Laser-Scribed Graphene-Based Electrochemical Sensors: A Review
Abstract
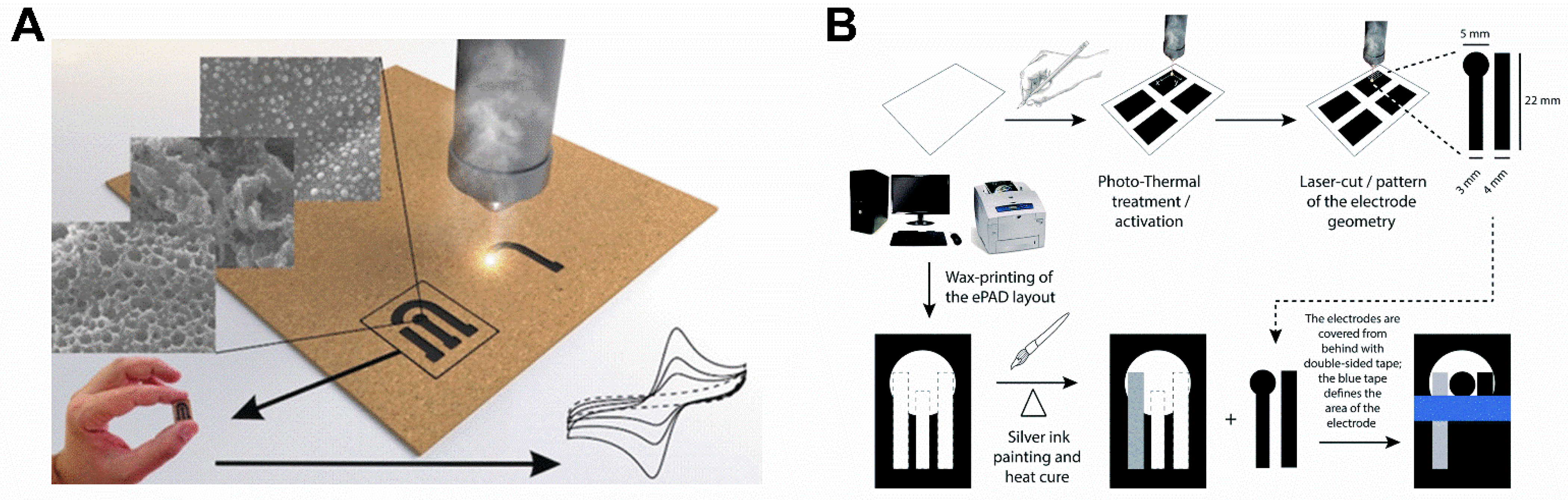
:1. Introduction
2. Graphene Preparation Methods
3. Fabrication of the LSG Electrodes
3.1. LSG from Polymer Treatment
3.2. LSG from 3D-Printed Electrode
3.3. LSG from Phenolic Resin
3.4. LSG from Graphene Ink
3.5. Element Doped LSG
4. Approaches to Modify LSG Electrochemical Sensors
4.1. LSG Modified by Nanostructured Materials and Polymers and Its Use as a Modifier of Conventional Electrodes
4.2. LSG Electrodes Modified with Biological Molecules
5. Analytical Methods
5.1. Electrochemical Tools
5.2. Data Analysis Technology
5.2.1. Artificial Neural Network
5.2.2. Support Vector Machine
5.2.3. Decision Trees
6. LSG-Based Electrodes—Applications to Chemical and Biochemical Sensing
6.1. Environmental Monitoring
6.2. Food Safety
6.3. Health Monitoring
6.4. Clinical Diagnosis
6.5. LSG Electrodes Integrated with Machine Learning Models
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Bo, X. Laser-enabled flexible electrochemical sensor on finger for fast food security detection. J. Hazard. Mater. 2022, 423, 127014. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- de Araujo, W.R.; Frasson, C.M.R.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R. Single-Step Reagentless Laser Scribing Fabrication of Electrochemical Paper-Based Analytical Devices. Angew. Chem. Int. Ed. 2017, 56, 15113–15117. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Gautam, A.; Silotia, P.; Malik, S.; de Oliveira Hansen, R.; Khalid, M.; Khosla, A.; Kaushik, A.; Mishra, Y.K. Internet-of-nano-things (IoNT) driven intelligent face masks to combat airborne health hazard. Mater. Today 2022, in press. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Hu, C. Laser-scribed graphene sensors on nail polish with tunable composition for electrochemical detection of nitrite and glucose. Sens. Actuators B Chem. 2022, 357, 131394. [Google Scholar] [CrossRef]
- Li, Q.; Bai, R.; Gao, Y.; Wu, R.; Ju, K.; Tan, J.; Xuan, F. Laser Direct Writing of Flexible Sensor Arrays Based on Carbonized Carboxymethylcellulose and Its Composites for Simultaneous Mechanical and Thermal Stimuli Detection. ACS Appl. Mater. Interfaces 2021, 13, 10171–10180. [Google Scholar] [CrossRef]
- Nasser, J.; Groo, L.A.; Zhang, L.; Sodano, H. Laser induced graphene fibers for multifunctional aramid fiber reinforced composite. Carbon 2020, 158, 146–156. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Kepić, D.; Sandoval, S.; del Pino, P.; György, E.; Cabana, L.; Ballesteros, B.; Tobias, G. Nanosecond Laser-Assisted Nitrogen Doping of Graphene Oxide Dispersions. ChemPhysChem 2017, 18, 935–941. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Rauf, S.; Beduk, T.; Durmus, C.; Aljedaibi, A.; Timur, S.; Alshareef, H.N.; Amine, A.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors and biosensors using laser-derived graphene: A comprehensive review. Biosens. Bioelectron. 2020, 168, 112565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Dale, C.; Hedley, J.; Kowal, M.D.; Kaner, R.B.; Keegan, N. Laser-scribed graphene presents an opportunity to print a new generation of disposable electrochemical sensors. Nanoscale 2014, 6, 13613–13622. [Google Scholar] [CrossRef] [Green Version]
- Kavai, M.S.; de Lima, L.F.; de Araujo, W.R. A disposable and low-cost laser-scribed graphene electrochemical sensor for simultaneous detection of hydroquinone, paracetamol and methylparaben. Mater. Lett. 2022, 330, 133211. [Google Scholar] [CrossRef]
- Johnson, Z.T.; Williams, K.; Chen, B.; Sheets, R.; Jared, N.; Li, J.; Smith, E.A.; Claussen, J.C. Electrochemical Sensing of Neonicotinoids Using Laser-Induced Graphene. ACS Sens. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Edberg, J.; Brooke, R.; Hosseinaei, O.; Fall, A.; Wijeratne, K.; Sandberg, M. Laser-induced graphitization of a forest-based ink for use in flexible and printed electronics. NPJ Flex. Electron. 2020, 4, 17. [Google Scholar] [CrossRef]
- Ataide, V.N.; Ameku, W.A.; Bacil, R.P.; Angnes, L.; de Araujo, W.R.; Paixão, T.R.L.C. Enhanced performance of pencil-drawn paper-based electrodes by laser-scribing treatment. RSC Adv. 2021, 11, 1644–1653. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, Y.; Zou, L.; Zhu, H.; Cao, R.; Zhao, G. Combination of machine learning and intelligent sensors in real-time quality control of alcoholic beverages. Food Sci. Technol. 2022, 42, 1–9. [Google Scholar] [CrossRef]
- Beduk, D.; Ilton de Oliveira Filho, J.; Beduk, T.; Harmanci, D.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; et al. ‘All In One’ SARS-CoV-2 variant recognition platform: Machine learning-enabled point of care diagnostics. Biosens. Bioelectron. X 2022, 10, 100105. [Google Scholar] [CrossRef]
- Ha, N.; Xu, K.; Ren, G.; Mitchell, A.; Ou, J.Z. Machine Learning-Enabled Smart Sensor Systems. Adv. Intell. Syst. 2020, 2, 2000063. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wang, J.; James, D.K.; Narkhede, P.; Singh, S.P.; Jassby, D.; Tour, J.M.; Arnusch, C.J. Laser-induced graphene and carbon nanotubes as conductive carbon-based materials in environmental technology. Mater. Today 2020, 34, 115–131. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Afsarimanesh, N.; Mukhopadhyay, S.C.; Kundu, S.; Xu, Y. Laser-Assisted Printed Flexible Sensors: A Review. Sensors 2019, 19, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Han, Q.; Cheng, Z.; Jiang, L.; Qu, L. Integrated graphene systems by laser irradiation for advanced devices. Nano Today 2017, 12, 14–30. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, e1803621. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Nayak, P.; Alshareef, H.N. Laser-derived graphene: A three-dimensional printed graphene electrode and its emerging applications. Nano Today 2019, 24, 81–102. [Google Scholar] [CrossRef]
- Li, G. Direct laser writing of graphene electrodes. J. Appl. Phys. 2020, 127, 8725–8729. [Google Scholar] [CrossRef] [Green Version]
- Gillani, N.; Arslan, T. Intelligent Sensing Technologies for the Diagnosis, Monitoring and Therapy of Alzheimer’s Disease: A Systematic Review. Sensors 2021, 21, 4249. [Google Scholar] [CrossRef]
- Çorman, M.E.; Ozcelikay, G.; Cetinkaya, A.; Kaya, S.I.; Armutcu, C.; Özgür, E.; Uzun, L.; Ozkan, S.A. Metal-organic frameworks as an alternative smart sensing platform for designing molecularly imprinted electrochemical sensors. TrAC Trends Anal. Chem. 2022, 150, 116573. [Google Scholar] [CrossRef]
- Namuduri, S.; Narayanan, B.N.; Davuluru, V.S.P.; Burton, L.; Bhansali, S. Review—Deep Learning Methods for Sensor Based Predictive Maintenance and Future Perspectives for Electrochemical Sensors. J. Electrochem. Soc. 2020, 167, 037552. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Lahcen, A.A.; Tashkandi, N.; Salama, K.N. One-step electrosynthesized molecularly imprinted polymer on laser scribed graphene bisphenol a sensor. Sens. Actuators B Chem. 2020, 314, 128026. [Google Scholar] [CrossRef]
- Wan, Z.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.-T.; Trinchi, A.; Shiddiky, M.J.A.; Gao, Y.; Li, Q. Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]
- Lei, Y.; Alshareef, A.H.; Zhao, W.; Inal, S. Laser-Scribed Graphene Electrodes Derived from Lignin for Biochemical Sensing. ACS Appl. Nano Mater. 2020, 3, 1166–1174. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, F.; Zhang, Y.; Sui, Y.; Liu, P.; Zhang, Z.; Xu, C.; Yang, C. High-performance hydrogen peroxide micro-sensors based on laser-induced fabrication of graphene@Ag electrodes. Appl. Surf. Sci. 2021, 565, 150565. [Google Scholar] [CrossRef]
- Ghanam, A.; Lahcen, A.A.; Beduk, T.; Alshareef, H.N.; Amine, A.; Salama, K.N. Laser scribed graphene: A novel platform for highly sensitive detection of electroactive biomolecules. Biosens. Bioelectron. 2020, 168, 112509. [Google Scholar] [CrossRef]
- Rocha, D.P.; Ataide, V.N.; de Siervo, A.; Gonçalves, J.M.; Muñoz, R.A.; Paixão, T.R.; Angnes, L. Reagentless and sub-minute laser-scribing treatment to produce enhanced disposable electrochemical sensors via additive manufacture. Chem. Eng. J. 2021, 425, 130594. [Google Scholar] [CrossRef]
- Mendes, L.F.; de Siervo, A.; de Araujo, W.R.; Paixão, T.R.L.C. Reagentless fabrication of a porous graphene-like electrochemical device from phenolic paper using laser-scribing. Carbon 2020, 159, 110–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light laser-induced graphene from phenolic resin: A new approach for directly writing graphene-based electrochemical devices on various substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Kothuru, A.; Goel, S. Laser induced graphene on phenolic resin and alcohol composite sheet for flexible electronics applications. Flex. Print. Electron. 2020, 5, 042001. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Yang, C.; Tao, S. Preparation of multifunctional porous carbon electrodes through direct laser writing on a phenolic resin film. J. Mater. Chem. A 2019, 7, 21168–21175. [Google Scholar] [CrossRef]
- Loguercio, L.F.; Thesing, A.; Noremberg, B.D.S.; Lopes, B.V.; Maron, G.K.; Machado, G.; Pope, M.A.; Carreno, N.L.V. Direct Laser Writing of Poly(furfuryl Alcohol)/Graphene Oxide Electrodes for Electrochemical Determination of Ascorbic Acid. ChemElectroChem 2022, 9, e202200334. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, J.; Ling, Y.; Yu, S.; Li, S.; Wu, X.; Zhang, Z. A facile and efficient nitrite electrochemical sensor based on N, O co-doped porous graphene film. Microchem. J. 2022, 178, 107361. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical sensor for nitrite detection in water samples using flexible laser-induced graphene electrodes functionalized by CNT decorated by Au nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Sharma, S.; Ganeshan, S.K.; Pattnaik, P.K.; Kanungo, S.; Chappanda, K.N. Laser induced flexible graphene electrodes for electrochemical sensing of hydrazine. Mater. Lett. 2020, 262, 127150. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, P.; Xue, T.; Xu, J.; Qiu, D.; Sheng, Y.; Li, W.; Lu, X.; Ge, Y.; Wen, Y. Facile and rapid one-step mass production of flexible 3D porous graphene nanozyme electrode via direct laser-writing for intelligent evaluation of fish freshness. Microchem. J. 2021, 162, 105855. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Q.; Wang, W.; Wen, Y.; Ji, K.; Liu, X.; He, P.; Janegitz, B.C.; Tang, K. The fabrication of a flexible and portable sensor based on home-made laser-induced porous graphene electrode for the rapid detection of sulfonamides. Microchem. J. 2022, 182, 107898. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Z.; Zhang, Y.; Liu, B.; Mo, G.; Li, J.; Ye, J. A glassy carbon electrode modified with a bismuth film and laser etched graphene for simultaneous voltammetric sensing of Cd(II) and Pb(II). Microchim. Acta 2018, 185, 438. [Google Scholar] [CrossRef]
- Rajaji, U.; Govindasamy, M.; Sha, R.; Alshgari, R.A.; Juang, R.-S.; Liu, T.-Y. Surface engineering of 3D spinel Zn3V2O8 wrapped on sulfur doped graphitic nitride composites: Investigation on the dual role of electrocatalyst for simultaneous detection of antibiotic drugs in biological fluids. Compos. Part B Eng. 2022, 242, 110017. [Google Scholar] [CrossRef]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef] [PubMed]
- Rajaji, U.; Ganesh, P.-S.; Kim, S.-Y.; Govindasamy, M.; Alshgari, R.A.; Liu, T.-Y. MoS2 Sphere/2D S-Ti3C2 MXene Nanocatalysts on Laser-Induced Graphene Electrodes for Hazardous Aristolochic Acid and Roxarsone Electrochemical Detection. ACS Appl. Nano Mater. 2022, 5, 3252–3264. [Google Scholar] [CrossRef]
- Ge, L.; Guo, C.; Li, H.; Xia, X.; Chen, L.; Ning, D.; Liu, X.; Li, F. Direct-laser-writing of electrochemiluminescent electrode on glassy carbon for iodide sensing in aqueous solution. Sens. Actuators B Chem. 2021, 337, 129766. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Z.; Dai, W.; Liu, B.; Zhang, Y.; Li, J.; Ye, J. Laser engraved nitrogen-doped graphene sensor for the simultaneous determination of Cd(II) and Pb(II). J. Electroanal. Chem. 2018, 828, 41–49. [Google Scholar] [CrossRef]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly Efficient Laser Scribed Graphene Electrodes for On-Chip Electrochemical Sensing Applications. Adv. Electron. Mater. 2016, 2, 1600185. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, R.; Zhu, X.; Wang, X.; Geng, X.; Xiong, Y.; Chen, T.; Wen, Y.; Ai, S. Intelligent analysis of maleic hydrazide using a simple electrochemical sensor coupled with machine learning. Anal. Methods 2021, 13, 4662–4673. [Google Scholar] [CrossRef]
- Li, D.; Shao, Y.; Zhang, Q.; Qu, M.; Ping, J.; Fu, Y.; Xie, J. A flexible virtual sensor array based on laser-induced graphene and MXene for detecting volatile organic compounds in human breath. Analyst 2021, 146, 5704–5713. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, L.; Wu, R.; Zhu, Y.; Sheng, Y.; Nie, P.; Liu, P.; Xu, L.; Wen, Y. Portable wireless intelligent sensing of ultra-trace phytoregulator α-naphthalene acetic acid using self-assembled phosphorene/Ti3C2-MXene nanohybrid with high ambient stability on laser induced porous graphene as nanozyme flexible electrode. Biosens. Bioelectron. 2021, 179, 113062. [Google Scholar] [CrossRef]
- Li, M.; Zhou, P.; Wang, X.; Wen, Y.; Xu, L.; Hu, J.; Huang, Z.; Li, M. Development of a simple disposable laser-induced porous graphene flexible electrode for portable wireless intelligent votammetric nanosensing of salicylic acid in agro-products. Comput. Electron. Agric. 2021, 191, 106502. [Google Scholar] [CrossRef]
- Getachew, B.A.; Bergsman, D.S.; Grossman, J.C. Laser-Induced Graphene from Polyimide and Polyethersulfone Precursors as a Sensing Electrode in Anodic Stripping Voltammetry. ACS Appl. Mater. Interfaces 2020, 12, 48511–48517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Q.; Chang, C.; Wang, R.; Wang, Q.; Shan, X. Highly efficient detection of Cd(II) ions by a stannum and cerium bimetal-modified laser-induced graphene electrode in water. Chem. Eng. J. 2022, 433, 133791. [Google Scholar] [CrossRef]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Abdullah, A.F.L.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/ porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef] [PubMed]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A machine learning-based multimodal electrochemical analytical device based on eMoSx-LIG for multiplexed detection of tyrosine and uric acid in sweat and saliva. Anal. Chim. Acta 2022, 1232, 340447. [Google Scholar] [CrossRef] [PubMed]
- Madhuvilakku, R.; Yen, Y.-K.; Yan, W.-M.; Huang, G.-W. Laser-scribed Graphene Electrodes Functionalized with Nafion/Fe3O4 Nanohybrids for the Ultrasensitive Detection of Neurotoxin Drug Clioquinol. ACS Omega 2022, 7, 15936–15950. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Nayak, P.; Hirsch, T.; Wolfbeis, O.S.; Alshareef, H.N.; Baeumner, A.J. Laser-Scribed Graphene Electrodes for Aptamer-Based Biosensing. ACS Sens. 2017, 2, 616–620. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, H.; Zhao, Y.; Wang, Y.; Li, F. Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens. Bioelectron. 2022, 199, 113906. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Li, J.; Cui, C.; Zhou, Z.; Wen, L. Surface Engineering of Laser-Induced Graphene Enables Long-Term Monitoring of On-Body Uric Acid and pH Simultaneously. Nano Lett. 2022, 22, 5451–5458. [Google Scholar] [CrossRef]
- Rossato, J.H.H.; Oliveira, M.E.; Lopes, B.V.; Gallo, B.B.; La Rosa, A.B.; Piva, E.; Barba, D.; Rosei, F.; Carreño, N.L.V.; Escote, M.T. A Flexible Electrochemical Biosensor Based on NdNiO3 Nanotubes for Ascorbic Acid Detection. ACS Appl. Nano Mater. 2022, 5, 3394–3405. [Google Scholar] [CrossRef]
- Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Ko, S.G.; Yoon, H.; Nah, J.S.; Xuan, X.; Park, J.Y. A Polyallylamine Anchored Amine-Rich Laser-Ablated Graphene Platform for Facile and Highly Selective Electrochemical IgG Biomarker Detection. Adv. Funct. Mater. 2020, 30, 1907297. [Google Scholar] [CrossRef]
- Johnson, Z.T.; Jared, N.; Peterson, J.K.; Li, J.; Smith, E.A.; Walper, S.A.; Hooe, S.L.; Breger, J.C.; Medintz, I.L.; Gomes, C.; et al. Enzymatic Laser-Induced Graphene Biosensor for Electrochemical Sensing of the Herbicide Glyphosate. Glob. Chall. 2022, 6, 2200057. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-Induced Graphene-Based Wearable Epidermal Ion-Selective Sensors for Noninvasive Multiplexed Sweat Analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Arantes, I.V.S.; Paixão, T.R.L.C. Couple batch-injection analysis and microfluidic paper-based analytical device: A simple and disposable alternative to conventional BIA apparatus. Talanta 2022, 240, 123201. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Xuan, X.; Kim, J.; Park, J.Y. A highly flexible and selective dopamine sensor based on Pt-Au nanoparticle-modified laser-induced graphene. Electrochimica Acta 2019, 328, 135066. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Huang, L.; Chen, Y.; Li, Y. A laser-induced graphene electrochemical immunosensor for label-free CEA monitoring in serum. Analyst 2021, 146, 6631–6642. [Google Scholar] [CrossRef]
- Beduk, T.; Beduk, D.; de Oliveira Filho, J.I.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; Salama, K.N.; et al. Rapid Point-of-Care COVID-19 Diagnosis with a Gold-Nanoarchitecture-Assisted Laser-Scribed Graphene Biosensor. Anal. Chem. 2021, 93, 8585–8594. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Puthongkham, P.; Wirojsaengthong, S.; Suea-Ngam, A. Machine learning and chemometrics for electrochemical sensors: Moving forward to the future of analytical chemistry. Analyst 2021, 146, 6351–6364. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021, 47, 173–184. [Google Scholar] [CrossRef]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef] [PubMed]
- Warra, A.A.; Prasad, M.N.V. African perspective of chemical usage in agriculture and horticulture—Their impact on human health and environment. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 401–436. ISBN 9780081030172. [Google Scholar]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, M.; Yu, H.; She, Y.; Cao, Z.; Ye, J.; El-Aty, A.M.A.; Hacimuftuoglu, A.; Wang, J.; Lao, S. An Overview on the Mechanisms and Applications of Enzyme Inhibition-Based Methods for Determination of Organophosphate and Carbamate Pesticides. J. Agric. Food Chem. 2020, 68, 7298–7315. [Google Scholar] [CrossRef] [PubMed]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Melchor Martínez, E.M.; Sosa-Hernández, J.E.; Bilal, M.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Highly hazardous pesticides and related pollutants: Toxicological, regulatory, and analytical aspects. Sci. Total Environ. 2022, 807, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Persson, S.; Hallén, N.; Ericsson, Å.; Thielke, D.; Lindgren, P.; Carlsson, K.S.; Jendle, J. Costs of diabetes complications: Hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia 2020, 63, 2582–2594. [Google Scholar] [CrossRef] [PubMed]
- Speth, J.D. Neanderthals, vitamin C., and scurvy. Quat. Int. 2018, 500, 172–184. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Y.; Wang, M.; Xiao, G.; Gao, F.; Li, Z.; Tao, G.; Zhuang, P.; Yue, F.; Chan, P.; et al. Real-time simultaneous recording of electrophysiological activities and dopamine overflow in the deep brain nuclei of a non-human primate with Parkinson’s disease using nano-based microelectrode arrays. Microsyst. Nanoeng. 2018, 4, 17070. [Google Scholar] [CrossRef] [Green Version]
- Alba, A.F.; Totoricaguena-Gorriño, J.; Sánchez-Ilárduya, M.B.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; del Campo, F.J. Laser-activated screen-printed carbon electrodes for enhanced dopamine determination in the presence of ascorbic and uric acid. Electrochim. Acta 2021, 399, 139374. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Farjam, M.; Iraji, A. Synthesis and biological activity of pyrimidines-containing hybrids: Focusing on pharmacological application. J. Mol. Struct. 2021, 1230, 129833. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and application of electrochemical sensor for analyzing hydrogen peroxide in food system and biological samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Gourama, H. Foodborne Pathogens. In Food Safety Engineering; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 25–49. ISBN 978-3-030-42660-6. [Google Scholar]
- Mutalik, V.K.; Adler, B.A.; Rishi, H.S.; Piya, D.; Zhong, C.; Koskella, B.; Kutter, E.M.; Calendar, R.; Novichkov, P.S.; Price, M.N.; et al. High-throughput mapping of the phage resistance landscape in E. coli. PLoS Biol. 2020, 18, e3000877. [Google Scholar] [CrossRef]







| Electrode | Substrate Material | Laser Source | Laser Wavelength | Laser Power | Scan Rate | z-Distance | Ambient Condition | Electrochemical Application | Reference |
|---|---|---|---|---|---|---|---|---|---|
| NP-LIG or Cu2+/NP-LIG | NP | NA | 405 nm | 3 W | NA | 60 mm | air | Chemical sensing | [5] |
| LSG | PEI | CO2 | NA | 10.5% | 40 mm s−1 | 10 mm | NA | Chemical sensing | [13] |
| LIG | PI | CO2 | NA | 5.25 W | 271.9 mm s−1 | 2 mm | NA | Chemical sensing | [14] |
| LIG | PI | CO2 | NA | 4% | 7% | 74 mm | air | Biochemical sensing | [15] |
| ePAD | GRA | CO2 | 10.6 µm | 500 mW | 25 mm s−1 | 10 mm | air | Chemical sensing | [17] |
| ACE2-AuNP/LSG | PI | CO2 | 10.6 µm | 3.2 W | 2.8 cm s−1 | 2.5 mm | N2 gas | Biochemical sensing | [19] |
| LSG/MIP | PI | CO2 | 10.6 µm | 3.2 W | 2.8 cm s−1 | 2.5 mm | air | Chemical sensing | [32] |
| N-LIG | PI | CO2 | 10.6 µm | 32 W | 108 cm s−1 | NA | air | Biochemical sensing | [33] |
| Enzyme/Ti3C2Tx/PB/N-LSG | Lign/PVA/UR | CO2 | 10.6 µm | 4.8% | 3% | 2.4 mm | air | Biochemical sensing | [34] |
| LIG@Ag | PI | NA | NA | 4.2 W | 50 mm s−1 | 10 mm | NA | Chemical sensing | [35] |
| LSG | PI | CO2 | 10.6 µm | 3.2 W | 2.8 cm s−1 | 2.5 mm | Inert gas | Biochemical sensing | [36] |
| 3D-printed CB/PLA | CB/PLA | CO2 | 10.6 µm | 350 mW | 20 mm s−1 | 10 mm | NA | Chemical sensing | [37] |
| LS-ET-PR | PR | CO2 | 10.6 µm | 2.1 W | 8.5 mm s−1 | 12.0 mm | air | Chemical sensing | [38] |
| PCE | PR | CO2 | 10.6 µm | 12 W | 300 mm s−1 | NA | NA | Chemical sensing | [41] |
| PFA/GO | PFA/GO | CO2 | NA | 3.88 W | 100 mm s−1 | NA | NA | Chemical sensing | [42] |
| N, O doped LIG | PI | NA | 355 nm | 2.5 W | 30 mm s−1 | NA | air | Chemical sensing | [43] |
| LSG/f-MWCNT-AuNPs | PI | NA | 405 nm | 2W | 50 mm s−1 | NA | air | Chemical sensing | [44] |
| LSG | PI | CO2 | 10.6 µm | 1.65 W | 3.5 mm s−1 | NA | air | Chemical sensing | [45] |
| Fe3O4/MWCNTS/LSG/CS/GCE | PI | NA | NA | 40 W | 100 mm s−1 | 0.1 mm | air | Chemical sensing | [46] |
| LSG | PI | NA | 450 nm | 5.23 W | 5.5 mm s−1 | NA | air | Chemical sensing | [47] |
| h-BN/LIPG0 | PI | NA | 450 nm | NA | NA | NA | air | Chemical sensing | [48] |
| LEGCN | PI | CO2 | NA | 6.4 W | 50 mm s−1 | NA | NA | Chemical sensing | [49] |
| ZVO/SGN-LGE | PI | CO2 | 10.6 µm | 3.2 W | 2.8 cm s−1 | 2.5 mm | NA | Chemical sensing | [50] |
| AuNPs-LIG | PI | NA | NA | 2.20–3.85 W | 1–3 cm s−1 | NA | air | Biochemical sensing | [51] |
| MoS2/S-Ti3C2/LGE | PI | CO2 | 10.6 µm | 3.2 W | 2.8 cm s−1 | 2.5 mm | NA | Biochemical sensing | [52] |
| LI-CdS-G@GC | GCE | CO2 | 10.6 µm | 6.0 W | 166 mm s−1 | NA | air | Chemical sensing | [53] |
| N@LEG/GCE | PI | CO2 | NA | 5 W | 50 mm s−1 | NA | air | Chemical sensing | [54] |
| Pt/LSG | PI | CO2 | 10.6 µm | 15% | 10% | 2 mm | air | Chemical sensing | [55] |
| PEDOT-LSG | PI | CO2 | 10.6 µm | 4.6 W | 0.56 cm s−1 | 2 mm | air | Chemical sensing | [56] |
| LIPG | PI | NA | NA | 5.2 W | 0.4 cm s−1 | NA | NA | Chemical sensing | [57] |
| Ti3C2Tx-LIG-IDE | PI | NA | NA | 3.85 W | 2 cm s−1 | NA | NA | Chemical sensing | [58] |
| Ti3C2-MXene/BP/LIPG | PI | NA | NA | 5.2W | NA | 10 mm | air | Chemical sensing | [59] |
| LIPG | PI | NA | NA | 5.5 W | 0.5 cm s−1 | NA | NA | Chemical sensing | [60] |
| PI-LIG | PI | NA | 10.6 µm | 50% | 50% | NA | NA | Chemical sensing | [61] |
| SnO2/CeO2/LIG | PI | NA | NA | NA | NA | NA | NA | Chemical sensing | [62] |
| LIG–CS–AuNPs | PI | CO2 | 10.6 µm | 1.8 W | 160 mm s−1 | 6 mm | air | Chemical sensing | [63] |
| eMoSx-LIG | PI | CO2 | NA | 10.5% | 5.5% | NA | NA | Biochemical sensing | [64] |
| Nafion/Fe3O4/LSG | PI | NA | 10.6 µm | 5.4 W | NA | 5 mm | NA | Chemical sensing | [65] |
| LSG | PI | NA | 10.6 μm | 0.81 W | 5.8 cm·s−1 | 2 mm | air | Biochemical sensing | [66] |
| AuNPs-LIG | PI | CO2 | NA | 8% | NA | NA | NA | Biochemical sensing | [67] |
| Co3O4 NPs-LIG | PI | CO2 | NA | NA | NA | NA | NA | Chemical sensing | [68] |
| Chit-Au-LIG | PI | CO2 | NA | 5% | NA | 6 mm | air | Biochemical sensing | [69] |
| DLEG | PI | CO2 | NA | 3.9 W | NA | 5 mm | air | Chemical sensing | [70] |
| PtNPs/PAAMI/LAG | PI | CO2 | NA | 17 W | 60 mm s−1 | NA | air | Biochemical sensing | [71] |
| Pt-LIG | PI | CO2 | NA | 7% | 7% | 2 mm | NA | Biochemical sensing | [72] |
| LIG | PI | CO2 | NA | 60% | 15% | NA | NA | Biochemical sensing | [73] |
| μPAD | CP | CO2 | 10.6 μm | 6.5% | 12 mm s− 1 | 12 mm | air | Chemical sensing | [74] |
| Pt-AuNPs/LIG/PDMS | PI/PDMS | CO2 | NA | 7.6 W | 200 mm/s | NA | NA | Chemical sensing | [75] |
| Electrode | Analyte | Technique | LOD | Dynamic Range | Machine Learning Model | Reference |
|---|---|---|---|---|---|---|
| NP-LIG or Cu2+/NP-LIG | Nit and GLU | Nit: DPV GLU: AMP | 0.9 μM | 2.0–1000 μM | NA | [5] |
| PEI-LSG | HQ, PA, MP | SWV | HQ: 9.42 × 10−8 mol L−1 PA: 3.23 × 10−7 mol L−1 MP: 2.95 × 10−7 mol L−1 | HQ, PA, MP: 10–50 µ mol L−1 | NA | [13] |
| LIG | CLO, IMD, TMX, DNT | SWV | CLO: 823 nM IMD: 384 nM TMX: 338 nM DNT: 682 nM | 10−40 μM | NA | [14] |
| LIG | S. enterica | EIS | 13 ± 7 CFU mL−1 | 25–105 CFU mL−1 | NA | [15] |
| ePADs | FUR | DPV | 2.4 × 10−7 M | 25–196 mM | NA | [17] |
| ACE2-AuNP/LSG | S1 and S2 | DPV | S1: 5.14 ng/mL S2: 2.09 ng/mL | 1.0–200 ng/mL | DNN | [19] |
| MIP/PPy@LSG | BPA | DPV | 8 nM | 0.5–5 mM | NA | [32] |
| N- LIG | miRNA | DPV | 10 fM | 10 fM–10 nM | NA | [33] |
| Enzyme/Ti3C2Tx/PB/N-LSG | LAC ALC GLU | AMP | LAC:0.5 μM ALC: NA GLU: 0.3 μM | 0–20 mM 0–50 mM 10 μM–5.3 mM | NA | [34] |
| AgNPs-LIG | H2O2 | AMP | 2.8 μM | 0.01–2.61 mM | NA | [35] |
| LSG | PAR | SWV | 31 nM | 0.1 μM–10 μM | NA | [36] |
| 3D-printed CB/PLA | Cd2+, Pb2+, Cu2+ | SWASV | Cd2+: 1.34 µg L−1, Pb2+: 1.32 µg L−1, Cu2+: 0.31 µg L−1 | Cd2+, Pb2+, Cu2+: 25–125 µg L−1 | NA | [37] |
| LSG | β-ED | DPV | 12.1 mM | 0.1–1 mM | NA | [38] |
| PCE | Nit DA | CV | Nit: 0.173 mM DA: 0.136 mM | Nit: 0.2–1 mM DA: 0.2–1 mM | NA | [41] |
| PFA/GO | AA | AMP | 1.0 μmol cm2 L−1 | 50–5000 μmol L−1 | NA | [42] |
| N, O doped LIG | Nit | DPV | 0.8 μmol/L | 5–450 μmol/L | NA | [43] |
| LIG/f-MWCNT-AuNPs | NO2− | SWV | 0.9 µM | 10–140 µM | NA | [44] |
| LSG | HYD | CV | 70 µM | 0.1–0.5 mM | NA | [45] |
| Fe3O4/MWCNTS/LSG/CS/GCE | Cd2+ Pb2+ | SWASV | Cd2+: 0.1 μg L−1 Pb2+: 0.07 μg L−1 | 1–200 μg L−1 | NA | [46] |
| LSG | XT HX | DPV | XT: 0.26 μMHX: 0.18 μM | XT: 0.3–179.9 μM HX: 0.3–159.9 μM | ANN | [47] |
| h-BN/LIPG0 | SMZ | DPV | 0.011 µM | 0.5–362.5 µM | NA | [48] |
| LEGCN | Cd2+ Pb2+ | SWASV | Cd2+: 0.47 μg L−1 Pb2+: 0.41 μg L−1 | Cd2+: 7–120 μg L−1 Pb2+: 5–120 μg L−1 | NA | [49] |
| ZVO/SGN-LGE | NFT CAP | DPV | NFT: 2.4 nM CAP: 1.5 nM | NFT: 0.005–325.5 μM CAP: 0.005–187.5 μM | NA | [50] |
| AuNPs-LIG | E. coli 0157:H7 | EIS | 1 × 102 CFU mL−1 | 1 × 102–1 × 108 CFU mL−1 | NA | [51] |
| MoS2/S-Ti3C2/LGE | ARA ROX | DPV | ARA: 1.65 nM ROX: 2.31 nM | 0.01−875.01 μM | NA | [52] |
| LI-CdS-G@GC | I− | ECL | 4.0 nM | 10.0–2500 nM | NA | [53] |
| N@LEG/GCE | Cd2+ Pb2+ | SWASV | Cd2+: 1.08 μgL−1 Pb2+: 0.16 μgL−1 | Cd2+: 5–10 μg L−1 and 10–380 μg L−1 Pb2+: 0.5–10 μgL−1 and 10–380 μgL−1 | NA | [54] |
| Pt/LSG | AA DA UA | DPV | AA: 6.1 × 10−6 M DA: 0.07 × 10−6 M UA: 0.22 × 10−6 M | AA: 10–890 × 10−6 M DA: 0.5–56 × 10−6 M UA: 1–63 × 10−6 M | NA | [55] |
| PEDOT-LSG | DA | DPV | 0.33 µM | 1–150 µM | NA | [56] |
| LIPG | MH | DPV | NA | NA | LSSVM | [57] |
| Ti3C2Tx-LIG-IDE | MeOH, EtOH, IPA, ACET | EIS | NA | 100–800 ppm | PCA, LDA and PLS | [58] |
| Ti3C2-MXene/BP/LIPG | NAA | LSV | 1.6 nM | 0.02–40 μM | ANN | [59] |
| LIPG | SA | LSV | 0.16 μM | 0.5 μM–500 μM | LSSVM | [60] |
| PI-LIG | Pb(II) | ASV | 50 ppb | NA | NA | [61] |
| SnO2/CeO2/LIG | Cd(II) | DPASV | 0.01 μg/L | 0.1–160 μg/L | NA | [62] |
| LIG–CS–AuNPs | UA | DPAdSV | 0.33 μmol L−1 | 1.0–30 μmol L−1 and 30–100 μmol L−1 | NA | [63] |
| eMoSx-LIG | UA and TYR | DPV | TYR: 100 nM UA: 10 nM | NA | DT | [64] |
| Nafion/Fe3O4/LSG | CQL | DPV | 0.73 nmol L−1 | 1 nmol L−1–100 mol L−1 | NA | [65] |
| LSG | TRB | DPV | 1 pmol L−1 | 1–100 pmol L−1 | NA | [66] |
| AuNPs-LIG | PRX | DPV | 1.2 ng mL−1 | 3–4000 ng mL−1 | NA | [67] |
| Co3O4 NPs-LIG | GLU | AMP | 0.41 μM | 1 μM–9 mM | NA | [68] |
| Chit-Au-LIG | UA | DPV UA | 0.5 M | NA | NA | [69] |
| DLEG | AA | CV | 3.8 mol L−1 | 30–1100 mol L−1 | NA | [70] |
| PtNPs/PAAMI/LAG | IgG | DPV | 6 pg mL−1 | 0.012–352 ng mL−1 | NA | [71] |
| Pt-LIG | Gly | AMP | 3.03 μM | 10–260 μM | NA | [72] |
| LIG | pH, Na+, and K+ | OCP | NA | pH: 4–7 Na+: 0.1–100 mM K+: 0.1–100 mM | NA | [73] |
| μPAD | PAR | AMP | 0.046–0.154 mmol L−1 | 50–5000 μmol L−1 | NA | [74] |
| Pt-AuNPs/LIG/PDMS | DA | DPV | 75 nM | 9.5 × 10−7–3 × 10−5 M | NA | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameku, W.A.; Negahdary, M.; Lima, I.S.; Santos, B.G.; Oliveira, T.G.; Paixão, T.R.L.C.; Angnes, L. Laser-Scribed Graphene-Based Electrochemical Sensors: A Review. Chemosensors 2022, 10, 505. https://doi.org/10.3390/chemosensors10120505
Ameku WA, Negahdary M, Lima IS, Santos BG, Oliveira TG, Paixão TRLC, Angnes L. Laser-Scribed Graphene-Based Electrochemical Sensors: A Review. Chemosensors. 2022; 10(12):505. https://doi.org/10.3390/chemosensors10120505
Chicago/Turabian StyleAmeku, Wilson A., Masoud Negahdary, Irlan S. Lima, Berlane G. Santos, Thawan G. Oliveira, Thiago R. L. C. Paixão, and Lúcio Angnes. 2022. "Laser-Scribed Graphene-Based Electrochemical Sensors: A Review" Chemosensors 10, no. 12: 505. https://doi.org/10.3390/chemosensors10120505
APA StyleAmeku, W. A., Negahdary, M., Lima, I. S., Santos, B. G., Oliveira, T. G., Paixão, T. R. L. C., & Angnes, L. (2022). Laser-Scribed Graphene-Based Electrochemical Sensors: A Review. Chemosensors, 10(12), 505. https://doi.org/10.3390/chemosensors10120505









