Abstract
Calprotectin (CP) is an established biomarker that allows the noninvasive evaluation of inflammation levels in the gastrointestinal tract of patients with inflammatory bowel disease and is helpful for the diagnosis and management of the disease. Herein, we demonstrate that CP can effectively suppress the activity of 17E DNAzyme (17E) by chelating Zn(Ⅱ), which is the cofactor of 17E. As the inhibition efficiency of CP on the DNAzyme is proportional to the concentration of CP, the detection of CP can be readily achieved by assessing the activity of 17E.
1. Introduction
Inflammatory bowel disease (IBD), mainly encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is a group of idiopathic, chronic, and recurrent conditions involving inflammation and ulceration of the gastrointestinal (GI) tract [1,2]. Compared with healthy individuals, patients with IBD often have poorer quality of life and a higher risk of developing colorectal cancer [3]. IBD imposes a heavy burden on patients’ physical and mental health [4]. The incidence of IBD has risen steadily over the past few decades [5]. To date, endoscopy with histological biopsies remains the gold standard for the diagnosis and management of individuals with IBD [6]. However, it has poor patient acceptability as it is invasive and needs bowel preparation and sedation prior to the operation. Given the requirement of multiple endoscopies throughout the lifetime of an IBD patient, there is an urgent demand for the development of alternative diagnostic modalities.
A liquid biopsy uses the molecular analysis of biofluids from patients, such as blood, urine, stool, and saliva, for the diagnosis and prognosis of various diseases [7,8]. Due to their noninvasive nature, liquid biopsies are suitable for repeat sampling to monitor and predict disease progression. Due to the direct contact between fecal samples and the site of GI inflammation, tests for fecal biomarkers are readily available as helpful diagnostic tools for IBD [9]. The biomarkers that are explicitly found in the stool samples of IBD patients are mainly leukocyte proteins. Calprotectin (CP), belonging to the S100 protein family and released by neutrophils and epithelial cells in response to inflammation, has been proven to be a powerful biomarker for IBD [9,10,11]. The fecal CP test is helpful in identifying the level of inflammation in the GI tract. Currently, the commonly used method for CP detection, enzyme-linked immunosorbent assays (ELISA) [9,12], suffers the drawbacks of tediously long test times, complex washing steps, and expensive label elements [13]. Additionally, it employs protein enzymes as catalysts for signal amplification, resulting in poor stability and reduced activity under harsh conditions [14,15]. Consequently, a convenient, stable, and cost-effective assay for CP detection is urgently demanded.
Deoxyribozymes, also entitled DNAzymes, refer to in vitro selected DNA oligonucleotides with the catalytic capability to accelerate a variety of biochemical reactions. Owing to their inherent advantages over protein enzymes, including low cost, excellent thermal and chemical stability, ease of synthesis and functionalization, and programmable structure, DNAzymes have been widely used in the field of biosensing, with nearly three decades of development [16]. Numerous DNAzymes, especially RNA-cleaving DNAzymes, generally require divalent metal ions (e.g., Mn2+, Ca2+, and Zn2+) as cofactors to enable their catalytic characteristics. On account of their high metal ion selectivity, DNAzymes have found practical applications in metal ion sensing [17,18]. Interestingly, CP is a transition-metal-chelating protein of the calcium-binding S100 family. It is a heterodimer of two subunits, S100A8 and S100A9 [19,20]. It has been revealed that CP possesses two chelating sites for divalent, first-row transition metal ions (e.g., Mn2+ or Zn2+) at the S100A8/S100A9 interface [21,22]. Actually, CP can inhibit the growth of pathogens through the tight adsorption of metal ions, such as Mn(Ⅱ) and Zn(Ⅱ) [23]. Resultingly, it is reasonable to suppose that CP can inhibit the catalytic activities of metal-ion-dependent DNAzymes by sequestering the metal ion cofactors. In this work, we demonstrate that CP-Zn(Ⅱ) binding interactions can inhibit the activity of 17E DNAzyme (17E), a Zn(Ⅱ)-dependent DNAzyme, by decreasing the effective concentration of Zn(Ⅱ) [24,25]. Since the inhibition efficiency depends on the concentration of CP, 17E can serve as a useful signal amplification method for detecting CP by simply monitoring the overall observed activity of the DNAzyme.
2. Materials and Methods
2.1. Materials
The oligonucleotides were synthesized and HPLC-purified by Sangon Biotech Co., Ltd. (Shanghai, China). The nucleotide sequences are listed in Table 1. Sodium chloride (NaCl), 4-hydroxyethylpiperazine ethyl sulfonic acid (HEPES), and 0.5 M ethylenediaminetetraacetic acid (EDTA), pH 8.0, were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Zinc chloride (ZnCl2) and sodium hydroxide (NaOH) were obtained from Aladdin Reagent Company (Shanghai, China). Human calprotectin was acquired from CUSABIO (Wuhan, China).

Table 1.
Sequences of the oligonucleotides used in this work.
2.2. Apparatus
The fluorescence spectra were obtained by F97Pro fluorophotometer (Lengguang Technology Co., Ltd., Shanghai, China) with excitation wavelength of 494 nm. The enzymatic reactions were carried out using HCM100-PRO constant temperature oscillating metal bath (DLAB Scientific Co., Ltd., Beijing, China).
2.3. Procedure of the Assay
Different concentrations of CP were incubated with ZnCl2 (4 µM) at 25 °C for 30 min before the addition of 17E to make sure Zn(Ⅱ) was fully sequestered. Subsequently, the mixture was incubated at 25 °C for an additional 30 min after 17E DNAzyme, and its substrate were added for the cleavage assay. The experiments were carried out in the buffer condition of NaCl (50 mM) and HEPES (50 mM, pH 7.0~9.0 as needed), with a final volume of 500 µL. After 25 µL EDTA (0.5 M pH 8.0) was added to stop the enzymatic reaction, the fluorescence intensity (FI) was measured with excitation at 494 nm and emission at 519 nm.
3. Results and Discussion
3.1. Principle of the Assay
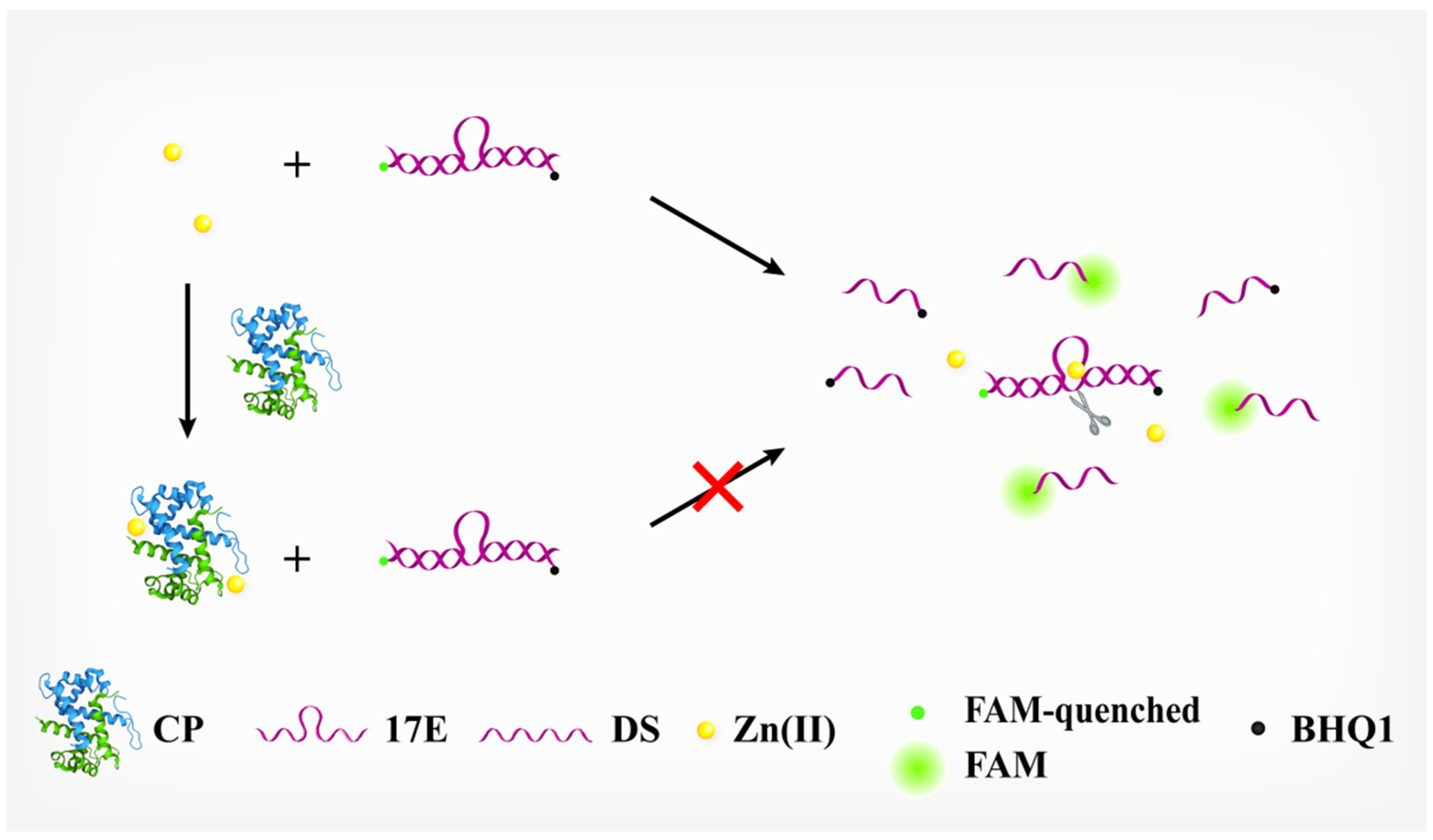
The principle of our DNAzyme-based assay for CP is illustrated in Figure 1. The DNAzyme employed here is 17E, which is a kind of RNA-cleaving DNAzyme that recruits metal ions as cofactors to exert its enzymatic activity. In the presence of its cofactor, Zn(Ⅱ), 17E can cleave the dual-labeled substrate (DS) at the ribonucleotide site and recover its fluorescence. If incubated with CP first, the Zn(Ⅱ) will be tightly bound to CP since CP owns two chelating sites for Zn(Ⅱ). In principle, the effective concentration of free Zn(Ⅱ) will be decreased correspondingly as the concentration of CP is increased. Consequently, the activity of 17E (i.e., cleavage of the DS) is decreased, and less DS can be hydrolyzed within a certain time, leading to a reduction in the FI of the format. Obviously, we can readily determine the concentration of CP by monitoring the activity of 17E.
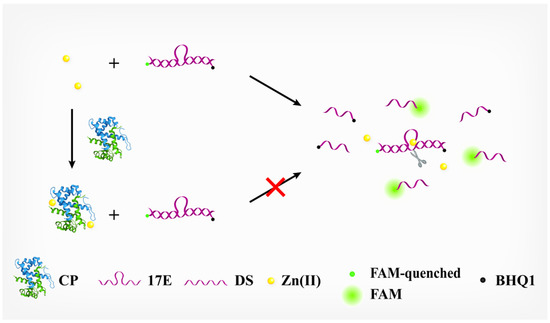
Figure 1.
Schematic illustration of calprotectin detection based on the inhibitory effect of calprotectin against the activity of 17E DNAzyme.
3.2. Verifying the Inhibitory Effect of CP upon the Activity of 17E DNAzyme
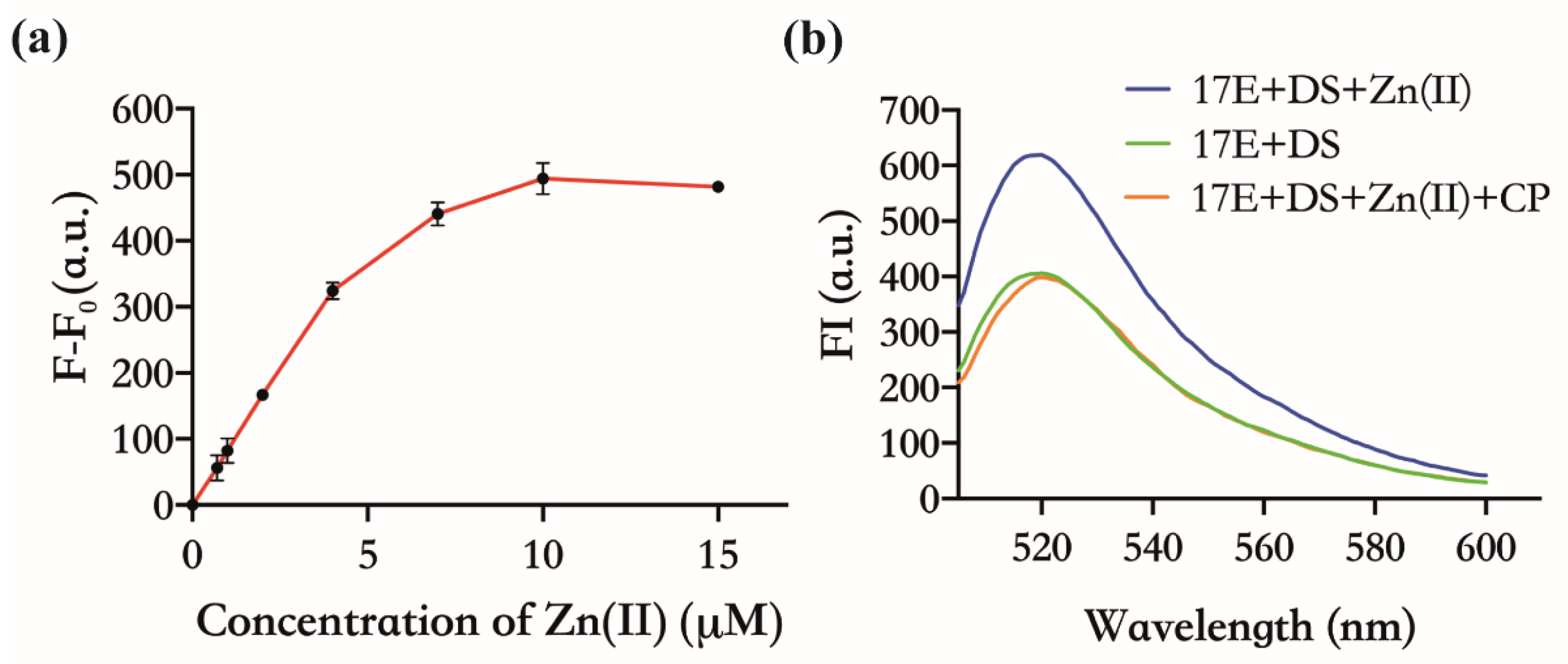
Both CP and 17E DNAzyme have already been proven to have an affinity for Zn(Ⅱ) [25,26,27]. In principle, CP can indirectly inhibit the activity of 17E by binding to Zn(Ⅱ) competitively with 17E. As shown in Figure 2a, there was an obvious FI enhancement when the mixtures of 17E and DS were incubated with different concentrations of Zn(Ⅱ), in contrast to those without Zn(Ⅱ). The FI increased with the increasing amount of Zn(Ⅱ) (0~10 µM), and the maximum slope of the curve was obtained at the concentration of 4 µM, which is the concentration that will be used for the subsequent feasibility test. This concentration-dependent manner implies that Zn(Ⅱ) is a necessary cofactor for 17E. When CP with half the concentration of Zn(Ⅱ) was added, which was the saturation concentration expected to bind exactly all the Zn(Ⅱ) (4 µM) in the system, the FI was almost the same as that of without Zn(Ⅱ) (Figure 2b). These results confirm that CP does suppress the enzymatic cleavage of DS by sequestering the Zn(Ⅱ) necessary for the activity of 17E.
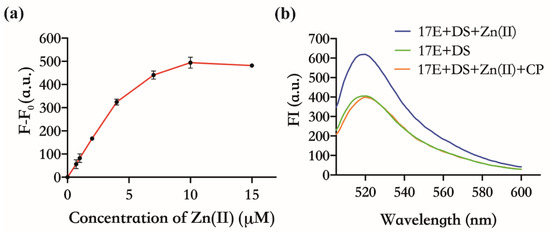
Figure 2.
The inhibitory effect of CP against the activity of 17E DNAzyme. Reaction conditions: 30 nM 17E, 150 nM DS, pH 7.5, 30 min. (a) Activity of 17E: FI enhancement with the addition of Zn(Ⅱ). F indicates the FI in the presence of Zn(Ⅱ) at 0.7 µM, 1 µM, 4 µM, 7 µM, 10 µM, and 15 µM, respectively; F0 indicates the FI without Zn(Ⅱ). (b) Inhibitory effect of CP on the activity of 17E. Green, without Zn(Ⅱ); Blue, 4 µM Zn(Ⅱ); Orange, 4 µM Zn(Ⅱ) and 2 µM CP.
3.3. Optimizing the Experimental Conditions of the Assay
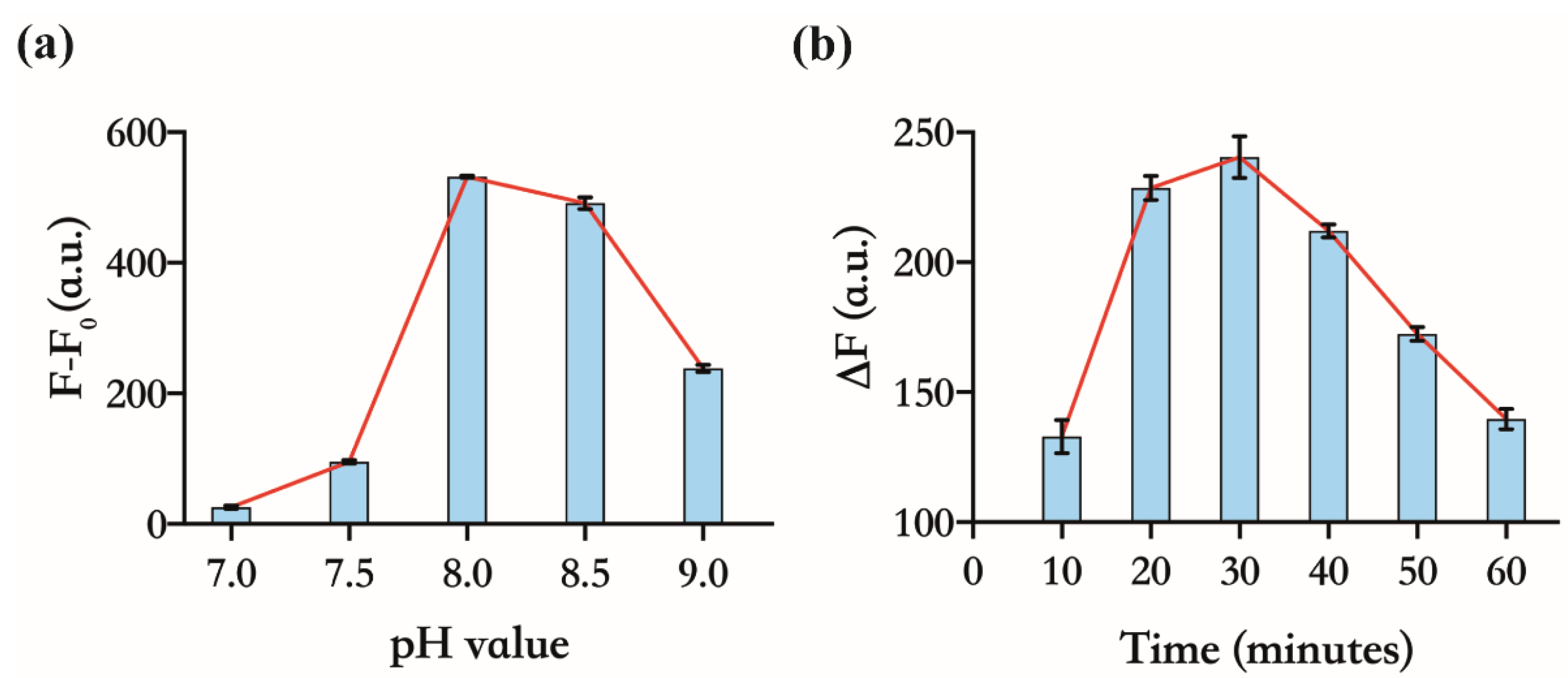
Based on the sensing principle, the more sensitively 17E responds to variations in the concentration of unbound Zn(Ⅱ), the better the above assay will perform in the detection of CP. We further investigated the response of the FL of DS to Zn(Ⅱ) under different reaction parameters, such as pH value and the reaction time of enzymatic cleavage. As shown in Figure 3a, the maximum difference of FL between the 17E/DS mixtures, with or without Zn(Ⅱ), was observed at pH 8.0. It is indicated that a certain amount of Zn(Ⅱ) can induce the maximum cleavage of DS at pH 8.0. Thus, we selected a buffer with a pH of 8.0 for the subsequent experiments. Although the higher level of Zn(Ⅱ) can stimulate faster cleavage of DS, with the extension of the reaction time, the cleavage reaction would reach the endpoint regardless of zinc(Ⅱ) concentration, meaning all substrates have already been cleaved. As we will use 4 µM of Zn(Ⅱ) in the detection of CP, we choose 4 µM and 1 µM as the high and low levels of Zn(Ⅱ), respectively, to determine the time in which the maximum fluorescence intensity difference (ΔF) between them was achieved (Figure 3b). Finally, we determined 30 min as the cleavage time. These optimized experimental conditions are applied to the following operation.
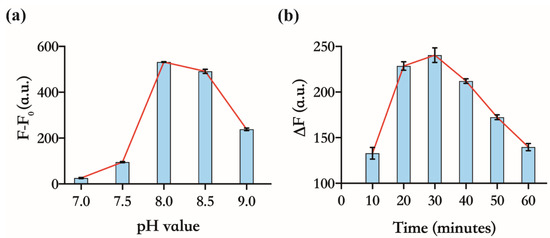
Figure 3.
Optimizing experimental conditions of the assay. All the solutions contain 30 nM 17E and 150 nM DS. (a) Activity of 17E at different pH values, 7.0, 7.5, 8.0, 8.5, and 9.0. F and F0 indicate the FI of the system with or without 4 µM Zn(Ⅱ) for 30 min, respectively; (b) FI difference of the system with two different levels of Zn(Ⅱ), 4 µM and 1 µM, and at different time intervals of enzymatic cleavage.
3.4. Quantitative Detection of CP using 17E DNAyzme
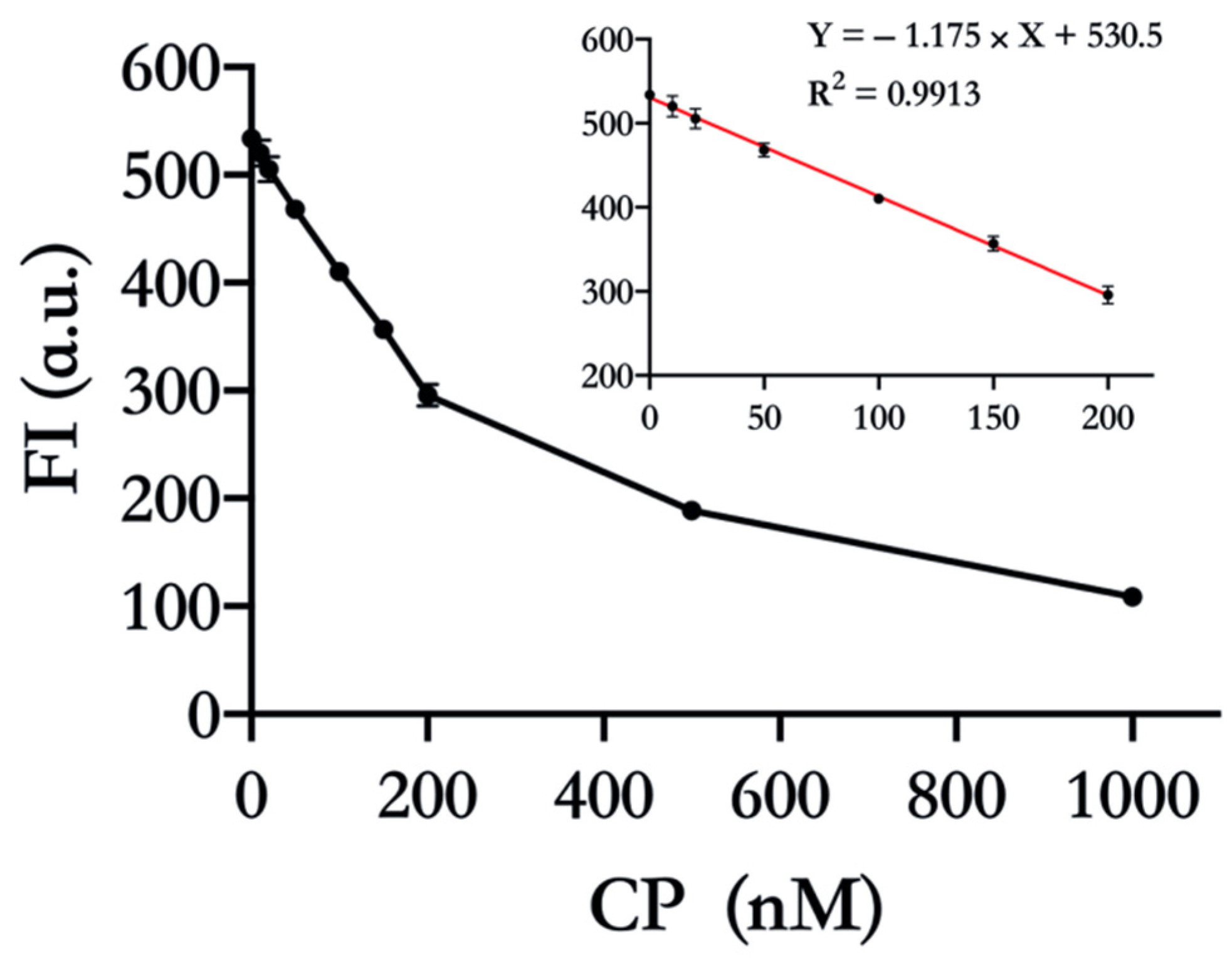
We further employed 17E as a probe to detect CP quantitatively. As expected, the FI gradually decreased with the increasing CP concentration, which indicated that the activity of more 17E was suppressed (Figure 4). The linear range of detection was from 10 nM to 200 nM with R2 = 0.9913. The limit of detection (LOD) was calculated to be 9.89 nM, which is below the cut-off value (tens of nM) of fecal CP used to distinguish IBD patients from functional GI disorders patients [28]. This concentration-dependent behavior further testifies that CP indeed inhibits the catalytic activity of 17E by chelating the cofactor, Zn(Ⅱ).
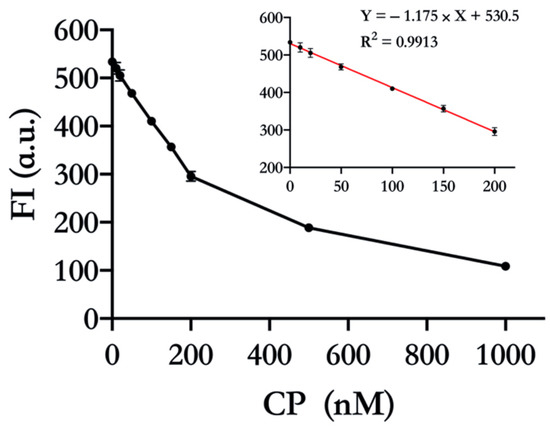
Figure 4.
Detection of CP by 17E DNAzyme. Zn(Ⅱ) was mixed with different concentrations of CP (0, 10 nM, 20 nM, 50 nM, 100 nM, 150 nM, 200 nM, 500 nM, and 1000 nM) for 30 min. Then the 17E and DS were added and incubated for cleavage over another 30 min, and FI was recorded. Other ingredients: pH 8.0, 4 µM Zn(Ⅱ), 30 nM 17E, and 150 nM DS. Note that the FI of 150 nM undegraded DS was deducted as a background signal.
4. Conclusions
In summary, to the best of our knowledge, we have demonstrated for the first time that CP can indirectly suppress the catalytic activity of 17E by complexing with Zn(Ⅱ) competitively with 17E. On the basis of this finding, we developed a turn-off fluorescent format for CP detection by evaluating the activity of 17E. In contrast to their proteinic counterparts, DNAzyme-based probes possess several inherent advantages. For example, their excellent thermal and chemical stabilities make DNAzymes especially suitable for point-of-care testing. Beyond the catalytic ability of 17E, the cleavage products of 17E can be aided with a variety of nucleic acid amplification methods for further improvement of the sensitivity. Their programmable structure and ease of functionalization allow DNAzyme probes to be adapted to various detection formats to meet different needs. We envision that this DNAzyme-based method will be a promising platform for the in vitro diagnosis of CP in clinical samples when combined with effective fishing methods.
Author Contributions
Conceptualization, C.Z.; investigation, J.S.; resources, X.S., D.Z. and Y.F.; writing—original draft preparation, J.S.; writing—review and editing, C.Z. and W.Z.; supervision, C.Z. and X.S.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Commission of Shanghai Municipality (Grant No. 22ZR1412000).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The completed data of this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chams, S.; Badran, R.; Sayegh, S.E.; Chams, N.; Shams, A.; Inaya, H.H. Inflammatory bowel disease: Looking beyond the tract. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Gao, X.; Hu, N.; Huang, M.; Ran, Z.; Liu, Z.; Zhong, J.; Zou, D.; Wu, X.; et al. Current diagnosis and management of Crohn’s disease in China: Results from a multicenter prospective disease registry. BMC Gastroenterol. 2019, 19, 145. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal Calprotectin is a Surrogate Marker for Endoscopic Lesions in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef]
- Rokkas, T.; Portincasa, P.; Koutroubakis, I.E. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: A diagnostic accuracy meta-analysis. J. Gastrointest. Liver Dis. 2018, 27, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Havelka, A.; Sejersen, K.; Venge, P.; Pauksens, K.; Larsson, A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci. Rep. 2020, 10, 4208. [Google Scholar] [CrossRef] [PubMed]
- Grützner, N.; Heilmann, R.M.; Suchodolski, J.S.; Steiner, J.M.; Holzenburg, A. Cold-microwave enhanced enzyme-linked immunosorbent assays--a path to high-throughput clinical diagnostics. Anal. Biochem. 2014, 457, 65–73. [Google Scholar] [CrossRef]
- Hu, R.; Liu, T.; Zhang, X.B.; Yang, Y.; Chen, T.; Wu, C.; Liu, Y.; Zhu, G.; Huan, S.; Fu, T.; et al. DLISA: A DNAzyme-Based ELISA for Protein Enzyme-Free Immunoassay of Multiple Analytes. Anal. Chem. 2015, 87, 7746–7753. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Argosf, P. Engineering protein thermal stability: Sequence statistics point to residue substitutions in α-helices. J. Mol. Biol. 1989, 206, 397–406. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- McGhee, C.E.; Loh, K.Y.; Lu, Y. DNAzyme sensors for detection of metal ions in the environment and imaging them in living cells. Curr. Opin. Biotechnol. 2017, 45, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Lake, R.J.; Yang, Z.; Zhang, J.; Lu, Y. DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions. Acc. Chem. Res. 2019, 52, 3275–3286. [Google Scholar] [CrossRef]
- Leukert, N.; Sorg, C.; Roth, J. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14). Biol. Chem. 2005, 386, 429–434. [Google Scholar] [CrossRef]
- Pröpper, C.; Huang, X.; Roth, J.; Sorg, C.; Nacken, W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J. Biol. Chem. 1999, 274, 183–188. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Stephan, J.R.; Cunden, L.S.; Brophy, M.B.; Wommack, A.J.; Keegan, B.C.; Shearer, J.M.; Nolan, E.M. The Hexahistidine Motif of Host-Defense Protein Human Calprotectin Contributes to Zinc Withholding and Its Functional Versatility. J. Am. Chem. Soc. 2016, 138, 12243–12251. [Google Scholar] [CrossRef] [PubMed]
- Silvers, R.; Stephan, J.R.; Griffin, R.G.; Nolan, E.M. Molecular Basis of Ca(II)-Induced Tetramerization and Transition-Metal Sequestration in Human Calprotectin. J. Am. Chem. Soc. 2021, 143, 18073–18090. [Google Scholar] [CrossRef] [PubMed]
- Damo, S.M.; Kehl-Fie, T.E.; Sugitani, N.; Holt, M.E.; Rathi, S.; Murphy, W.J.; Zhang, Y.; Betz, C.; Hench, L.; Fritz, G.; et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 3841–3846. [Google Scholar] [CrossRef]
- Moon, W.J.; Yang, Y.; Liu, J. Zn2+-Dependent DNAzymes: From Solution Chemistry to Analytical, Materials and Therapeutic Applications. ChemBioChem 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Kwon, A.H.; Lu, Y. In vitro selection and characterization of a highly efficient Zn(II)-dependent RNA-cleaving deoxyribozyme. Nucleic Acids Res. 2000, 28, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.B.; Hayden, J.A.; Nolan, E.M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 2012, 134, 18089–18100. [Google Scholar] [CrossRef] [PubMed]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Lozoya Angulo, M.E.; de Las Heras Gómez, I.; Martinez Villanueva, M.; Noguera Velasco, J.A.; Avilés Plaza, F. Faecal calprotectin, an useful marker in discriminating between inflammatory bowel disease and functional gastrointestinal disorders. Gastroenterol. Y Hepatol. 2017, 40, 125–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).