Recent Progress on the Applications of Nanozyme in Surface-Enhanced Raman Scattering
Abstract
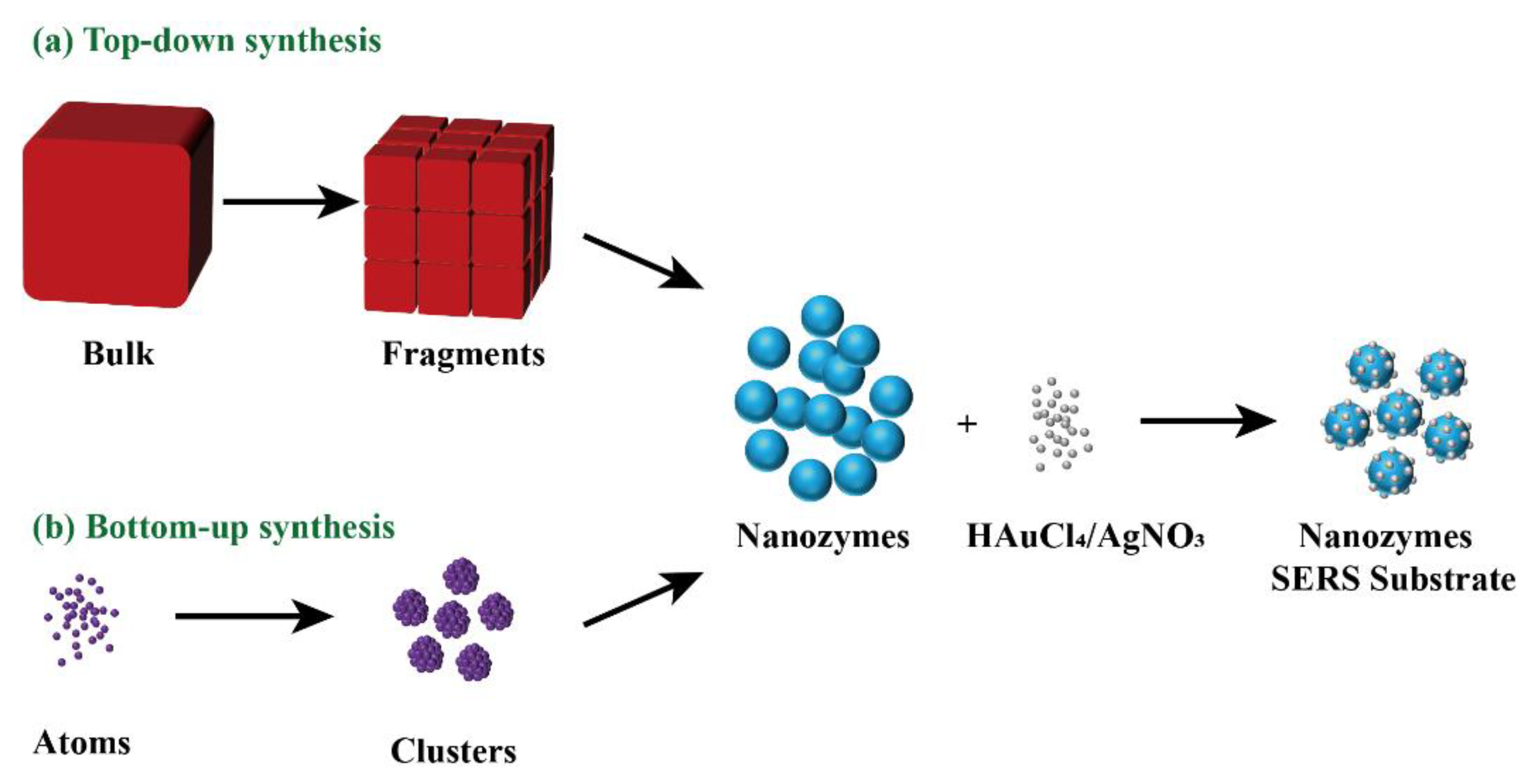
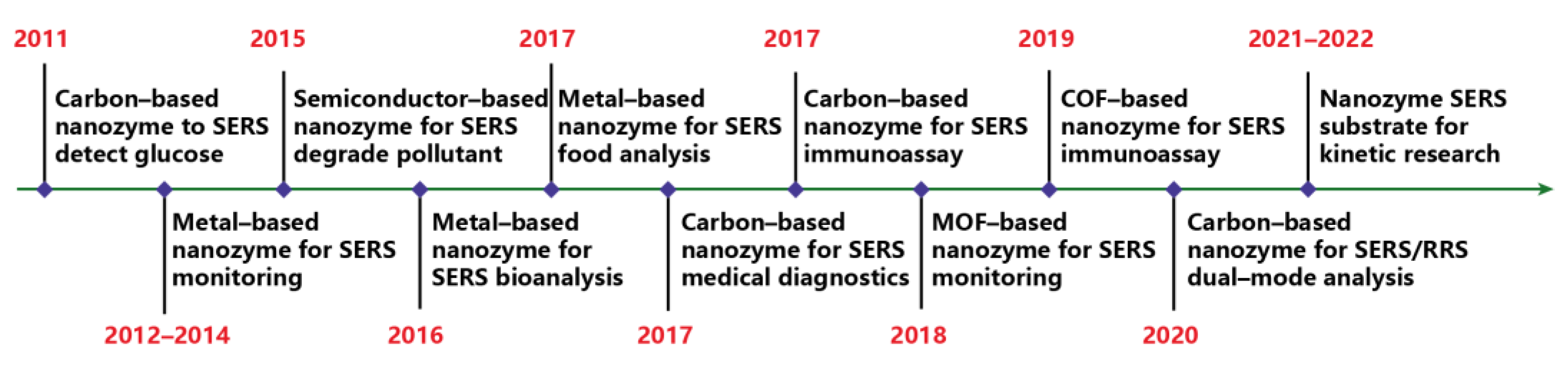
:1. Introduction
2. Classification of Nanozymes in SERS
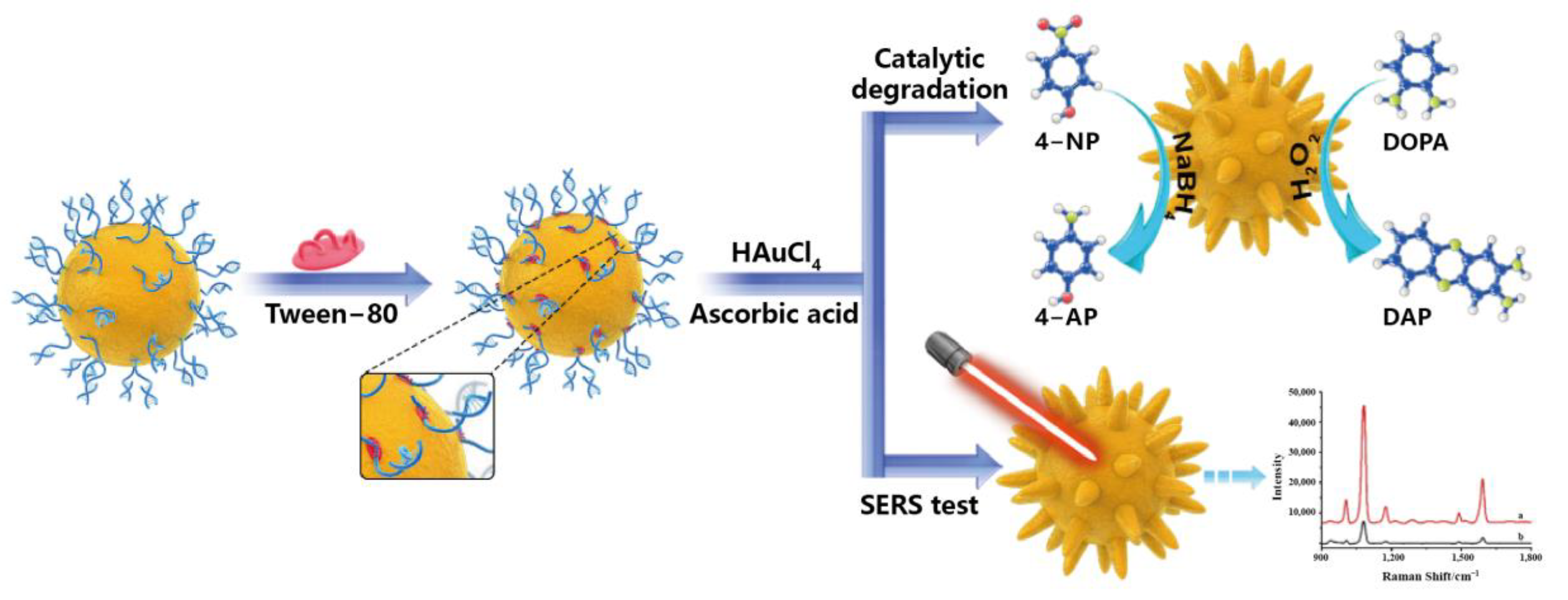
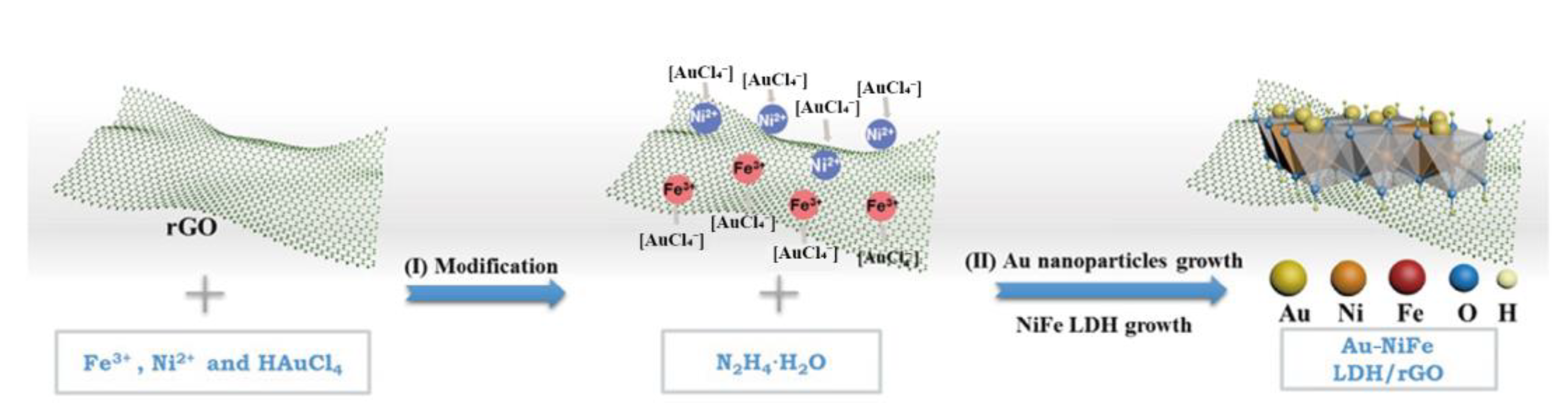
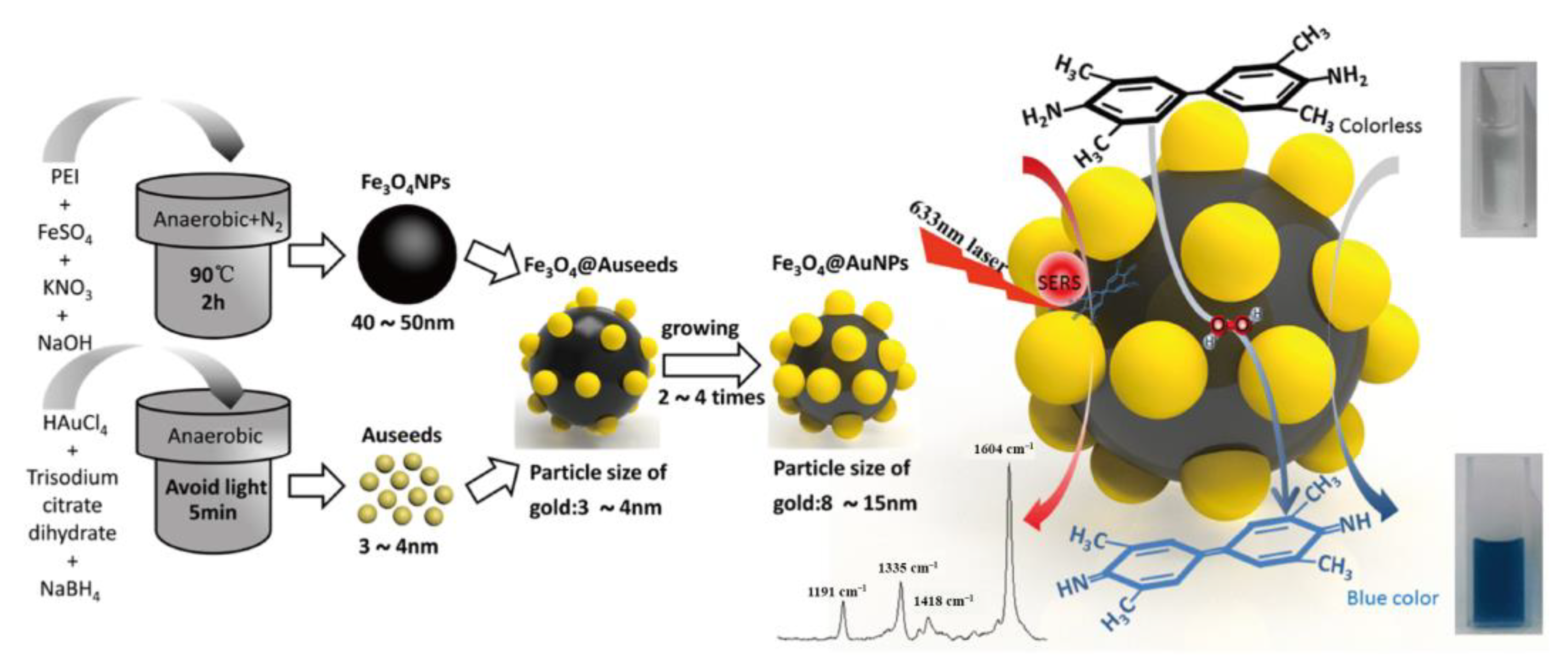
2.1. Metal-Based Nanozymes
2.2. Carbon-Based Nanozymes
2.3. COF/MOF-Based Nanozymes
2.4. Semiconductor-Based Nanozymes
3. Nanozyme SERS Application
3.1. Disease Diagnosis
3.2. Food Safety
3.3. Environmental Safety
3.4. Others
4. Conclusions and Prospects
- (1)
- Boost the catalytic performance of nanozymes in SERS. The majority of nanozymes in SERS offer both SERS properties as well as enzyme-like catalytic properties. Among them, the introduction of SERS active materials may obstruct the catalytic active sites on the surface of nanozymes, decreasing the catalytic effectiveness; on the other hand, the introduction of nanozyme components and even the occurrence of enzymatic processes may also alter the SERS signal. Even though numerous methodologies for the rational design of nanozymes in SERS have been devised, the catalytic efficiency of existing nanozymes in SERS is still typically lower than that of natural enzymes. Future research may focus on merging machine learning with high-throughput trials to hasten the discovery of highly catalytically active nanozymes in SERS.
- (2)
- Although nanozyme-based SERS analysis technology has been developed in a variety of analysis areas, the reported research mostly focuses on the detection of simple environmental and biological samples, with the detection of complex system samples being rare. For this reason, expanding the use of nanozymes in SERS requires significant improvements in the pretreatment of analytes during sample preparation. The application of nanozymes in SERS for complex systems can be promoted by designing devices with integrated sample pretreatment functions or by designing “all in one” nanozymes that integrate multiple functions such as separation and enrichment capabilities. In addition, realizing the detection of multiple targets in complicated systems is likewise a foreseeable tendency.
- (3)
- Expand the variety of nanozymes in SERS. Currently, the published nanozymes in SERS are dominated by peroxidase-like enzymes, which are inadequate to cover all major enzymatic reactions. Therefore, it is essential for researchers to widen the kinds of nanozymes in SERS as well as further clarify the definition and properties of related nanozymes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Du, D.; Ni, L.; Pan, J.; Niu, X. Emerging Applications of Nanozymes in Environmental Analysis: Opportunities and Trends. Trac-Trend. Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Chong, Y.; Liu, Q.; Ge, C. Advances in Oxidase-Mimicking Nanozymes: Classification, Activity Regulation and Biomedical Applications. Nano Today 2021, 37, 101076. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Wang, X. Functionalized Gold Nanomaterials as Biomimetic Nanozymes and Biosensing Actuators. Trac-Trend. Anal. Chem. 2021, 143, 116376. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes Based on Metal-Organic Frameworks: Construction and Prospects. Trac-Trend. Anal. Chem. 2020, 133, 116080. [Google Scholar] [CrossRef]
- Tang, R.; Xia, X.; Zhang, X.; Jiang, H.; Wang, B.; Zhang, P.; Zhang, Y.; Tang, Y.; Zhou, Y. Synergistic Function of Au NPs/GeO2 Nanozymes with Enhanced Peroxidase-like Activity and SERS Effect to Detect Choline Iodide. Spectrochim. Acta. A 2022, 266, 120467. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Y.; Su, A.; Wang, Y. Synthesis of Catalytically Active Carbon Quantum Dots and Its Application for Colorimetric Detection of Glutathione. Sens. Actuators B Chem. 2018, 273, 1098–1102. [Google Scholar] [CrossRef]
- Xie, W.; Walkenfort, B.; Schlücker, S. Label-Free SERS Monitoring of Chemical Reactions Catalyzed by Small Gold Nanoparticles Using 3D Plasmonic Superstructures. J. Am. Chem. Soc. 2013, 135, 1657–1660. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, D.; Mei, Y.; Sun, F. Ag–Cu@FA Hydrogel-Nanocomposites as Catalyst and SERS Substrate for the Oxidation of TMB. Compos. Commun. 2020, 22, 100485. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, H.; Wang, B.; Zhang, X. MoS2-Based Composite Nanozymes with Superior Peroxidase-like Activity for Ultrasensitive SERS Detection of Glucose. New J. Chem. 2021, 45, 19593–19604. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Huang, Y.; Yang, Y.; Xie, A.; Shen, Y. A Novel Porous Flower-like HA/Ag Nanocomposite: One Pot Preparation and Excellent Performances as Both SERS Nanosensor and Catalyst. Micropor. Mesopor. Mat. 2018, 258, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, C.; Ma, R.; Xu, Z.; Ozaki, Y. In Situ SERS Monitoring of Intracellular H2O2 in Single Living Cells Based on Label-Free Bifunctional Fe3O4@Ag Nanoparticles. Analyst 2022, 147, 1815–1823. [Google Scholar] [CrossRef]
- Wong, E.L.S.; Vuong, K.Q.; Chow, E. Nanozymes for Environmental Pollutant Monitoring and Remediation. Sensors 2021, 21, 408. [Google Scholar] [CrossRef]
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes—Hitting the Biosensing “Target”. Sensors 2021, 21, 5201. [Google Scholar] [CrossRef]
- Yan, M.; Chen, G.; She, Y.; Ma, J.; Hong, S.; Shao, Y.; Abd El-Aty, A.M.; Wang, M.; Wang, S.; Wang, J. Sensitive and Simple Competitive Biomimetic Nanozyme-Linked Immunosorbent Assay for Colorimetric and Surface-Enhanced Raman Scattering Sensing of Triazophos. J. Agric. Food Chem. 2019, 67, 9658–9666. [Google Scholar] [CrossRef]
- Tang, R.; Lei, Z.; Weng, Y.; Xia, X.; Zhang, X. Self-Assembly Synthesis of Ag@PANI Nanocomposites as a Tandem Enzyme Utilizing a Highly Efficient Label-Free SERS Method to Detect Saccharides. New J. Chem. 2020, 44, 16384–16389. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Yuan, R.; Chai, Y. A Novel Ratiometric SERS Biosensor with One Raman Probe for Ultrasensitive MicroRNA Detection Based on DNA Hydrogel Amplification. J. Mater. Chem. B 2019, 7, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pu, H.; Huang, L.; Sun, D. Plasmonic Nanoparticles on Metal-Organic Framework: A Versatile SERS Platform for Adsorptive Detection of New Coccine and Orange II Dyes in Food. Food Chem. 2020, 328, 127105. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.; Zhuang, X.; Sun, Y.; Wang, J.; Ren, H.; Zhang, S.; Zhang, Y.; Qiu, H.; Hu, Y. Construction of an Enzyme-Free Biosensor Utilizing CuO Nanoparticles Enriched in DNA Polymer to Catalyze a Click Chemistry Reaction for SERS Detection of the P53 Gene. Anal. Chim. Acta 2022, 1222, 339958. [Google Scholar] [CrossRef]
- Bai, X.; Zeng, Y.; Zhou, X.; Wang, X.; Shen, A.; Hu, J. Environmentally Safe Mercury(II) Ions Aided Zero-Background and Ultrasensitive SERS Detection of Dipicolinic Acid. Anal. Chem. 2017, 89, 10335–10342. [Google Scholar] [CrossRef]
- Sakthikumar, K.; Anantharaj, S.; Ede, S.R.; Karthick, K.; Kundu, S. A Highly Stable Rhenium Organosol on a DNA Scaffold for Catalytic and SERS Applications. J. Mater. Chem. C 2016, 4, 6309–6320. [Google Scholar] [CrossRef]
- Song, W.; Hildebrandt, P.; Weidinger, I.M. Plasmonic Cu/CuCl/Cu2S/Ag and Cu/CuCl/Cu2S/Au Supports with Peroxidase-Like Activity: Insights from Surface Enhanced Raman Spectroscopy. Z. Phys. Chem. 2018, 232, 1541–1550. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional Nanozymes: Enzyme-like Catalytic Activity Combined with Magnetism and Surface Plasmon Resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Liu, F.; Wang, Y.; Li, N.; Ding, B. Enzyme Mimic Nanomaterials and Their Biomedical Applications. ChemBioChem 2020, 21, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, B.; Lien, C.; Chu, H.; Huang, C. A Review on Metal Nanozyme-Based Sensing of Heavy Metal Ions: Challenges and Future Perspectives. J. Hazard. Mater. 2021, 401, 123397. [Google Scholar] [CrossRef] [PubMed]
- Nehra, K.; Pandian, S.K.; Byram, C.; Moram, S.S.B.; Soma, V.R. Quantitative Analysis of Catalysis and SERS Performance in Hollow and Star-Shaped Au Nanostructures. J. Phys. Chem. C 2019, 123, 16210–16222. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Shan, G.; Chen, Y.; Tong, T.; Liu, Y. Laser-Induced Formation of Au/Pt Nanorods with Peroxidase Mimicking and SERS Enhancement Properties for Application to the Colorimetric Determination of H2O2. Microchim. Acta 2018, 185, 445. [Google Scholar] [CrossRef]
- Wang, A.; Guan, C.; Shan, G.; Chen, Y.; Wang, C.; Liu, Y. A Nanocomposite Prepared from Silver Nanoparticles and Carbon Dots with Peroxidase Mimicking Activity for Colorimetric and SERS-Based Determination of Uric Acid. Microchim. Acta 2019, 186, 644. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Luo, Y.; Jiang, Z. Silver Nanosol SERS Quantitative Analysis of Ultratrace Biotin Coupled N-Doped Carbon Dots Catalytic Amplification with Affinity Reaction. Food Chem. 2020, 317, 126433–126440. [Google Scholar] [CrossRef]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücker, S. Synthesis of Bifunctional Au/Pt/Au Core/Shell Nanoraspberries for in Situ SERS Monitoring of Platinum-Catalyzed Reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yashchenok, A.; Li, L.; Möhwald, H.; Bargheer, M. Mechanistic Study on Reduction Reaction of Nitro Compounds Catalyzed by Gold Nanoparticles Using in Situ SERS Monitoring. Colloid. Surf. A 2015, 470, 108–113. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, C.; Liu, Q.; Wen, G.; Liang, A.; Jiang, Z. Resonance Rayleigh Scattering and SERS Spectral Detection of Trace Hg(II) Based on the Gold Nanocatalysis. Nanomaterials 2017, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Sloan-Dennison, S.; Laing, S.; Shand, N.C.; Graham, D.; Faulds, K. A Novel Nanozyme Assay Utilising the Catalytic Activity of Silver Nanoparticles and SERRS. Analyst 2017, 142, 2484–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Peng, Y.; Wang, H.; Liang, A.; Jiang, Z. A Nanosol SERS Method for Quantitative Analysis of Trace Potassium Based on Aptamer Recognition and Silver Nanorod Catalysis of Ag(I)-Glucose Reaction. Sens. Actuators B Chem. 2019, 281, 53–59. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Gao, W.; Han, Z.; Liu, T.; Li, C.; Wang, Z.; He, E.; Zheng, H. Ag-Au Alloy Nanoparticles: Synthesis and in Situ Monitoring SERS of Plasmonic Catalysis. Sens. Actuators B Chem. 2016, 231, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Zhan, F.; Yin, J.; Zhang, A.; Zhou, J.; Wang, M.; Jiao, T. Controllable Morphology and Highly Efficient Catalytic Performances of Pd–Cu Bimetallic Nanomaterials Prepared via Seed-Mediated Co-Reduction Synthesis. Appl. Surf. Sci. 2020, 527, 146719. [Google Scholar] [CrossRef]
- Kundu, S.; Dai, W.; Chen, Y.; Ma, L.; Yue, Y.; Sinyukov, A.M.; Liang, H. Shape-Selective Catalysis and Surface Enhanced Raman Scattering Studies Using Ag Nanocubes, Nanospheres and Aggregated Anisotropic Nanostructures. J. Colloid. Interf. Sci. 2017, 498, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, L.; Zhang, Z.; Li, G. Synthesis of Sea Urchin-Shaped Au Nanocrystals by Double-Strand Diblock Oligonucleotides for Surface-Enhanced Raman Scattering and Catalytic Application. Nanotechnology 2021, 32, 175501. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Wang, L.; Shen, A.; Hu, J. Simultaneous Enzymatic and SERS Properties of Bifunctional Chitosan-Modified Popcorn-like Au-Ag Nanoparticles for High Sensitive Detection of Melamine in Milk Powder. Talanta 2015, 140, 204–211. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Wang, J.; Lao, S.; Abd El-Aty, A.M. Determination of Dichlorvos in Pears by Surface-Enhanced Raman Scattering (SERS) with Catalysis by Platinum Coated Gold Nanoparticles. Anal. Lett. 2022, 55, 427–437. [Google Scholar] [CrossRef]
- Wu, J.; Qin, K.; Yuan, D.; Tan, J.; Qin, L.; Zhang, X.; Wei, H. Rational Design of Au@Pt Multibranched Nanostructures as Bifunctional Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 12954–12959. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, L.; Zhang, G.; Zhou, X.; Shen, A.; Hu, J. Core–Shell Fructus Broussonetia-like Au@Ag@Pt Nanoparticles as Highly Efficient Peroxidase Mimetics for Supersensitive Resonance-Enhanced Raman Sensing. Anal. Methods 2016, 8, 2097–2105. [Google Scholar] [CrossRef]
- Li, L.; Jin, J.; Liu, J.; Yang, J.; Song, W.; Yang, B.; Zhao, B. Accurate SERS Monitoring of the Plasmon Mediated UV/Visible/NIR Photocatalytic and Photothermal Catalytic Process Involving Ag@carbon Dots. Nanoscale 2021, 13, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Zhao, Y.; Zhang, H.; Liu, G.; Cai, W.; Li, Y.; Qu, L. Copper Nanoparticle@graphene Composite Arrays and Their Enhanced Catalytic Performance. Acta Mater. 2016, 105, 59–67. [Google Scholar] [CrossRef]
- Ouyang, H.; Liang, A.; Jiang, Z. Fullerol Nanocatalysis and Trimodal Surface Plasmon Resonance for the Determination of Isocarbophos. Front. Chem. 2020, 8, 673. [Google Scholar] [CrossRef]
- Murugan, E.; Santhoshkumar, S.; Govindaraju, S.; Palanichamy, M. Silver Nanoparticles Decorated g-C3N4: An Efficient SERS Substrate for Monitoring Catalytic Reduction and Selective Hg2+ ions Detection. Spectrochim. Acta. A 2021, 246, 119036. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, L.; Zhang, L.; Ma, J.; Peng, X.; Sun, R. Recycled Fiber Derived Carbon Dispersed Ag Nanoparticles as High-Performance Catalyst for 4-Nitrophenol Reduction and Substrate for Surface-Enhanced Raman Scattering. Cellulose 2020, 27, 1649–1659. [Google Scholar] [CrossRef]
- Han, B.; Gao, Y.; Zhu, L.; Ma, Z.; Zhang, X.; Ding, H.; Zhang, Y. In Situ Integration of SERS Sensors for On-Chip Catalytic Reactions. Adv. Mater. Technol.-US 2020, 5, 1900963. [Google Scholar] [CrossRef]
- Liang, A.; Li, C.; Wang, X.; Luo, Y.; Wen, G.; Jiang, Z. Immunocontrolling Graphene Oxide Catalytic Nanogold Reaction and Its Application to SERS Quantitative Analysis. ACS Omega 2017, 2, 7349–7358. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Luo, Y.; Liang, A.; Wen, G.; Jiang, Z. A Sensitive Gold Nanoplasmonic SERS Quantitative Analysis Method for Sulfate in Serum Using Fullerene as Catalyst. Nanomaterials 2018, 8, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Ma, L.; Ling, S.; Ouyang, H.; Liang, A.; Jiang, Z. Aptamer-Adjusted Carbon Dot Catalysis-Silver Nanosol SERS Spectrometry for Bisphenol A Detection. Nanomaterials 2022, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Luo, Y.; Jiang, Z. Preparation of Highly Catalytic N-Doped Carbon Dots and Their Application in SERS Sulfate Sensing. Materials 2018, 11, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, C.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. A Novel SERS Sensor for the Ultrasensitive Detection of Kanamycin Based on a Zn-Doped Carbon Quantum Dot Catalytic Switch Controlled by Nucleic Acid Aptamer and Size-Controlled Gold Nanorods. Food Chem. 2021, 362, 130261. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Wen, G.; Jiang, Z. A Dual-Model SERS and RRS Analytical Platform for Pb(II) Based on Ag-Doped Carbon Dot Catalytic Amplification and Aptamer Regulation. Sci. Rep. 2019, 9, 9991. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Li, C.; Wen, G.; Liang, A.; Jiang, Z. A Highly Sensitive and Accurate SERS/RRS Dual-Spectroscopic Immunosensor for Clenbuterol Based on Nitrogen/Silver-Codoped Carbon Dots Catalytic Amplification. Talanta 2020, 209, 120529. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Liang, A.; Wen, G.; Jiang, Z. Aptamer Turn-On SERS/RRS/Fluorescence Tri-Mode Platform for Ultra-Trace Urea Determination Using Fe/N-Doped Carbon Dots. Front. Chem. 2021, 9, 613083. [Google Scholar] [CrossRef]
- Guo, Y.; Tao, Y.; Ma, X.; Jin, J.; Wen, S.; Ji, W.; Song, W.; Zhao, B.; Ozaki, Y. A Dual Colorimetric and SERS Detection of Hg2+ Based on the Stimulus of Intrinsic Oxidase-like Catalytic Activity of Ag-CoFe2O4/Reduced Graphene Oxide Nanocomposites. Chem. Eng. J. 2018, 350, 120–130. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ma, X.; Jin, J.; Ji, W.; Wang, X.; Song, W.; Zhao, B.; He, C. Fabrication of Ag–Cu2O/Reduced Graphene Oxide Nanocomposites as Surface-Enhanced Raman Scattering Substrates for in Situ Monitoring of Peroxidase-Like Catalytic Reaction and Biosensing. ACS Appl. Mater. Interfaces 2017, 9, 19074–19081. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Y.; Wang, Y.; Zhang, H.; Ma, X.; Wen, S.; Jin, J.; Song, W.; Zhao, B.; Ozaki, Y. A Nanozyme-Based Enhanced System for Total Removal of Organic Mercury and SERS Sensing. J. Hazard. Mater. 2021, 405, 124642. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, S.; Song, Y.; Zhao, H.; Zhang, Z.; Guo, Y.; Li, J.; Song, W.; Yang, B.; Zhao, B. Precisely Controllable Core–Shell Ag@Carbon Dots Nanoparticles: Application to in Situ Super-Sensitive Monitoring of Catalytic Reactions. ACS Appl. Mater. Interfaces 2016, 8, 27956–27965. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Han, W.; Fu, Z.; Wang, L. The Chain-like Au/Carbon Dots Nanocomposites with Peroxidase-like Activity and Their Application for Glucose Detection. Colloid. Surface B 2021, 199, 111553. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Song, W.; Wang, J.; Li, L.; Tian, Y.; Zhu, S.; Zhang, Y.; Xu, S.; Yang, B.; Zhao, B. A Highly Sensitive SERS Platform Based on Small-Sized Ag/GQDs Nanozyme for Intracellular Analysis. Chem. Eng. J. 2022, 430, 132687. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, C.; Li, L.; Yang, T.; Wu, Y.; Ma, C.; Gu, J.; Gao, H.; Yang, Z.; Wang, Z.; et al. TMB-AgNPs@COF Based SERS Probe for the Rapid Detection of Glucose in Drinks. Vib. Spectrosc. 2022, 122, 103411. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, C.; Han, L.; Liao, X.; Wang, C. SERS and Dark-Field Scattering Dual-Mode Detection of Intracellular Hydrogen Peroxide Using Biocompatible Au@COF Nanosensor. Sens. Actuators B Chem. 2022, 373, 132770. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Yu, B.; Liang, A.; Jiang, Z. A Highly Sensitive Gold Nanosol SERS Aptamer Assay for Glyphosate with a New COF Nanocatalytic Reaction of Glycol-Au(III). Sens. Actuators B Chem. 2021, 344, 130288. [Google Scholar] [CrossRef]
- Su, Y.; Wu, D.; Chen, J.; Chen, G.; Hu, N.; Wang, H.; Wang, P.; Han, H.; Li, G.; Wu, Y. Ratiometric Surface Enhanced Raman Scattering Immunosorbent Assay of Allergenic Proteins via Covalent Organic Framework Composite Material Based Nanozyme Tag Triggered Raman Signal “Turn-on” and Amplification. Anal. Chem. 2019, 91, 11687–11695. [Google Scholar] [CrossRef]
- Wen, G.; Pan, S.; Gan, M.; Liang, A.; Jiang, Z. Aptamer-Regulated Gold Nanosol Plasmonic SERS/RRS Dimode Assay of Trace Organic Pollutants Based on TpPa-Loaded PdNC Catalytic Amplification. ACS Appl. Bio Mater. 2021, 4, 4582–4590. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Li, G.; Zhang, R. A Composite Prepared from Gold Nanoparticles and a Metal Organic Framework (Type MOF-74) for Determination of 4-Nitrothiophenol by Surface-Enhanced Raman Spectroscopy. Microchim. Acta 2019, 186, 477. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Liang, A.; Jiang, Z. Highly Catalysis Amplification of MOFNd-Loaded Nanogold Combined with Specific Aptamer SERS/RRS Assay of Trace Glyphosate. Analyst 2022, 147, 2369–2377. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Liang, A.; Jiang, Z. Highly Catalysis MOFCe Supported Ag Nanoclusters Coupled with Specific Aptamer for SERS Quantitative Assay of Trace Dopamine. Talanta 2022, 245, 123468. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-Free Tandem Reaction Strategy for Surface-Enhanced Raman Scattering Detection of Glucose by Using the Composite of Au Nanoparticles and Porphyrin-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wen, S.; Xue, X.; Guo, Y.; Jin, J.; Song, W.; Zhao, B. Controllable Synthesis of SERS-Active Magnetic Metal–Organic Framework-Based Nanocatalysts and Their Application in Photoinduced Enhanced Catalytic Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 25726–25736. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.; Gao, Y.; Cai, Y.; Guo, X.; Wen, Y.; Yang, H. Fabrication and Characterization of the Stable Ag-Au-Metal-Organic-Frameworks: An Application for Sensitive Detection of Thiabendazole. Sens. Actuators B Chem. 2019, 293, 289–295. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Wen, S.; Xie, Q.; Li, L.; Jin, J.; Wang, X.; Zhao, B.; Song, W. Ultra-Sensitive SERS Detection, Rapid Selective Adsorption and Degradation of Cationic Dyes on Multifunctional Magnetic Metal-Organic Framework-Based Composite. Nanotechnology 2020, 31, 315501. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, H.; Gao, L.; Fu, M.; Luo, X.; Zhang, X.; Liu, Q.; Zeng, R. Fe-Doped Ag2S with Excellent Peroxidase-like Activity for Colorimetric Determination of H2O2. J. Alloys Compd. 2019, 785, 1189–1197. [Google Scholar] [CrossRef]
- Wen, S.; Ma, X.; Liu, H.; Chen, G.; Wang, H.; Deng, G.; Zhang, Y.; Song, W.; Zhao, B.; Ozaki, Y. Accurate Monitoring Platform for the Surface Catalysis of Nanozyme Validated by Surface-Enhanced Raman-Kinetics Model. Anal. Chem. 2020, 92, 11763–11770. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, Z.; Zhang, Y.; Liu, H.; Ma, X.; Li, L.; Song, W.; Zhao, B. Ultrasensitive Stimulation Effect of Fluoride Ions on a Novel Nanozyme–SERS System. ACS Sustain. Chem. Eng. 2020, 8, 11906–11913. [Google Scholar] [CrossRef]
- Ma, S.; Cai, Q.; Lu, K.; Liao, F.; Shao, M. Bi-Functional Au/FeS (Au/Co3O4) Composite for in Situ SERS Monitoring and Degradation of Organic Pollutants. J. Nanopart. Res. 2016, 18, 26. [Google Scholar] [CrossRef]
- Cai, Q.; Lu, S.; Liao, F.; Li, Y.; Ma, S.; Shao, M. Catalytic Degradation of Dye Molecules and in Situ SERS Monitoring by Peroxidase-like Au/CuS Composite. Nanoscale 2014, 6, 8117–8123. [Google Scholar] [CrossRef] [PubMed]
- McKeating, K.S.; Sloan-Dennison, S.; Graham, D.; Faulds, K. An Investigation into the Simultaneous Enzymatic and SERRS Properties of Silver Nanoparticles. Analyst 2013, 138, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaidi, I.; Gou, H.; Al-kazaz, A.K.A.; Aguilar, Z.P.; Melconian, A.K.; Zheng, P.; Wu, N. A Gold@silica Core–Shell Nanoparticle-Based Surface-Enhanced Raman Scattering Biosensor for Label-Free Glucose Detection. Anal. Chim. Acta 2014, 811, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.; Hahm, E.; Kim, T.; Kim, H.; Lee, S.; Lee, S.; Kang, H.; Lee, H.; Jeong, D.; Choi, H.; et al. Enzyme-Amplified SERS Immunoassay with Ag-Au Bimetallic SERS Hot Spots. Nano Res. 2020, 13, 3338–3346. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Hu, S.; Zhu, A.; Ying, Y.; Guo, X.; Wu, Y.; Wen, Y.; Yang, H. MnO2 Coated Au Nanoparticles Advance SERS Detection of Cellular Glutathione. Biosens. Bioelectron. 2022, 215, 114388. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, Y.; Liu, X.; Deng, T.; Dai, S.; Qu, J.; Yang, G.; Qu, L. Reusable Ring-like Fe3O4/Au Nanozymes with Enhanced Peroxidase-like Activities for Colorimetric-SERS Dual-Mode Sensing of Biomolecules in Human Blood. Biosens. Bioelectron. 2022, 209, 114253. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Q.; Miao, X.; Wen, S.; Yu, S.; Chu, Y.; Lu, X.; Jiang, L.; Zhu, J. Spatially Engineered Janus Hybrid Nanozyme toward SERS Liquid Biopsy at Nano/Microscales. ACS Appl. Mater. Interfaces 2019, 11, 41979–41987. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, L.; Qin, X.; Tian, J.; Lu, W.; Chang, G.; Sun, X. One-Pot Green Synthesis of Ag Nanoparticles-Graphene Nanocomposites and Their Applications in SERS, H2O2, and Glucose Sensing. RSC Adv. 2011, 2, 538–545. [Google Scholar] [CrossRef]
- Wang, X.; Xia, Z.; Fodjo, E.K.; Deng, W.; Li, D. A Dual-Responsive Nanozyme Sensor with Ultra-High Sensitivity and Ultra-Low Cross-Interference towards Metabolic Biomarker Monitoring. J. Mater. Chem. B 2022, 10, 3023–3031. [Google Scholar] [CrossRef]
- Pham, X.; Seong, B.; Bock, S.; Hahm, E.; Huynh, K.; Kim, Y.; Kim, W.; Kim, J.; Kim, D.; Jun, B. Nonenzymatic Hydrogen Peroxide Detection Using Surface-Enhanced Raman Scattering of Gold–Silver Core–Shell-Assembled Silica Nanostructures. Nanomaterials 2021, 11, 2748. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Liu, J.; Su, Z. Bio-Inspired Nanoenzyme Synthesis and Its Application in A Portable Immunoassay for Food Allergy Proteins. J. Agric. Food Chem. 2021, 69, 14751–14760. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Xu, E.; Jin, Z. Establishment of a Dual Mode Immunochromatographic Assay for Campylobacter Jejuni Detection. Food Chem. 2019, 289, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Hassan, M.M.; Ali, S.; Chen, Q. SERS Based Artificial Peroxidase Enzyme Regulated Multiple Signal Amplified System for Quantitative Detection of Foodborne Pathogens. Food Control 2021, 123, 107733. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Yu, X.; Jia, X.; Zhu, C.; Liu, J.; Zhang, F.; Chen, Z.; Yan, M.; Yang, Q. A Sensitive and Simple Competitive Nanozyme-Linked Apta-Sorbent Assay for the Dual-Mode Detection of Ochratoxin A. Analyst 2022, 147, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, P.; Xing, X.; Cheng, W.; Liu, S.; Lu, X.; Zhong, L. Colorimetry /SERS Dual-Sensor of H2O2 Constructed via TMB–Fe3O4@ AuNPs. Talanta 2022, 240, 123118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Hu, Y. Fabrication of Bimetallic Nanoparticles Modified Hollow Nanoporous Carbons Derived from Covalent Organic Framework for Efficient Degradation of 2,4-Dichlorophenol. Chin. Chem. Lett. 2021, 32, 2529–2533. [Google Scholar] [CrossRef]
- Logan, N.; Lou-Franco, J.; Elliott, C.; Cao, C. Catalytic Gold Nanostars for SERS-Based Detection of Mercury Ions (Hg2+) with Inverse Sensitivity. Environ. Sci. Nano 2021, 8, 2718–2730. [Google Scholar] [CrossRef]
- Song, C.; Li, J.; Sun, Y.; Jiang, X.; Zhang, J.; Dong, C.; Wang, L. Colorimetric/SERS Dual-Mode Detection of Mercury Ion via SERS-Active Peroxidase-like Au@AgPt NPs. Sens. Actuators B Chem. 2020, 310, 127849. [Google Scholar] [CrossRef]
- Ma, J.; Feng, G.; Ying, Y.; Shao, Y.; She, Y.; Zheng, L.; EI-Aty, A.M.A.; Wang, J. Sensitive SERS Assay for Glyphosate Based on the Prevention of L-Cysteine Inhibition of a Au–Pt Nanozyme. Analyst 2021, 146, 956–963. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, Z.; Zong, S.; Yang, K.; Zhu, K.; Cui, Y. Peroxidase-like Recyclable SERS Probe for the Detection and Elimination of Cationic Dyes in Pond Water. J. Hazard. Mater. 2020, 408, 124426. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.; Hu, Y. In Situ Loading of Well-Dispersed Silver Nanoparticles on Nanocrystalline Magnesium Oxide for Real-Time Monitoring of Catalytic Reactions by Surface Enhanced Raman Spectroscopy. Nanoscale 2015, 7, 16952–16959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, Y.; Zhu, S.; Song, Y.; Jin, J.; Ji, W.; Song, W.; Zhao, B.; Yang, B.; Ozaki, Y. Facile Synthesis of Silver Nanoparticles/Carbon Dots for a Charge Transfer Study and Peroxidase-like Catalytic Monitoring by Surface-Enhanced Raman Scattering. Appl. Surf. Sci. 2017, 410, 42–50. [Google Scholar] [CrossRef]
- Song, W.; Chi, M.; Gao, M.; Zhao, B.; Wang, C.; Lu, X. Self-Assembly Directed Synthesis of Au Nanorices Induced by Polyaniline and Their Enhanced Peroxidase-like Catalytic Properties. J. Mater. Chem. C 2017, 5, 7465–7471. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xia, L.; Li, G. Recent Progress on the Applications of Nanozyme in Surface-Enhanced Raman Scattering. Chemosensors 2022, 10, 462. https://doi.org/10.3390/chemosensors10110462
Li D, Xia L, Li G. Recent Progress on the Applications of Nanozyme in Surface-Enhanced Raman Scattering. Chemosensors. 2022; 10(11):462. https://doi.org/10.3390/chemosensors10110462
Chicago/Turabian StyleLi, Dan, Ling Xia, and Gongke Li. 2022. "Recent Progress on the Applications of Nanozyme in Surface-Enhanced Raman Scattering" Chemosensors 10, no. 11: 462. https://doi.org/10.3390/chemosensors10110462






