Rational Design of Ratiometric Fluorescent Probe for Zn2+ Imaging under Oxidative Stress in Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Intermediates 1–4 and Probes BDP-2BPEA, BDP-p-BPEA, and BDP-p-TMPA
2.2.1. Synthesis of 1
2.2.2. Synthesis of 2
2.2.3. Synthesis of 3
2.2.4. Synthesis of 4
2.2.5. Synthesis of BDP-2BPEA
2.2.6. Synthesis of BDP-p-BPEA
2.2.7. Synthesis of BDP-p-TMPA
2.3. Optical Experimental Method
2.4. Cell Culture
3. Results
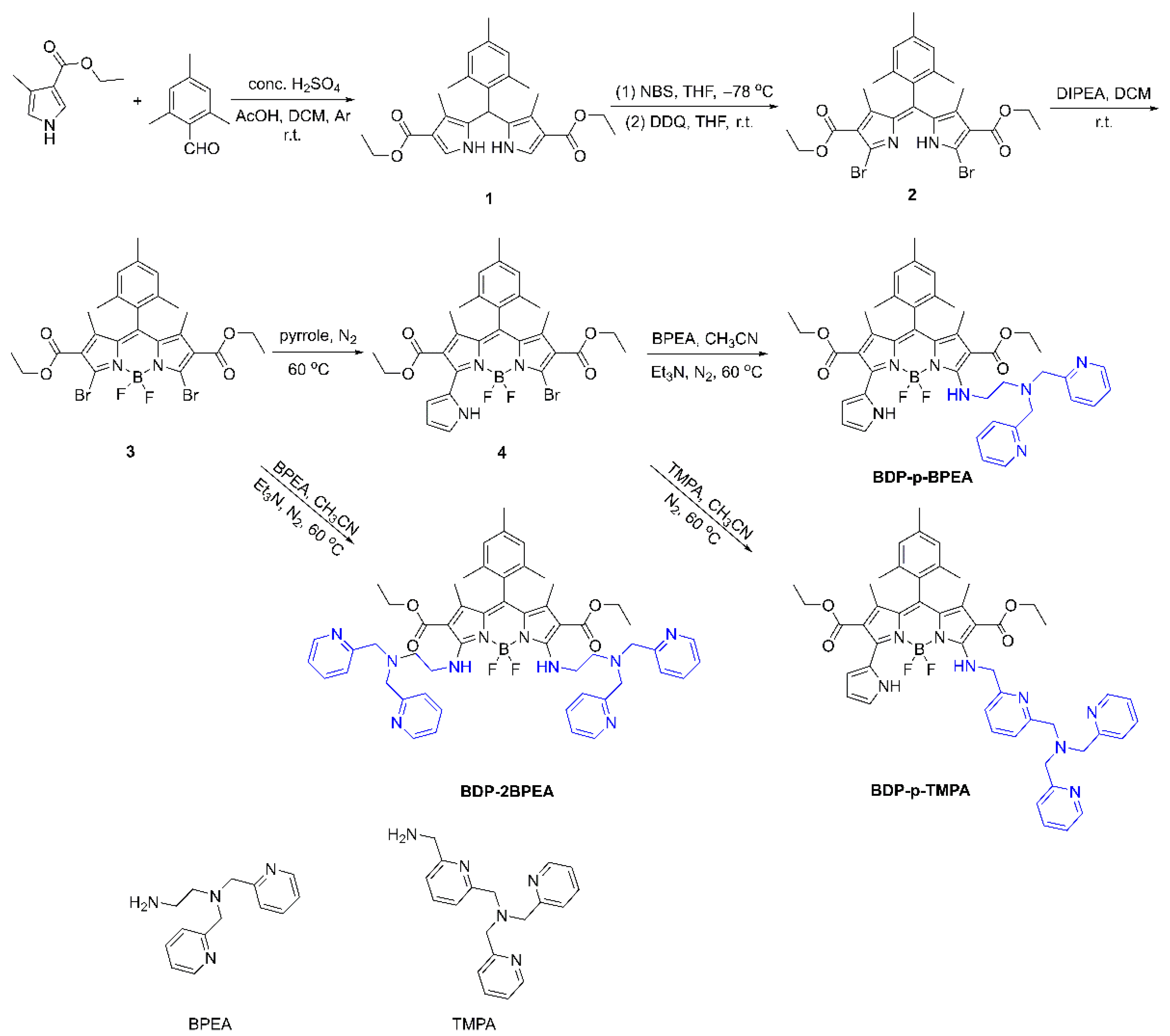
3.1. Design and Synthesis of the Three Probes
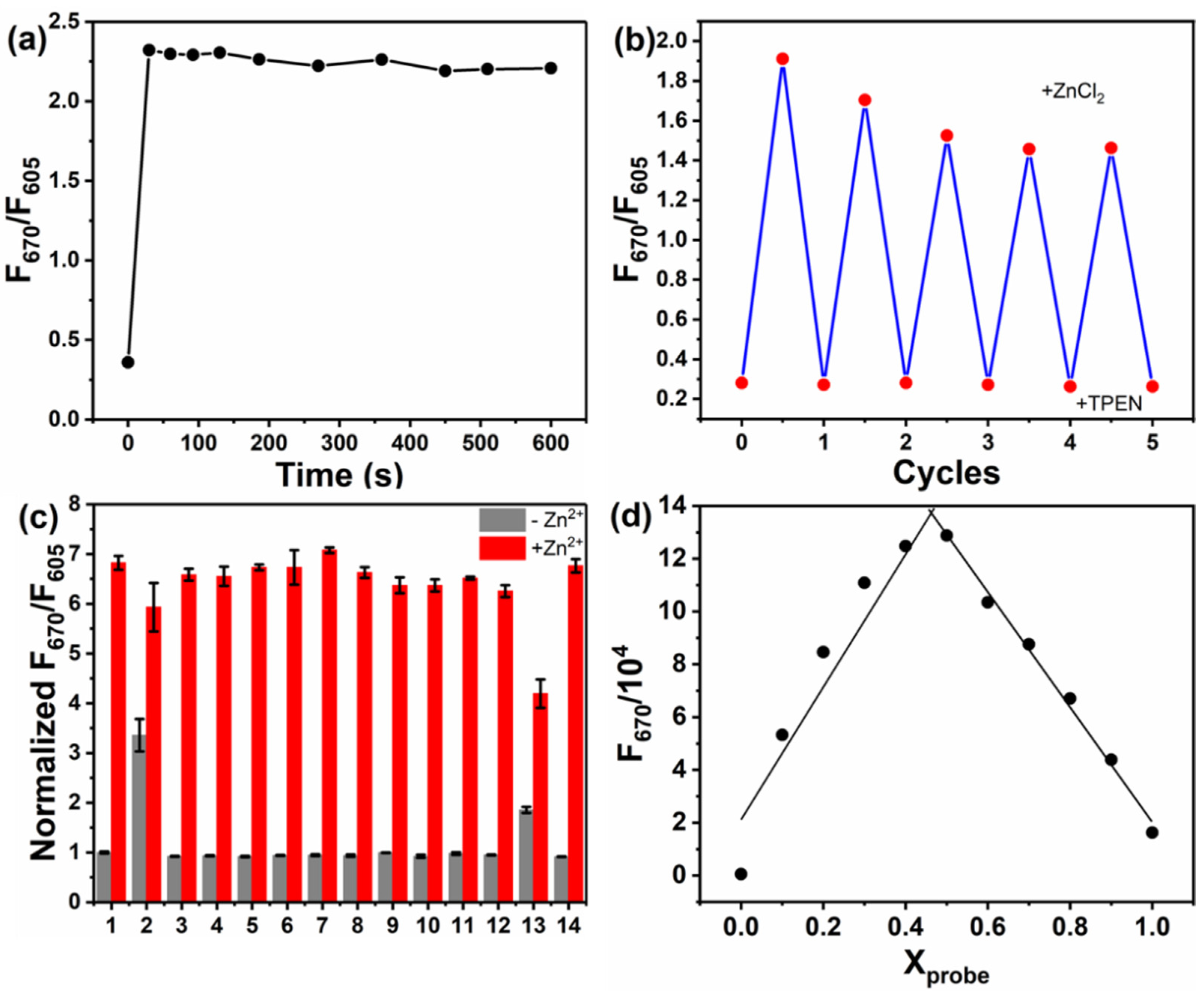
3.2. Spectral Response Towards Zn2+ and Screening of the Probes
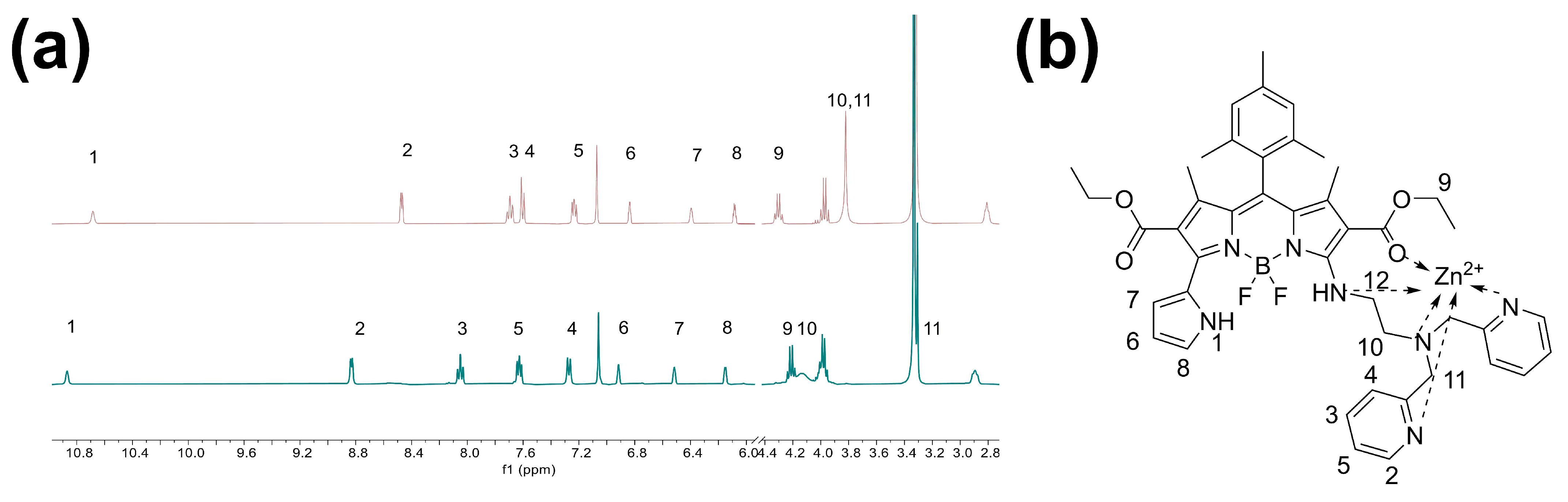
3.3. Exploring the Sensing Mechanism for BDP-p-BPEA
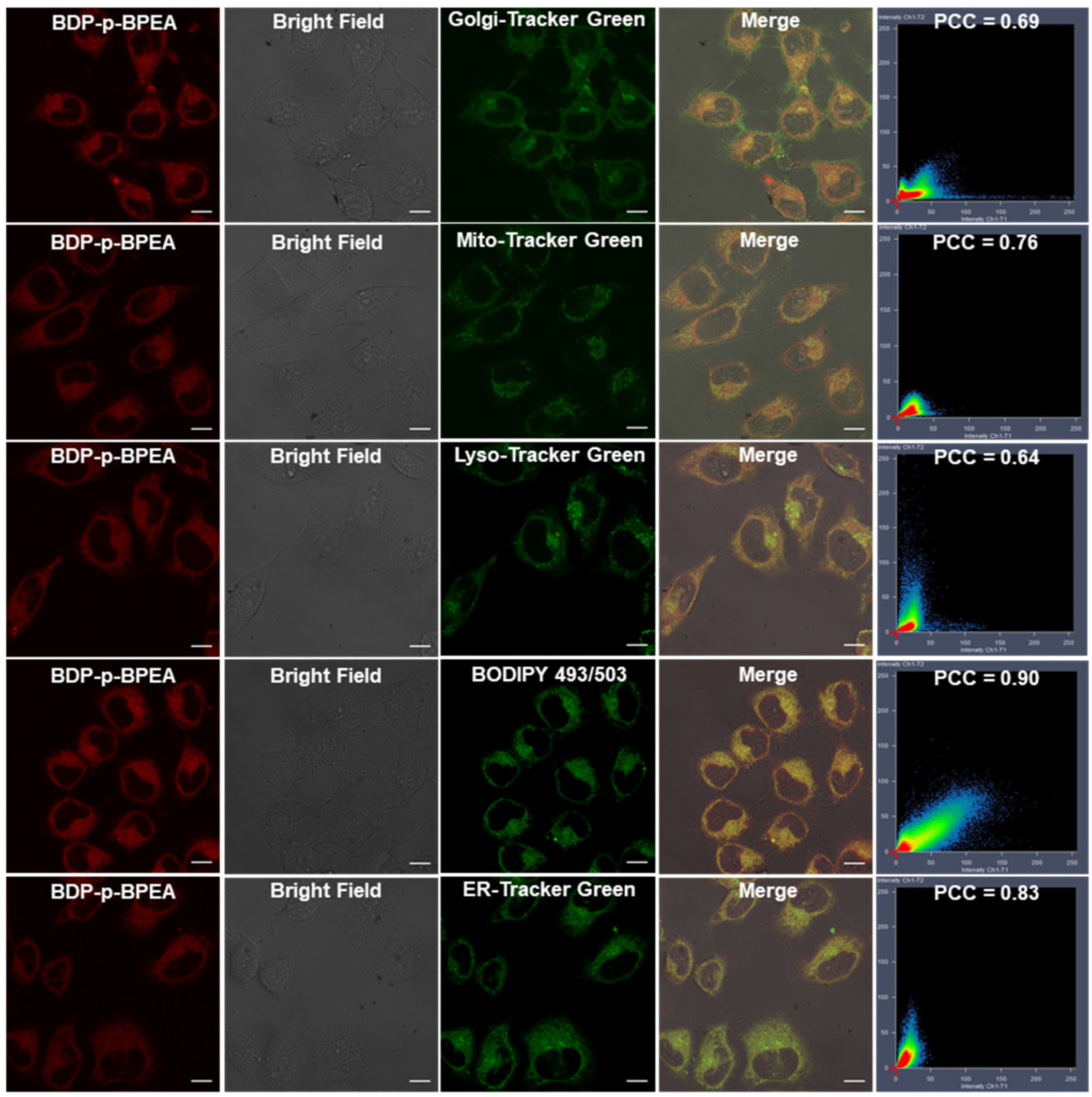
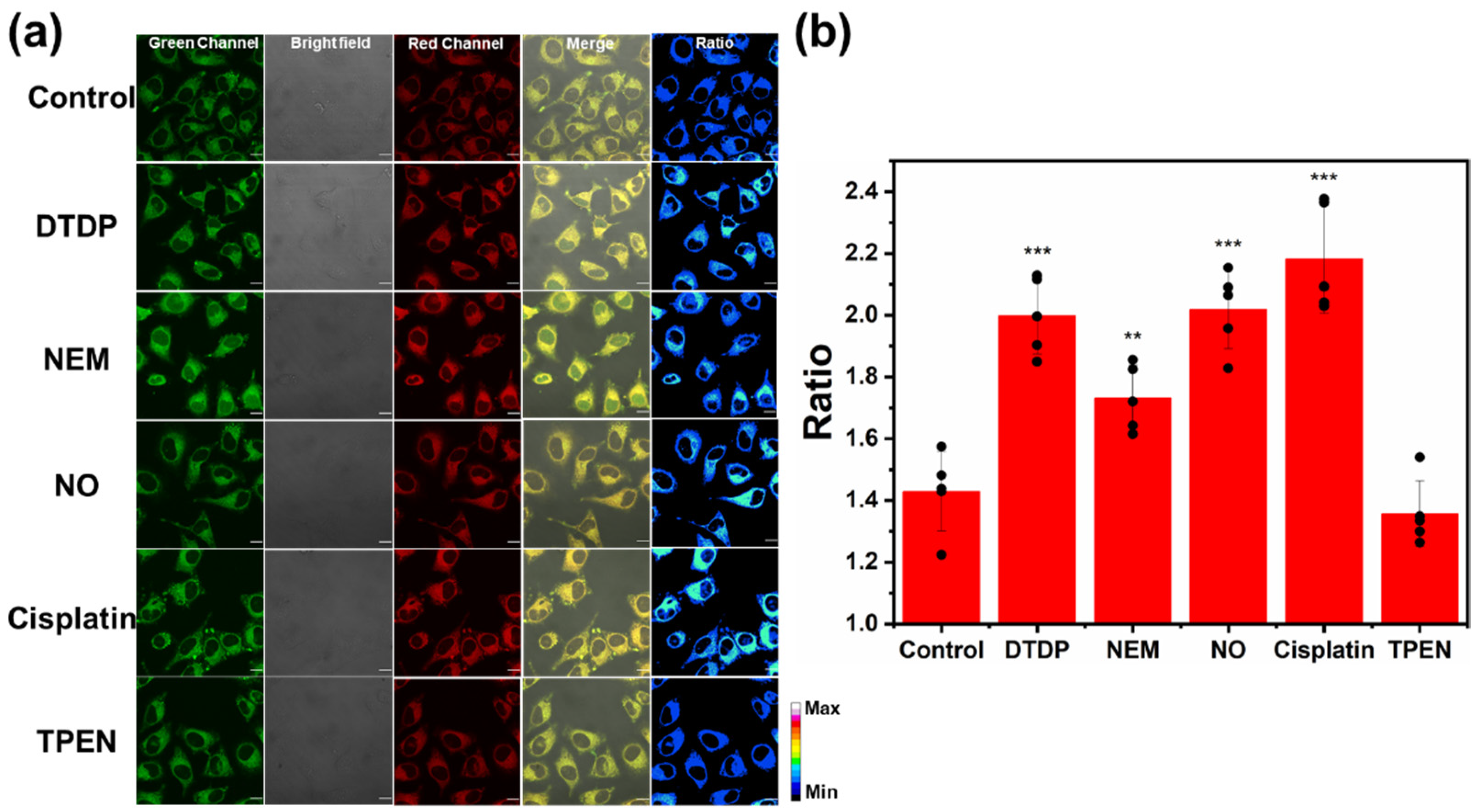
3.4. Imaging of Zn2+ Levels in Cellular Oxidative Stress
3.5. Comparison with the Previous Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, F.; Liu, Y.; Lu, N. Zinc and its effects on oxidative stress in Alzheimer’s disease. Neurol. Sci. 2014, 35, 923–928. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radical Bio. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Marreiro, D.d.N.; Climaco Cruz, K.J.; Silva Morais, J.B.; Beserra, J.B.; Severo, J.S.; Soares de Oliveira, A.R. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Aizenman, E.; Stout, A.K.; Hartnett, K.A.; Dineley, K.E.; McLaughlin, B.; Reynolds, I.J. Induction of neuronal apoptosis by thiol oxidation: Putative role of intracellular zinc release. J. Neurochem. 2000, 75, 1878–1888. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Maret, W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006, 273, 4300–4310. [Google Scholar] [CrossRef]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wang, K.; Kong, Q.; Wang, J.; Xi, D.; Gu, B.; Lu, S.; Wei, T.; Chen, X. Recent studies focusing on the development of fluorescence probes for zinc ion. Coord. Chem. Rev. 2021, 429, 213636. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Z.; Lake, R.J.; Zheng, C.; Lu, Y. Enzyme-mediated endogenous and bioorthogonal control of a DNAzyme fluorescent sensor for imaging metal ions in living cells. Angew. Chem. Int. Ed. 2019, 58, 17061–17067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Tong, L.; Zhong, H.; Xia, H.; Hua, R.; Chen, B. Improved LRET-based detection characters of Cu2+ using sandwich structured NaYF4@NaYF4:Er3+/Yb3+@NaYF4 nanoparticles as energy donor. Sens. Actuators B Chem. 2018, 257, 829–838. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, L.; Chen, Y.; Li, M.; Chen, W.; Wang, Y.; Wei, X.; Zhang, Y.; Zhou, Y.; Xu, M. Dual-ligand near-infrared luminescent lanthanide-based metal–organic framework coupled with in vivo microdialysis for highly sensitive ratiometric detection of Zn2+ in a mouse model of Alzheimer’s disease. Anal. Chem. 2022, 94, 11940–11948. [Google Scholar] [CrossRef]
- Liu, R.; Kowada, T.; Du, Y.; Amagai, Y.; Matsui, T.; Inaba, K.; Mizukami, S. Organelle-level labile Zn(2+) mapping based on targetable fluorescent sensors. ACS Sens. 2022, 7, 748–757. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Carroll, S.L.; Chen, J.; Wang, M.C.; Wang, J. Challenges and opportunities for small-molecule fluorescent probes in redox biology applications. Antioxid. Redox. Signal. 2018, 29, 518–540. [Google Scholar] [CrossRef]
- Hagimori, M.; Hara, F.; Mizuyama, N.; Fujino, T.; Saji, H.; Mukai, T. High-affinity ratiometric fluorescence probe based on 6-amino-2,2’-bipyridine scaffold for endogenous Zn(2+) and its application to living cells. Molecules 2022, 27, 1287. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, G.; Hu, J.; Wang, H.; Eriksson, P.; Zhang, R.; Zhang, Z.; Uvdal, K. Real-time visualizing the regulation of reactive oxygen species on Zn2+ release in cellular lysosome by a specific fluorescent probe. Sensor. Actuat. B Chem. 2018, 264, 419–425. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Zhang, S.; Cao, J.; Song, K.; Ge, D.; Dong, H.; Wang, J.; Peng, X. Ratiometric fluorescence imaging of lysosomal Zn(2+) release under oxidative stress in neural stem cells. Biomater. Sci. 2014, 2, 89–97. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Zhang, S.; Zhang, W.; Zhang, W.; Tang, B. Simultaneous fluorescence visualization of mitochondrial hydrogen peroxide and zinc ions in live cells and in vivo. Chem. Commun. 2016, 52, 12741–12744. [Google Scholar] [CrossRef] [PubMed]
- Trusso Sfrazzetto, G.; Satriano, C.; Tomaselli, G.A.; Rizzarelli, E. Synthetic fluorescent probes to map metallostasis and intracellular fate of zinc and copper. Coord. Chem. Rev. 2016, 311, 125–167. [Google Scholar] [CrossRef]
- Liu, M.; Ma, S.; She, M.; Chen, J.; Wang, Z.; Liu, P.; Zhang, S.; Li, J. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Deniz, E.; Isbasar, G.C.; Bozdemir, Ö.A.; Yildirim, L.T.; Siemiarczuk, A.; Akkaya, E.U. Bidirectional switching of near IR emitting boradiazaindacene fluorophores. Org. Lett. 2008, 10, 3401–3403. [Google Scholar] [CrossRef]
- Hiruta, Y.; Koiso, H.; Ozawa, H.; Sato, H.; Hamada, K.; Yabushita, S.; Citterio, D.; Suzuki, K. Near IR Emitting red-shifting ratiometric fluorophores based on borondipyrromethene. Org. Lett. 2015, 17, 3022–3025. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective manipulation of ICT and PET processes in styryl-bodipy derivatives: Applications in molecular logic and fluorescence sensing of metal ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef]
- Guliyev, R.; Ozturk, S.; Kostereli, Z.; Akkaya, E.U. From virtual to physical: Integration of chemical logic gates. Angew. Chem. Int. Ed. 2011, 50, 9826–9831. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Shen, J.; Wang, J.; Wang, H.; Fang, M.; Zhou, H.; Tanasova, M. Ratiometric fluorescent and colorimetric BODIPY-based sensor for zinc ions in solution and living cells. Sensor. Actuat. B Chem. 2018, 258, 1279–1286. [Google Scholar] [CrossRef]
- Kawashima, H.; Ukai, S.; Nozawa, R.; Fukui, N.; Fitzsimmons, G.; Kowalczyk, T.; Fliegl, H.; Shinokubo, H. Determinant factors of three-dimensional aromaticity in antiaromatic cyclophanes. J. Am. Chem. Soc. 2021, 143, 10676–10685. [Google Scholar] [CrossRef]
- Dai, E.; Pang, W.; Zhang, X.F.; Yang, X.; Jiang, T.; Zhang, P.; Yu, C.; Hao, E.; Wei, Y.; Mu, X.; et al. Synthesis and photophysics of BF2 -rigidified partially closed chain bromotetrapyrroles: Near infrared emitters and photosensitizers. Chem. Asian. J. 2015, 10, 1327–1334. [Google Scholar] [CrossRef]
- Zhang, S.; Adhikari, R.; Fang, M.; Dorh, N.; Li, C.; Jaishi, M.; Zhang, J.; Tiwari, A.; Pati, R.; Luo, F.T.; et al. Near-infrared fluorescent probes with large Stokes shifts for sensing Zn(II) ions in living cells. ACS Sens. 2016, 1, 1408–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaiyandi, L.M.; Dineley, K.E.; Reynolds, I.J. Divergent consequences arise from metallothionein overexpression in astrocytes: Zinc buffering and oxidant-induced zinc release. Glia 2004, 45, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Nguyen, L.V.; Makarem, M.; Dong, Y.; Shih, K.; Eirew, P.; Raouf, A.; Emerman, J.T.; Eaves, C.J. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc. Natl. Acad. Sci. USA 2014, 111, 7789–7794. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Jaworski, J.; Nolan, E.M.; Sheng, M.; Lippard, S.J. A tautomeric zinc sensor for ratiometric fluorescence imaging Application to nitric oxide-induced release of intracellular zinc. Proc. Natl. Acad. Sci. USA 2003, 101, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Chen, C.; Hou, J.; Xin, W.; Mori, A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim. Biophys. Acta 2000, 1498, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Xia, S.; Bi, J.; Wigstrom, T.P.; Valenzano, L.; Wang, J.; Tanasova, M.; Luck, R.L.; Liu, H. Detecting Zn(II) ions in live cells with near-infrared fluorescent probes. Molecules 2019, 24, 1592. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhu, C.; Chen, Y.; Bai, Y.; Han, Z.; Yao, S.; Jiao, Y.; Yuan, H.; He, W.; Guo, Z. A FRET-based fluorescent Zn(2+) sensor: 3D ratiometric imaging, flow cytometric tracking and cisplatin-induced Zn(2+) fluctuation monitoring. Chem. Sci. 2020, 11, 11037–11041. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.; Su, D.; Zhang, Y.; Li, P.; Lu, S.; Gong, Y.; Zhang, W.; Tang, B. Simultaneous fluorescence imaging reveals N-methyl-D-aspartic acid receptor dependent Zn(2+)/H(+) flux in the brains of mice with depression. Anal. Chem. 2020, 92, 4101–4107. [Google Scholar] [CrossRef]
- Fang, H.; Li, Y.; Yao, S.; Geng, S.; Chen, Y.; Guo, Z.; He, W. An endoplasmic reticulum-targeted ratiometric fluorescent molecule reveals Zn(2+) micro-dynamics during drug-induced organelle ionic disorder. Front. Pharmacol. 2022, 13, 927609. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, J.Z.; Mao, X.M.; Wang, Q.; Li, S.P.; Wang, C.Y. Real-time detection and imaging of exogenous and endogenous Zn(2+) in the PC12 cell model of depression with a NIR fluorescent probe. Analyst 2021, 146, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Dai, E.; Sheng, W.; Yu, C.; Hao, E.; Liu, W.; Wei, Y.; Jiao, L. Direct synthesis of dipyrrolyldipyrrins from SNAr reaction on 1,9-dihalodipyrrins with pyrroles and their NIR fluorescence “turn-on” response to Zn2+. Org. Lett. 2017, 19, 6244–6247. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Trigiante, G.; Crespo-Otero, R.; Hawes, C.S.; Philpott, M.P.; Jones, C.R.; Watkinson, M. Endoplasmic reticulum targeting fluorescent probes to image mobile Zn2+. Chem. Sci. 2019, 10, 10881–10887. [Google Scholar] [CrossRef] [PubMed]

| Detection Mode | λex (nm)/λem (nm) | Fluorophore | Linear Range (µM) | LOD (nM) | Testing Media | Kd(nM) | Ref. |
|---|---|---|---|---|---|---|---|
| Enhanced | 750/779 | cyanine | 0.1–5.0 | 11 | 25 mM HEPES | 1.42 × 103 | [21] |
| Enhanced | 687/703 | hemicyanine | 0–1.4 | 0.45 | HEPES buffer solution (10 mM, pH 7.0) | 1.05 × 103 | [38] |
| Ratiometric | 415/560 to 480 | coumarin & benzo[c][1,2,5]oxadiazole | 0–5 | 82 | DMSO: aqueous solution (50 mM HEPES 100 mM KNO3, pH = 7.40) v:v = 1:99 | 0.014 | [39] |
| Enhanced | 390/460 | coumarin & rhodamine | 0–10 | 3.2×102 | 0.1 M Tris, pH 7.4 | 3.94 × 103 | [40] |
| Ratiometric | 610 to 575/700 to 660 | BODIPY | 0–10 | 31.8 | DMSO: aqueous solution (50 mM HEPES 100 mM KNO3, pH = 7.2) v:v = 3:2 | 3.65 | [41] |
| Ratiometric | 430 to 475/660 | isophorone | 0–8 | 106 | DMSO: aqueous solution (10 mM HEPES, pH = 7.46) v:v = 1:1 | 7.7×103 | [42] |
| Enhanced | 544 to 607/634 | dipyrrolyldipyrrin | (No data) | 210 | DMF | 13 | [43] |
| Enhanced | 346/414 | naphthalimide | 0–0.003 | 0.047 | DMSO: aqueous solution (10 mM HEPES, pH 7.4) v:v = 1:9 | 4.70 | [44] |
| Ratiometric | 550/620 to 650 | BODIPY | 0–3 | 29 | MeCN: aqueous solution (50 mM HEPES 100 mM KNO3, pH 7.4) v:v = 1:1 | 3.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yao, S.; Fang, H.; He, W.; Chen, Y.; Guo, Z. Rational Design of Ratiometric Fluorescent Probe for Zn2+ Imaging under Oxidative Stress in Cells. Chemosensors 2022, 10, 477. https://doi.org/10.3390/chemosensors10110477
Li Y, Yao S, Fang H, He W, Chen Y, Guo Z. Rational Design of Ratiometric Fluorescent Probe for Zn2+ Imaging under Oxidative Stress in Cells. Chemosensors. 2022; 10(11):477. https://doi.org/10.3390/chemosensors10110477
Chicago/Turabian StyleLi, Yaheng, Shankun Yao, Hongbao Fang, Weijiang He, Yuncong Chen, and Zijian Guo. 2022. "Rational Design of Ratiometric Fluorescent Probe for Zn2+ Imaging under Oxidative Stress in Cells" Chemosensors 10, no. 11: 477. https://doi.org/10.3390/chemosensors10110477
APA StyleLi, Y., Yao, S., Fang, H., He, W., Chen, Y., & Guo, Z. (2022). Rational Design of Ratiometric Fluorescent Probe for Zn2+ Imaging under Oxidative Stress in Cells. Chemosensors, 10(11), 477. https://doi.org/10.3390/chemosensors10110477






