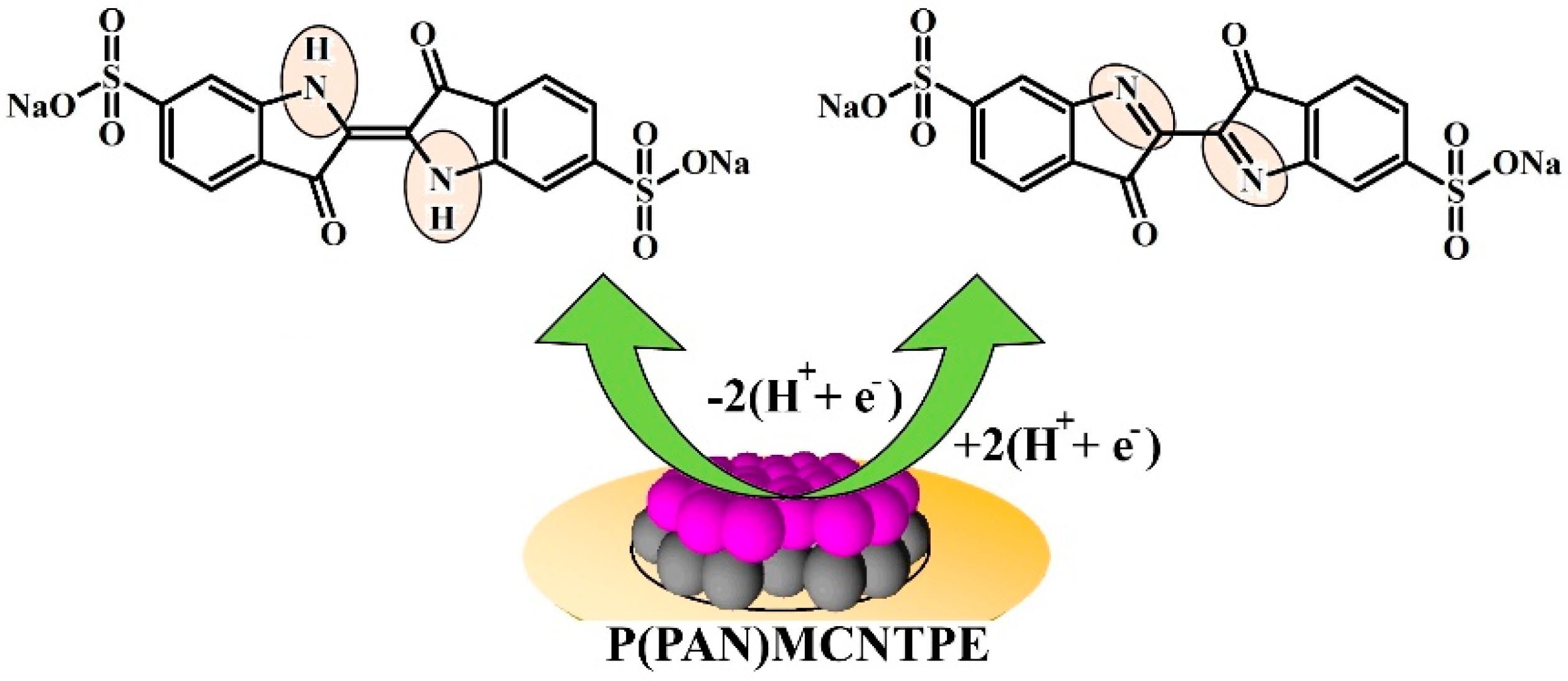
3.5. Electrochemical Nature of ICN
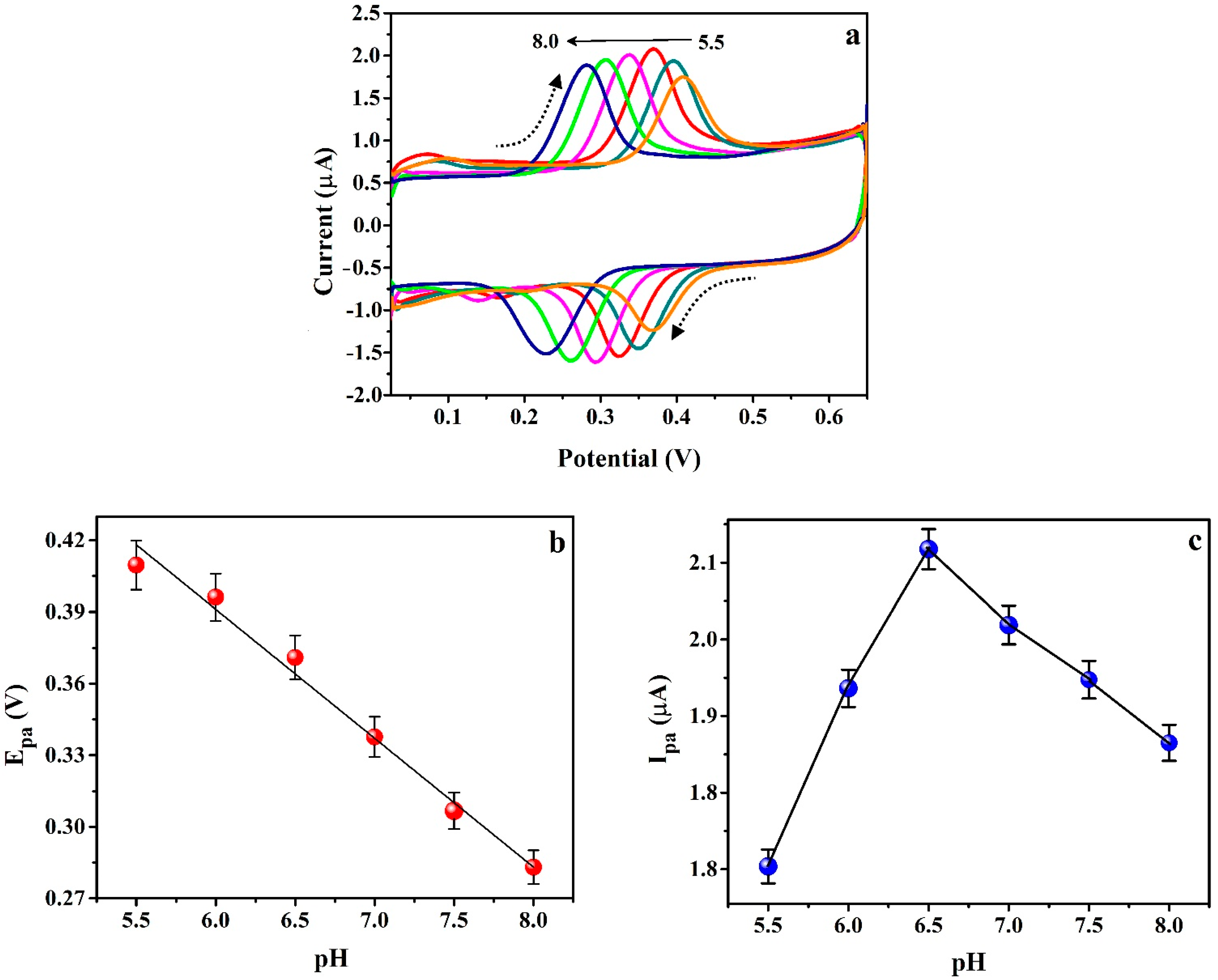
3.6. Effect of pH on ICN Electrochemical Activity
3.7. Scan Rate Impact on Peak Current and Potential
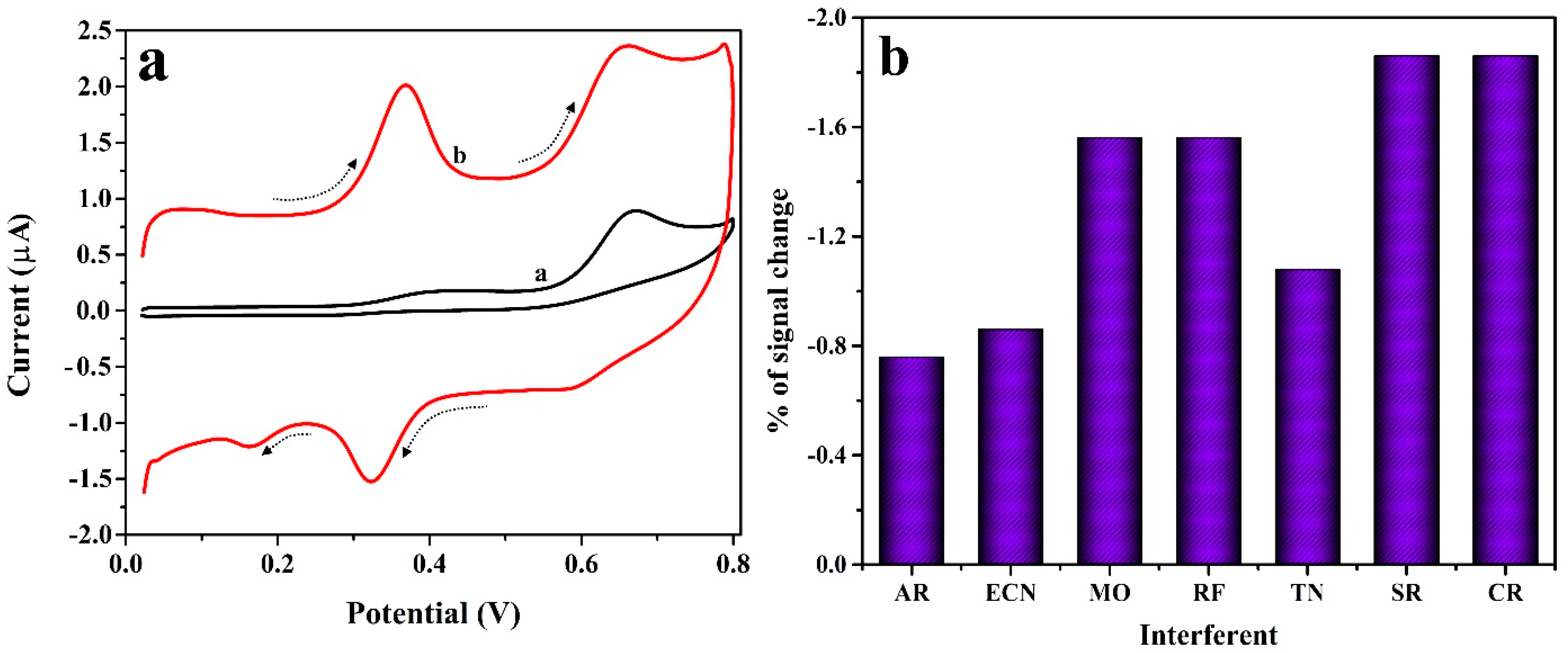
3.8. Simultaneous and Interference Analysis
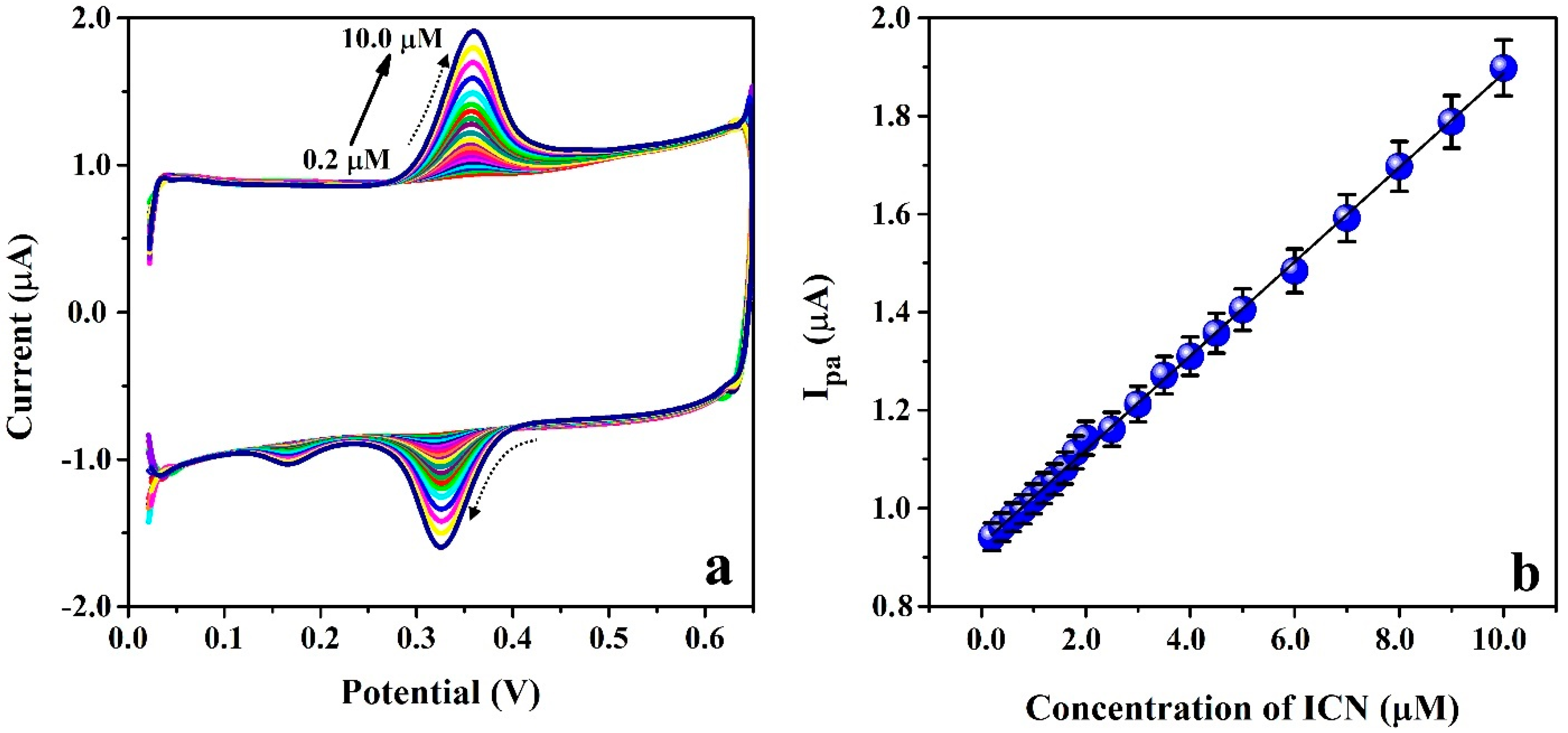
3.9. Limit of Detection and Quantification
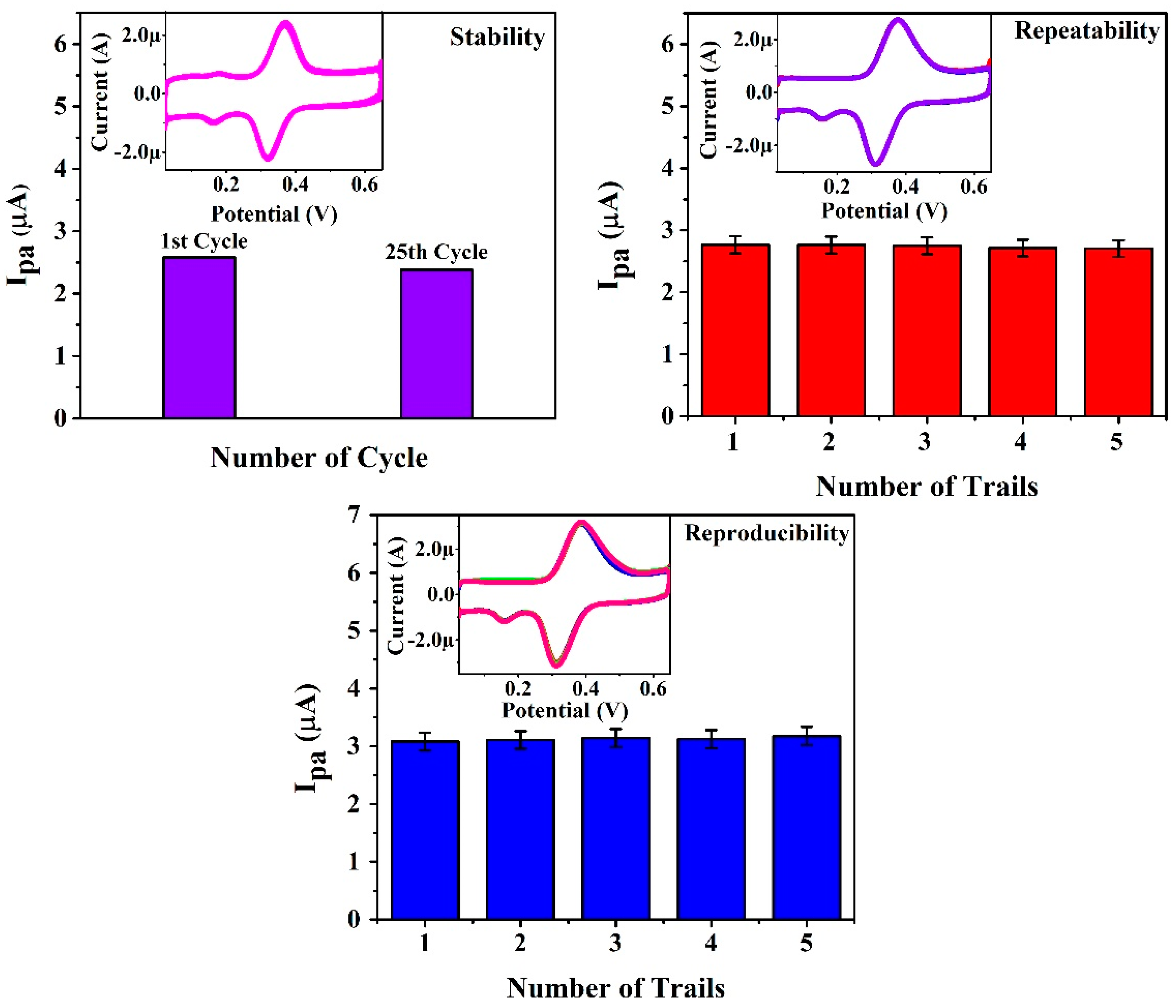
3.10. Stability, Repeatability and Reproducibility
3.11. Analysis of Water Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.V.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact; IntechOpen: London, UK, 2013; pp. 151–176. [Google Scholar]
- Ammar, S.; Abdelhedi, R.; Flox, C.; Arias, C.; Brillas, E. Electrochemical degradation of the dye indigo carmine at boron-doped diamond anode for wastewaters remediation. Environ. Chem. Lett. 2006, 4, 229–233. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, S.K. The use of indigo carmine in ureteral operations. Urol. J. 1967, 98, 669. [Google Scholar] [CrossRef]
- Ikeda, K.; Sannohe, Y.; Araki, S.; Inutsuka, S. A new trial in endoscopic diagnosis for stomach cancer: Intra-arterial dye (IAD) method. Gastrointest. Endosc. 1980, 26, 19801. [Google Scholar] [CrossRef]
- Fujita, M.; Kuroda, C.; Hosomi, N.; Inoue, E.; Kuriyama, K.; Ohhigashi, H.; Kishimoto, S.; Ishikawa, O.; Nakaizumi, A.; Vasc, J. Dye-Injection Method for Placement of an Infusion Catheter in Regional Hepatic Chemotherapy. Interv. Radiol. 1995, 6, 119. [Google Scholar] [CrossRef]
- Altok, M.; Sahin, A.F.; Gokce, M.I.; Ekin, G.R.; Divrik, R.T. Ureteral orifice involvement by urothelial carcinoma: Long term oncologic and functional outcomes. Int. Braz. J. Urol. 2017, 43, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.Y.; Datta, T.D.; Kirimli, B.I. Reaction to indigo carmine. J. Urol. 1976, 116, 132. [Google Scholar] [CrossRef]
- Lakshmi, U.R.; Srivastava, V.C.; Mall, I.D.; Lataye, D.H. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine dye. J. Environ. Manag. 2009, 90, 710. [Google Scholar] [CrossRef]
- Kennedy, W.K.; Wirjoatmadja, K.; Akamatsu, T.J.; Bonica, J.J. Cardiovascular and respiratory effects of indigo carmine. J. Urol. 1968, 100, 775. [Google Scholar] [CrossRef]
- Naitoh, J.; Fox, B.M. Severe hypotension, bronchospasm, and urticaria from intravenous indigo carmine. Urology 1994, 44, 271. [Google Scholar] [CrossRef]
- Berzas, J.J.; Flores, J.R.; Llerena, M.J.V.; Farinas, N.R. Spectrophotometric resolution of ternary mixtures of Tartrazine, Patent Blue V and Indigo Carmine in commercial products. Anal. Chim. Acta 1999, 391, 353. [Google Scholar] [CrossRef]
- Oka, H.; Ikai, Y.; Kawamura, K.; Yamada, M.; Inoue, H. Simple method for the analysis of food dyes on reversed-phase thin-layer plates. J. Chromatogr. A 1987, 411, 437. [Google Scholar] [CrossRef]
- Fereja, T.H.; Kitte, S.A.; Zafar, M.N.; Halawa, M.I.; Han, S.; Zhang, W.; Xu, G. Highly sensitive and selective non-enzymatic glucose detection based on indigo carmine/hemin/H2O2 chemiluminescence. Analyst 2020, 145, 1041. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Kuo, C.H.; Shih, D.Y.C. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 2015, 23, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.J.B.; Abedul, M.T.F.; Garcia, A.C. Flow amperometric detection of indigo for enzyme-linked immunosorbent assays with use of screen-printed electrodes. Anal. Chim. Acta 2002, 462, 31. [Google Scholar] [CrossRef]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103. [Google Scholar] [CrossRef]
- Kavieva, L.; Ziyatdinova, G. Voltammetric Sensor Based on SeO2 Nanoparticles and Surfactants for Indigo Carmine Determination. Sensors 2022, 22, 3224. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Teixeira, M.F.; Fatibello-Filho, O.; Dockal, E.R.; Bonifacio, V.G.; Marcolino-Junior, L.H. Electrochemical sensor for ranitidine determination based on carbon paste electrode modified with oxovanadium (IV) salen complex. Mater. Sci. Eng. C 2013, 33, 4081–4085. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Bahrami, H.; Karimi-Maleh, H.; Mallakpour, S. Carbon Paste Electrode Prepared from Chemically Modified Multiwall Carbon Nanotubes for the Voltammetric Determination of Isoprenaline in Pharmaceutical and Urine Samples. Chin. J. Catal. 2012, 33, 1919–1926. [Google Scholar] [CrossRef]
- Arvand, M.; Saberi, M.; Ardaki, M.S.; Mohammadi, A. Mediated electrochemical method for the determination of indigo carmine levels in food products. Talanta 2017, 173, 60–68. [Google Scholar] [CrossRef]
- Arbabi, N.; Beitollahi, H. A New Sensor Based on a La3+/Co3O4 Nanoflowers Modified Screen Printed Electrode for a Sensitive Simultaneous Determination of Levodopa and Tryptophan. Surf. Eng. Appl. Electrochem. 2022, 58, 305–312. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Liu, M.; Li, D.; Liu, G.; Zhao, Y.; Ding, Z.; Chen, F.; Wang, B.; Tan, X.; et al. Poly(glutamic acid) hydrogels crosslinked via native chemical ligation. New J. Chem. 2017, 41, 8656. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Alothman, Z.A.; Sillanpää, M. Simple and affordable graphene nano-platelets and carbon nanocomposite surface decorated with cetrimonium bromide as a highly responsive electrochemical sensor for rutin detection. J. Electroanal. Chem. 2022, 917, 116388. [Google Scholar] [CrossRef]
- Tajik, S.H. Beitollahi, Hydrothermal synthesis of CuFe2O4 nanoparticles for highly sensitive electrochemical detection of sunset yellow. Food Chem. Toxicol. 2022, 165, 113048. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.-I.; van Staden, J.F. Dot microsensors based on zinc porphyrins and zinc phthalocyanine for the determination of indigo carmine. ECS J. Solid State Sci. Technol. 2020, 9, 41015. [Google Scholar] [CrossRef]
- Amrutha, B.M.; Manjunatha, J.G.; Bhatt, A.S.; Nagarajappa, H. Sensitive and selective electrochemical detection of vanillin at graphene-based poly (methyl orange) modified electrode. J. Sci. Adv. Mater. Devices 2021, 6, 415–424. [Google Scholar]
- Hareesha, N.; Manjunatha, J.G.; Amrutha, B.M.; Pushpanjali, P.A.; Charithra, M.M.; Prinith, S.N. Electrochemical analysis of indigo carmine in food and water samples using a poly (glutamic acid) layered multi-walled carbon nanotube paste electrode. J. Electron. Mater. 2021, 50, 1230–1238. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Nagarajappa, H.; D’Souza, E.S.; Charithra, M.M.; Prinith, N.S. Voltammetric analysis of antihistamine drug cetirizine and paracetamol at poly(L-Leucine) layered carbon nanotube paste electrode. Surf. Interfaces 2021, 24, 101154. [Google Scholar] [CrossRef]
- Ma, X.; Chao, M. Electrocatalytic determination of maltol in food products by cyclic voltammetry with a poly(l-phenylalanine) modified electrode. Anal. Methods 2013, 5, 5823. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A novel poly (glycine) biosensor towards the detection of indigo carmine: A voltammetric study. J. Food Drug Anal. 2018, 26, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.A.; Pereira, G.F.; Fatibello-Filho, O.; Eguiluz, K.I.; Salazar-Banda, G.R. Electroanalytical sensing of indigo carmine dye in water samples using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2016, 769, 28–34. [Google Scholar] [CrossRef]
- Edwin, D.S.S.; Manjunatha, J.G.; Raril, C.; Girish, T.; Ravishankar, D.K.; Arpitha, H.J. Electrochemical analysis of indigo carmine using polyarginine modified carbon paste electrode. J. Electrochem. Sci. Eng. 2021, 11, 87–96. [Google Scholar] [CrossRef]
- Deroco, P.B.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Selective and simultaneous determination of indigo carmine and allura red in candy samples at the nano-concentration range by flow injection analysis with multiple pulse amperometric detection. Food Chem. 2018, 247, 66–72. [Google Scholar] [CrossRef] [PubMed]

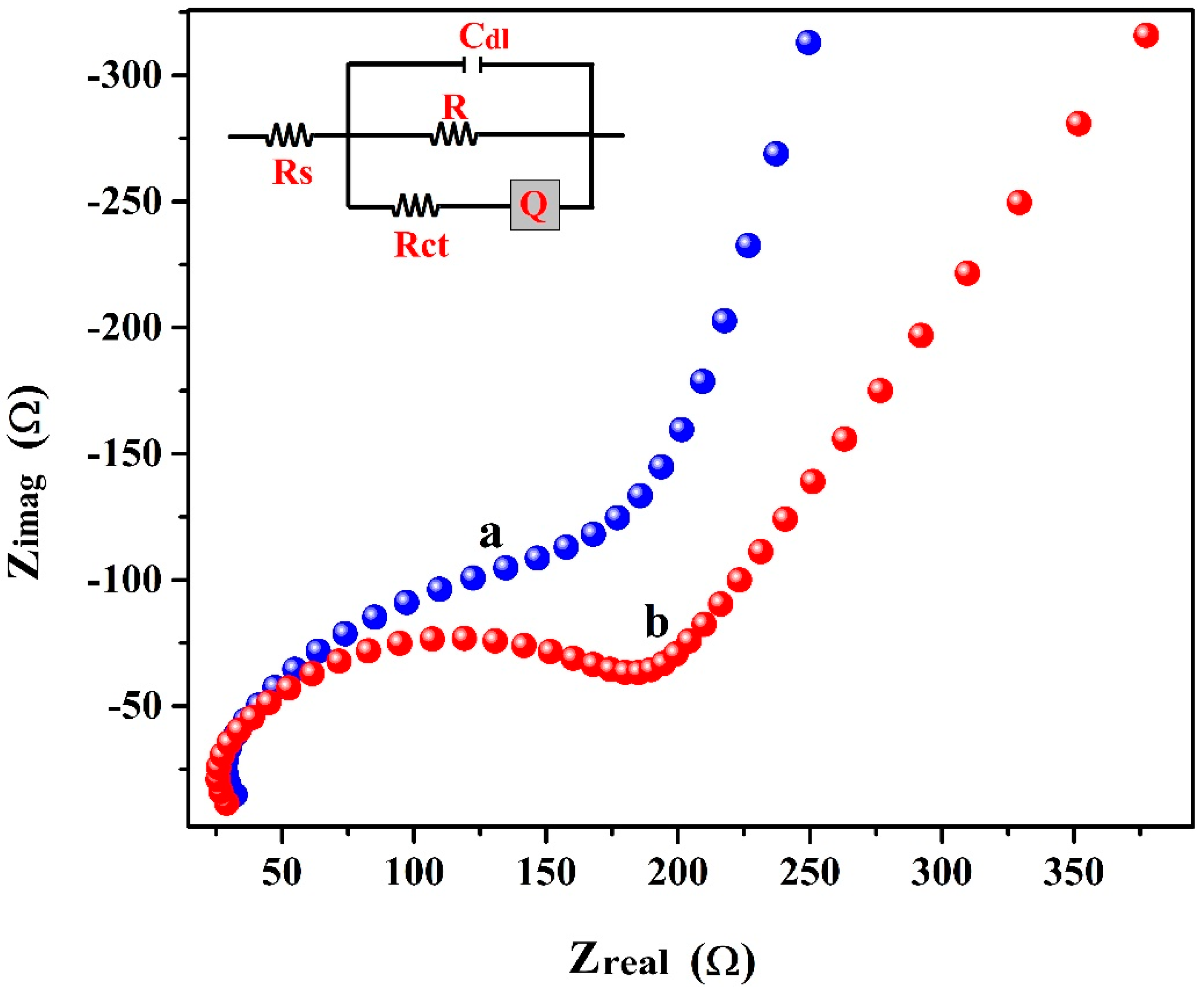


| Parameter | Electrode | |
|---|---|---|
| BCNTPE | P(PAN)LCNTPE | |
| Rs (Ω) | 26.9 | 24.28 |
| Rct (Ω) | 177.4 | 148.8 |
| Cdl (F) | 1.08 × 10−8 | 1.60 × 10−8 |
| Q (S.secn) | 6.456 × 10−7 | 1.27 × 10−8 |
| Technique | Electrode | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|
| Differential pulse voltammetry (DPV) | 4-(4-Nitrophenilazo)N-benzyl,N-ethylaniline-carbon paste electrode | 1.0–100.0 | 0.36 | 20 |
| DPV | P(GA)LMWCNTPE | 5.0–50.0 | 0.36 | 27 |
| CV | Poly (glycine) modified carbon paste electrode | 2.0–60.0 | 0.11 | [30] |
| SWV | Cathodically pre-treated boron-doped diamond (CPTBDE) | 0.5–84.1 | 0.058 | [31] |
| DPV | Poly(arginine)/carbon paste electrode | 0.2–1.0 | 0.036 | [32] |
| Flow injection analysis with multiple pulse amperometry | CPTBDE | 0.07–1.0 | 0.04 | [33] |
| CV | P(PAN)LCNTPE | 0.2–10.0 | 0.021 | Present work |
| Sample | Added (µm) | Found (µm) | Recovery (%) |
|---|---|---|---|
| Tap water | 1.0 (n = 3) | 1.004 ± 0.001 | 100.40 ± 0.001 |
| 2.0 (n = 3) | 1.967 ± 0.003 | 98.35 ± 0.003 | |
| 3.0 (n = 3) | 2.982 ± 0.001 | 99.40 ± 0.001 | |
| 4.0 (n = 3) | 3.995 ± 0.0002 | 99.87 ± 0.0002 | |
| 5.0 (n = 3) | 4.890 ± 0.0005 | 97.80 ± 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhimaraya, K.; Manjunatha, J.G.; Nagarajappa, H.; Tighezza, A.M.; Albaqami, M.D.; Sillanpää, M. Electroanalytical Detection of Indigo Carmine in Presence of Tartrazine Using a Poly(dl-phenylalanine) Modified Carbon Nanotube Paste Electrode. Chemosensors 2022, 10, 461. https://doi.org/10.3390/chemosensors10110461
Bhimaraya K, Manjunatha JG, Nagarajappa H, Tighezza AM, Albaqami MD, Sillanpää M. Electroanalytical Detection of Indigo Carmine in Presence of Tartrazine Using a Poly(dl-phenylalanine) Modified Carbon Nanotube Paste Electrode. Chemosensors. 2022; 10(11):461. https://doi.org/10.3390/chemosensors10110461
Chicago/Turabian StyleBhimaraya, Kanthappa, Jamballi G. Manjunatha, Hareesha Nagarajappa, Ammar M. Tighezza, Munirah D. Albaqami, and Mika Sillanpää. 2022. "Electroanalytical Detection of Indigo Carmine in Presence of Tartrazine Using a Poly(dl-phenylalanine) Modified Carbon Nanotube Paste Electrode" Chemosensors 10, no. 11: 461. https://doi.org/10.3390/chemosensors10110461
APA StyleBhimaraya, K., Manjunatha, J. G., Nagarajappa, H., Tighezza, A. M., Albaqami, M. D., & Sillanpää, M. (2022). Electroanalytical Detection of Indigo Carmine in Presence of Tartrazine Using a Poly(dl-phenylalanine) Modified Carbon Nanotube Paste Electrode. Chemosensors, 10(11), 461. https://doi.org/10.3390/chemosensors10110461













