Au–Ru Composite for Enzyme-Free Epinephrine Sensing
Abstract
:1. Introduction
2. Materials and Methods
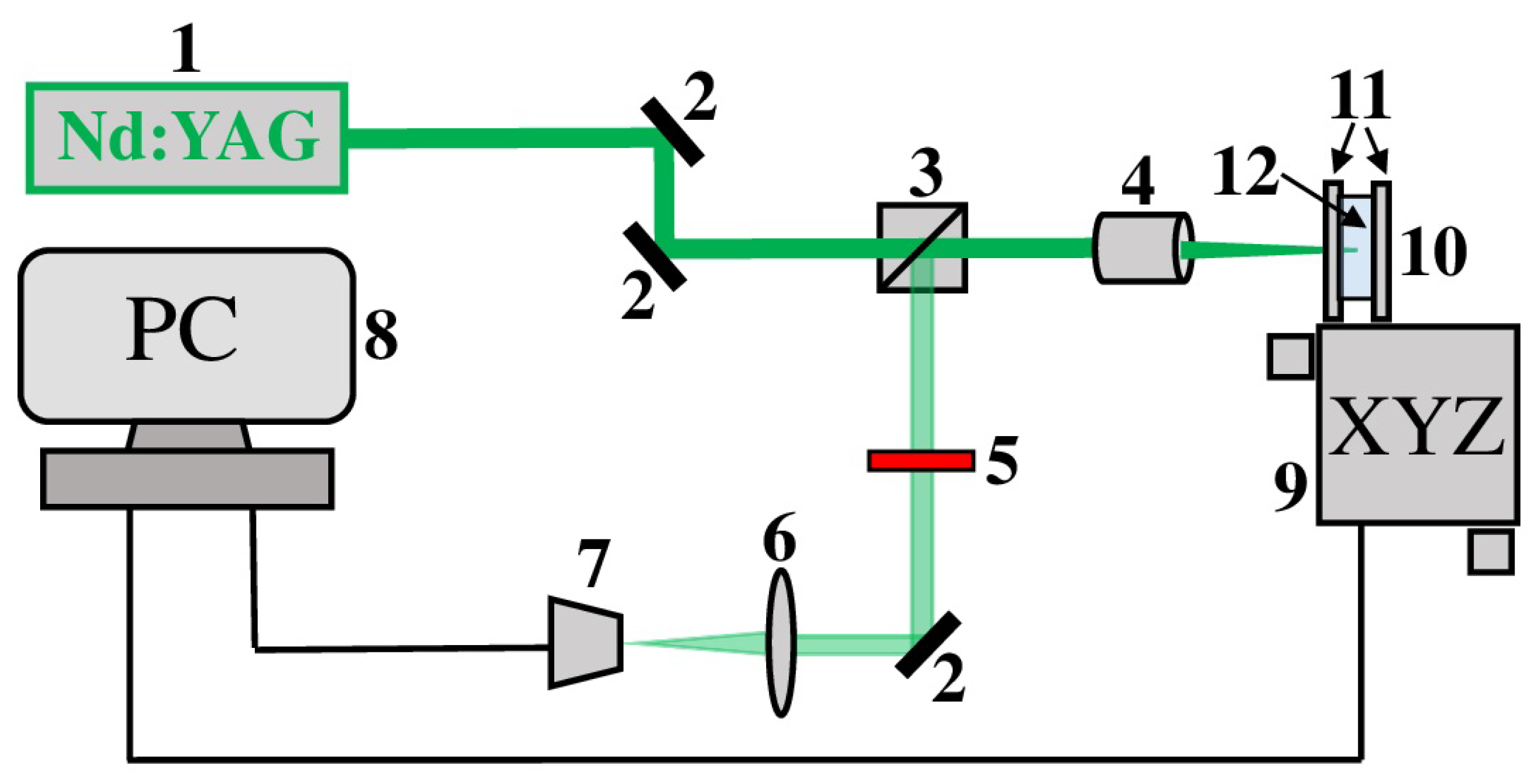
2.1. Laser-Assisted Synthesis of Au–Ru Composite
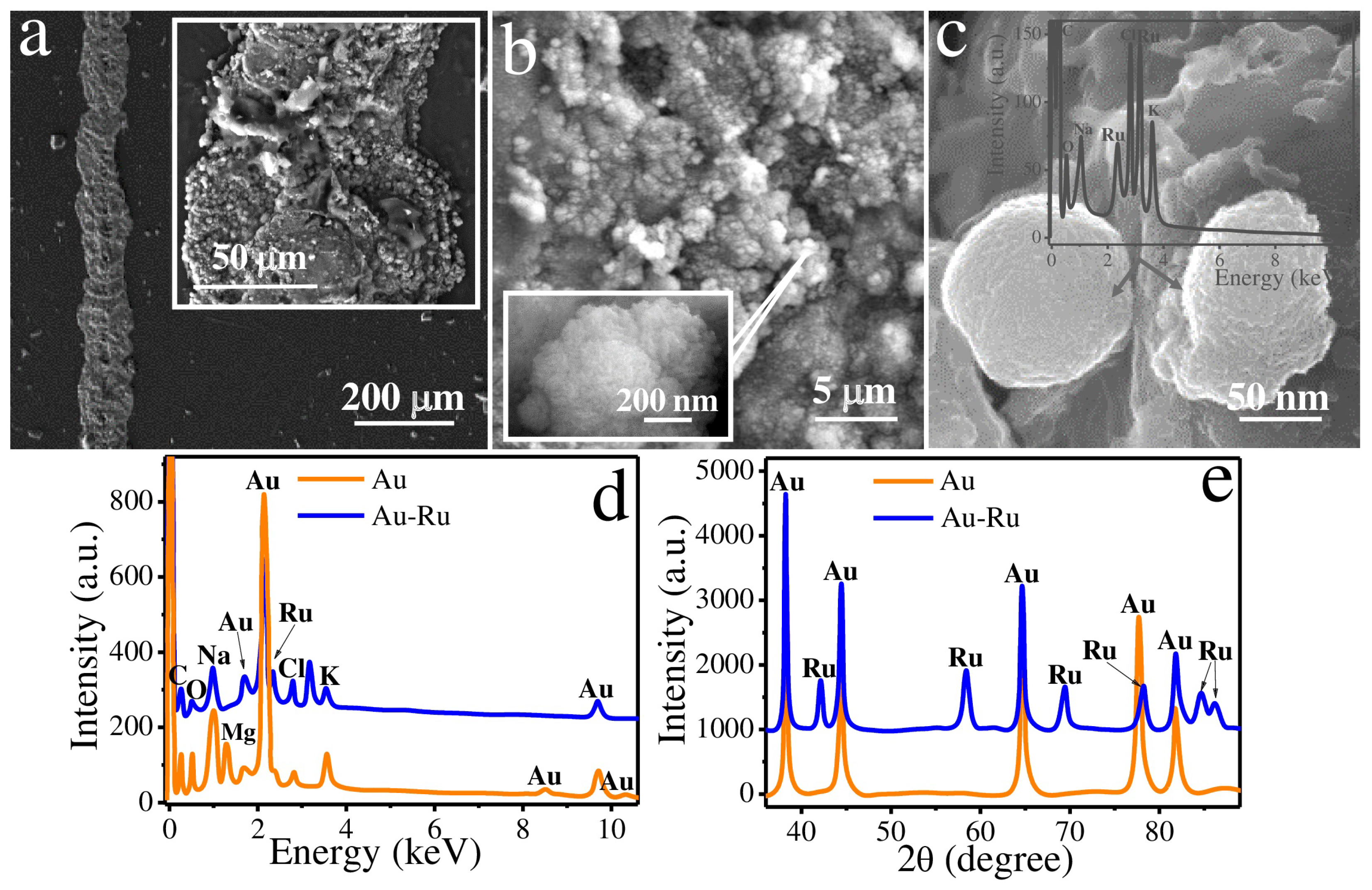
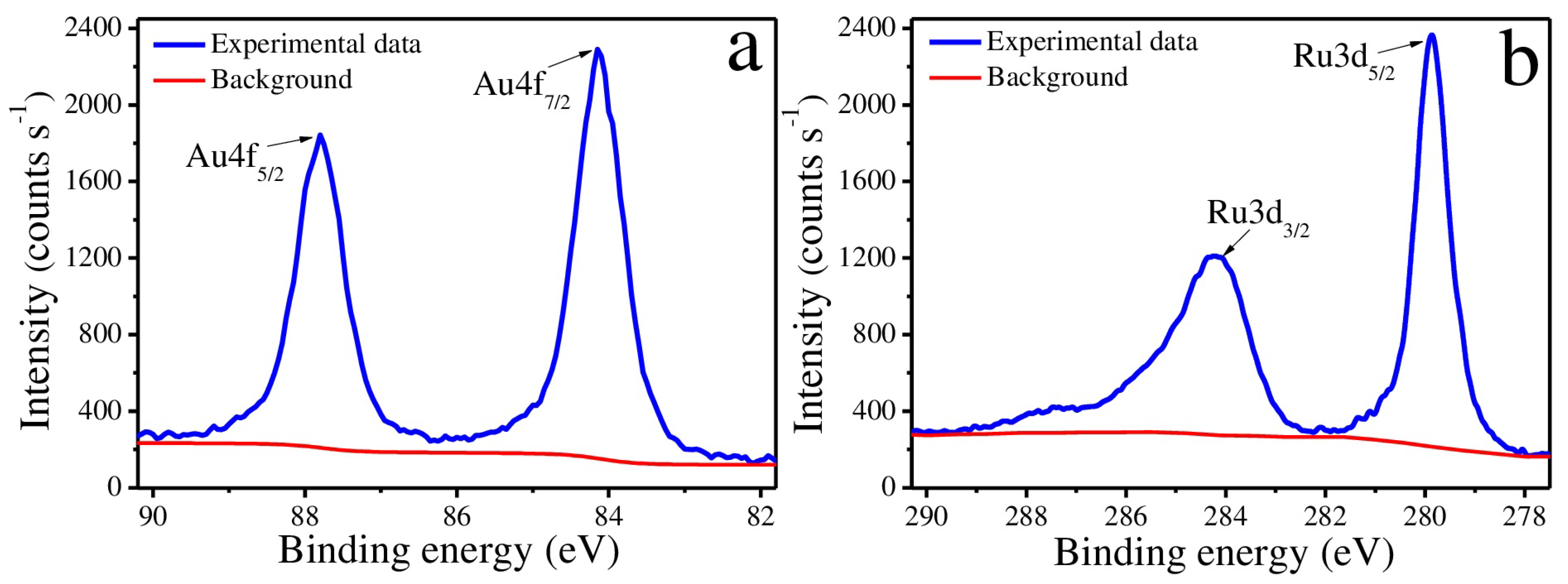
2.2. Morphology and Composition Analysis
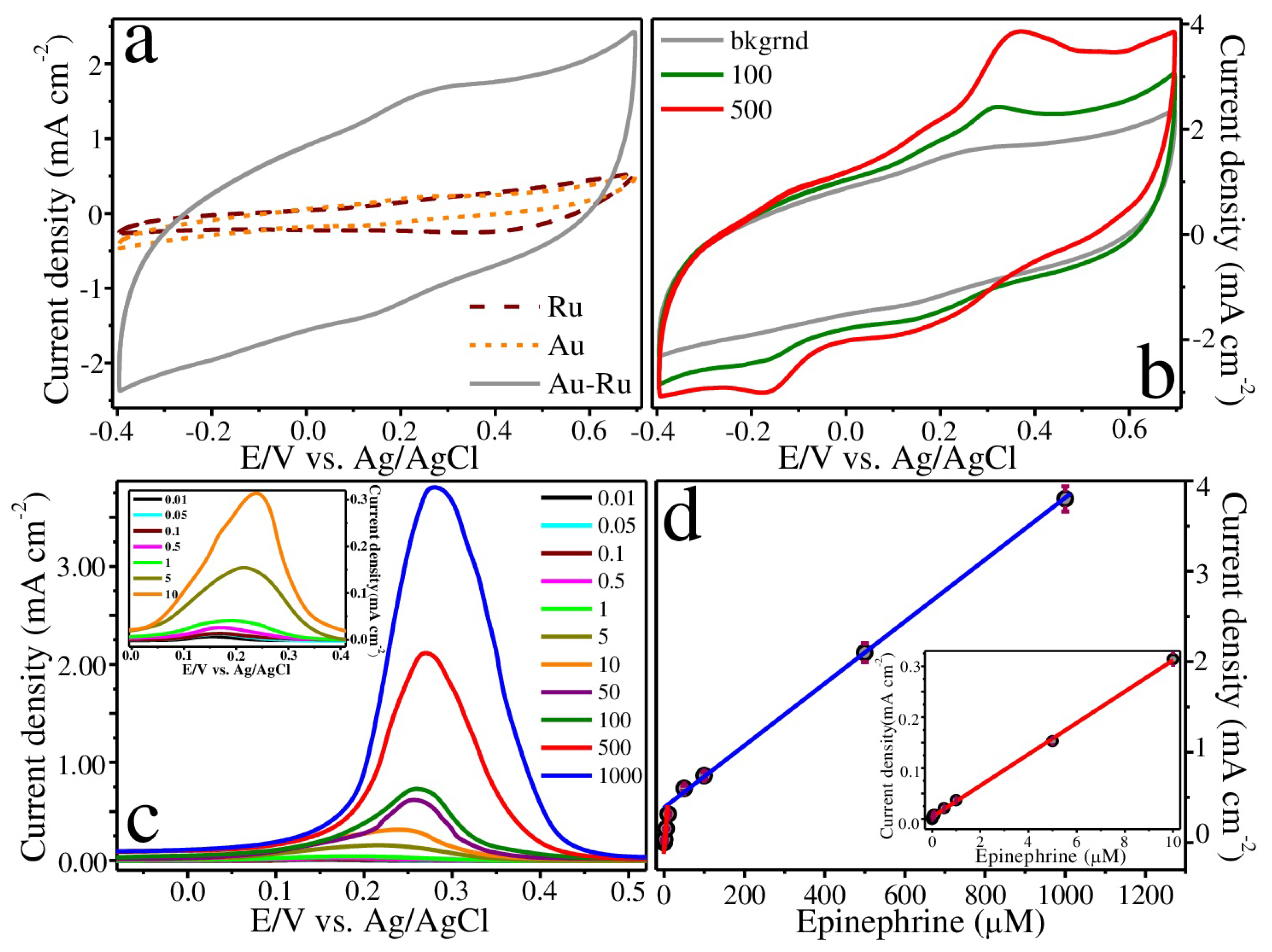
2.3. Electrochemical Studies
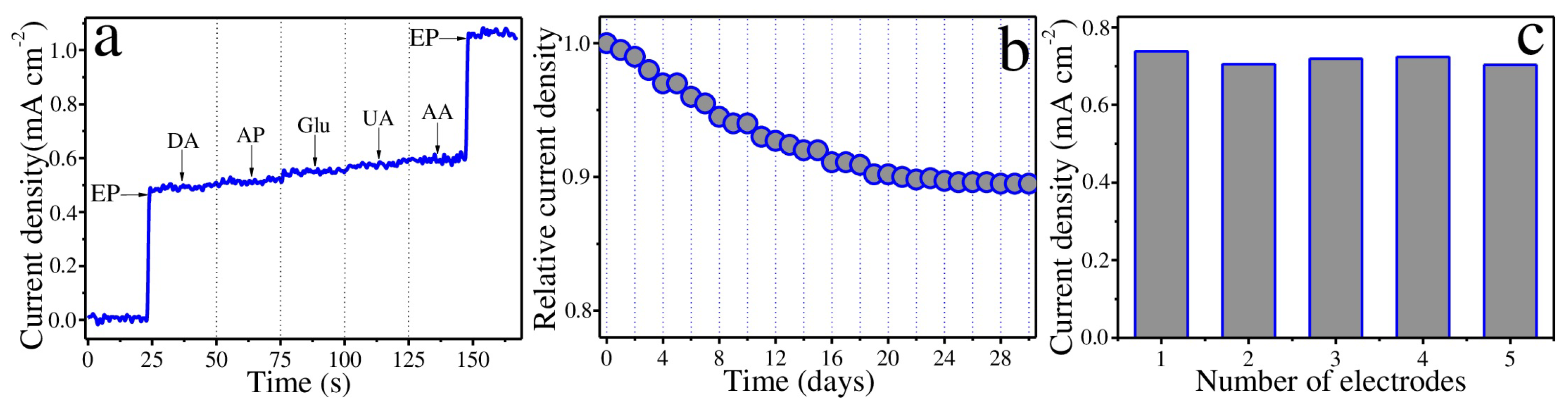
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Veerakumar, P.; Hung, S.T.; Hung, P.Q.; Lin, K.C. Review of the design of ruthenium-based nanomaterials and their sensing applications in electrochemistry. J. Agric. Food Chem. 2022, 70, 8523–8550. [Google Scholar] [CrossRef]
- Panahi, Z.; Custer, L.; Halpern, J.M. Recent advances in non-enzymatic electrochemical detection of hydrophobic metabolites in biofluids. Sens. Actuators Rep. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Sumathi, C.; Wilson, J.; Ravi, G. Enzymeless biosensor based on β-NiS@ rGO/Au nanocomposites for simultaneous detection of ascorbic acid, epinephrine and uric acid. RSC Adv. 2016, 6, 96467–96478. [Google Scholar] [CrossRef]
- Anithaa, A.; Asokan, K.; Sekar, C. Voltammetric determination of epinephrine and xanthine based on sodium dodecyl sulphate assisted tungsten trioxide nanoparticles. Electrochim. Acta 2017, 237, 44–53. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K.; Ghiasi, M. All-electrochemical synthesis of a three-dimensional mesoporous polymeric GC 3 N 4/PANI/CdO nanocomposite and its application as a novel sensor for the simultaneous determination of epinephrine, paracetamol, mefenamic acid, and ciprofloxacin. New J. Chem. 2020, 44, 3412–3424. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Szultka-Młyńska, M.; Buszewski, B.; Sulka, G.D. Epinephrine sensing at nanostructured Au electrode and determination its oxidative metabolism. Sens. Actuators Chem. 2016, 237, 206–215. [Google Scholar] [CrossRef]
- Gorczyński, A.; Kubicki, M.; Szymkowiak, K.; Łuczak, T.; Patroniak, V. Utilization of a new gold/Schiff-base iron (III) complex composite as a highly sensitive voltammetric sensor for determination of epinephrine in the presence of ascorbic acid. RSC Adv. 2016, 6, 101888–101899. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, L.; Zhang, X.; Wang, X.; Ji, W.; Zhao, J.; Ozaki, Y. CTAB-triggered Ag aggregates for reproducible SERS analysis of urinary polycyclic aromatic hydrocarbon metabolites. Chem. Commun. 2019, 55, 2146–2149. [Google Scholar] [CrossRef]
- Li, W.; Wu, F.; Dai, Y.; Zhang, J.; Ni, B.; Wang, J. Poly (octadecyl methacrylate-co-trimethylolpropane trimethacrylate) monolithic column for hydrophobic in-tube solid-phase microextraction of chlorophenoxy acid herbicides. Molecules 2019, 24, 1678. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Revathi, C. Enzymatic and nonenzymatic electrochemical biosensors. In Fundamentals and Sensing Applications of 2D Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–300. [Google Scholar]
- Florescu, M.; David, M. Tyrosinase-based biosensors for selective dopamine detection. Sensors 2017, 17, 1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: A bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.Y.; Zhang, H.J.; Huang, S.; Gao, X.; Song, S.S.; Wang, Z.; Wang, W.Q.; Guan, E.H. A novel non-enzymatic dopamine sensors based on NiO-reduced graphene oxide hybrid nanosheets. J. Mater. Sci. Mater. Electron. 2019, 30, 5000–5007. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Jiao, J.; Chen, Y.; Han, B.; Chen, Q. Synthesis of tetrahexahedral Au-Pd core–shell nanocrystals and reduction of graphene oxide for the electrochemical detection of epinephrine. J. Colloid Interface Sci. 2018, 512, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, C.; Wang, F.; Liao, M.; Jiang, G. Bimetallic nanoparticles decorated hollow nanoporous carbon framework as nanozyme biosensor for highly sensitive electrochemical sensing of uric acid. Biosens. Bioelectron. 2020, 150, 111869. [Google Scholar] [CrossRef]
- Tooley, C.A.; Gasperoni, C.H.; Marnoto, S.; Halpern, J.M. Evaluation of metal oxide surface catalysts for the electrochemical activation of amino acids. Sensors 2018, 18, 3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Sutradhar, S.C.; Lei, J.; Kim, J.; Kim, D.H.; Lee, Y.H.; Kim, W. Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens. Bioelectron. 2019, 126, 381–388. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Recent developments on graphene-based electrochemical sensors toward nitrite. J. Electrochem. Soc. 2019, 166, B881. [Google Scholar] [CrossRef]
- Arvand, M.; Khoshkholgh, Z.; Hemmati, S. Trace level detection of guanine and adenine and evaluation of damage to DNA using electro-synthesised ZnS@ CdS core-shell quantum dots decorated graphene oxide nanocomposite. J. Electroanal. Chem. 2018, 817, 149–159. [Google Scholar] [CrossRef]
- Li, G. Direct laser writing of graphene electrodes. J. Appl. Phys. 2020, 127, 010901. [Google Scholar] [CrossRef] [Green Version]
- Mizoshiri, M.; Nishitani, K.; Hata, S. Effect of heat accumulation on femtosecond laser reductive sintering of mixed CuO/NiO nanoparticles. Micromachines 2018, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Hsu, C.C.; Chang, T.L.; Kuo, L.S.; Chen, P.H. Study on wetting properties of periodical nanopatterns by a combinative technique of photolithography and laser interference lithography. Appl. Surf. Sci. 2010, 256, 3683–3687. [Google Scholar] [CrossRef]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J. Colloid Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Gao, J.; Shao, C.; Shao, S.; Bai, C.; Khalil, U.R.; Zhao, Y.; Jiang, L.; Qu, L. Laser-assisted multiscale fabrication of configuration-editable supercapacitors with high energy density. ACS Nano 2019, 13, 7463–7470. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Panov, M.S.; Logunov, L.S.; Tumkin, I.I.; Gordeychuk, D.I.; Kochemirovsky, V.A. Aluminum chloride reveals the catalytic activity towards laser-induced deposition of copper from water-based solutions. Int. J. Electrochem. Sci. 2015, 10, 6084–6091. [Google Scholar]
- Panov, M.S.; Khairullina, E.M.; Vshivtcev, F.S.; Ryazantsev, M.N.; Tumkin, I.I. Laser-induced synthesis of composite materials based on iridium, gold and platinum for non-enzymatic glucose sensing. Materials 2020, 13, 3359. [Google Scholar] [CrossRef]
- Baranauskaite, V.E.; Novomlinskii, M.O.; Tumkin, I.I.; Khairullina, E.M.; Mereshchenko, A.S.; Balova, I.A.; Panov, M.S.; Kochemirovsky, V.A. In situ laser-induced synthesis of gas sensing microcomposites based on molybdenum and its oxides. Compos. Part Eng. 2019, 157, 322–330. [Google Scholar] [CrossRef]
- Panov, M.S.; Vereshchagina, O.A.; Ermakov, S.S.; Tumkin, I.I.; Khairullina, E.M.; Skripkin, M.Y.; Mereshchenko, A.S.; Ryazantsev, M.N.; Kochemirovsky, V.A. Non-enzymatic sensors based on in situ laser-induced synthesis of copper-gold and gold nano-sized microstructures. Talanta 2017, 167, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Panov, M.S.; Grishankina, A.E.; Stupin, D.D.; Lihachev, A.I.; Mironov, V.N.; Strashkov, D.M.; Khairullina, E.M.; Tumkin, I.I.; Ryazantsev, M.N. In situ laser-induced fabrication of a ruthenium-based microelectrode for non-enzymatic dopamine sensing. Materials 2020, 13, 5385. [Google Scholar] [CrossRef] [PubMed]
- Stupin, D.D.; Abelit, A.A.; Mereshchenko, A.S.; Panov, M.S.; Ryazantsev, M.N. Copper–Ruthenium Composite as Perspective Material for Bioelectrodes: Laser-Assisted Synthesis, Biocompatibility Study, and an Impedance-Based Cellular Biosensor as Proof of Concept. Biosensors 2022, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Kochemirovsky, V.A.; Skripkin, M.Y.; Tveryanovich, Y.S.; Mereshchenko, A.S.; Gorbunov, A.O.; Panov, M.S.; Tumkin, I.I.; Safonov, S.V. Laser-induced copper deposition from aqueous and aqueous–organic solutions: State of the art and prospects of research. Russ. Chem. Rev. 2015, 84, 1059. [Google Scholar] [CrossRef]
- Khan, M.; Bouet, G.; Vierling, F.; Meullemeestre, J.; Schwing, M.J. Formation of cobalt (II), nickel (II) and copper (II) chloro complexes in alcohols and the Irving-Williams order of stabilities. Transit. Met. Chem. 1996, 21, 231–234. [Google Scholar] [CrossRef]
- Ryazantsev, M.N.; Jamal, A.; Maeda, S.; Morokuma, K. Global investigation of potential energy surfaces for the pyrolysis of C 1–C 3 hydrocarbons: Toward the development of detailed kinetic models from first principles. Phys. Chem. Chem. Phys. 2015, 17, 27789–27805. [Google Scholar] [CrossRef]
- Glebov, E.; Plyusnin, V.; Grivin, V.; Krupoder, S.; Liskovskaya, T.; Danilovich, V. Photochemistry of copper (II) polyfluorocarboxylates and copper (II) acetate as their hydrocarbon analogues. J. Photochem. Photobiol. Chem. 2000, 133, 177–183. [Google Scholar] [CrossRef]
- Parker, D.S.; Dangi, B.B.; Kaiser, R.I.; Jamal, A.; Ryazantsev, M.; Morokuma, K. Formation of 6-Methyl-1, 4-dihydronaphthalene in the Reaction of the p-Tolyl Radical with 1, 3-Butadiene under Single-Collision Conditions. J. Phys. Chem. 2014, 118, 12111–12119. [Google Scholar] [CrossRef]
- Bag, A.; Ghorai, P.K. Computational investigation of the ligand field effect to improve the photoacoustic properties of organometallic carbonyl clusters. RSC Adv. 2015, 5, 31575–31583. [Google Scholar] [CrossRef]
- El-Khoury, P.Z.; Tarnovsky, A.N.; Schapiro, I.; Ryazantsev, M.N.; Olivucci, M. Structure of the photochemical reaction path populated via promotion of CF2I2 into its first excited state. J. Phys. Chem. 2009, 113, 10767–10771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaletto, M.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A. XPS study of supported gold catalysts: The role of Au0 and Au+ δ species as active sites. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surfaces, Interfaces Thin Film. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Park, K.; Doub, J.; Gougousi, T.; Parsons, G. Microcontact patterning of ruthenium gate electrodes by selective area atomic layer deposition. Appl. Phys. Lett. 2005, 86, 051903. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Huang, P.; Wang, H.; Zhang, L.; Zhao, Y. Electrochemical behavior and determination of epinephrine at a penicillamine self-assembled gold electrode. Int. J. Electrochem. Sci. 2006, 1, 238–249. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Sulka, G.D. Nanoporous spongelike Au–Ag films for electrochemical epinephrine sensing. J. Electroanal. Chem. 2016, 762, 43–50. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, H.; Zhen, Q.; Xin, J.; Tan, L.; Zhang, C.; Wang, X.; Xiao, B. Hierarchical flower-like zinc oxide nanosheets in-situ growth on three-dimensional ferrocene-functionalized graphene framework for sensitive determination of epinephrine and its oxidation derivative. Appl. Surf. Sci. 2020, 526, 146721. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical biosensing of dopamine neurotransmitter: A review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Taei, M.; Hadadzadeh, H.; Hasanpour, F.; Tavakkoli, N.; Dolatabadi, M.H. Simultaneous electrochemical determination of ascorbic acid, epinephrine, and uric acid using a polymer film-modified electrode based on Au nanoparticles/poly (3,3’,5,5’-tetrabromo-m-cresolsulfonphthalein). Ionics 2015, 21, 3267–3278. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Jiang, W.; Liang, J.; Sun, Y.; Zhang, Y.; Wu, Q.; Zhang, G.; Song, X.M. Poly (ionic liquid) functionalized polypyrrole nanotubes supported gold nanoparticles: An efficient electrochemical sensor to detect epinephrine. Mater. Sci. Eng. 2017, 75, 495–502. [Google Scholar] [CrossRef] [PubMed]
| Electrode Material | Reagent, (mM) | Concentration, (mM) |
|---|---|---|
| Au | chloro(triphenylphosphine)gold(I) [(C6H5)3P]AuCl | 3 |
| Ru | triruthenium dodecacarbonyl Ru3(CO)12 | 5 |
| Electrode Material | Linear Range (M) | LOD (M) | Refs. |
|---|---|---|---|
| Au–Ru composite | 0.01–10 and 10–1000 | 0.009 and 0.02 | This work |
| Au nanotube | 10–150 | 2.8 | [7] |
| Au–Pd core-shell nanocrystals | 0.001–1000 | 0.0012 | [16] |
| Pen/Au electrode | 10–100 and 0.5–1 | 0.1 | [44] |
| Nanoporous spongelike Au–Ag films | 25–700 | 5.05 | [45] |
| Poly(BCG)AuNp/GCE | 4–903 | 0.01 | [48] |
| PPY/ZnO/AuNp/ RGO/GCE | 0.6–500 | 0.06 | [49] |
| Au-PILs-PPyNTs | 35–960 | 0.2989 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panov, M.S.; Zakharov, A.P.; Khairullina, E.M.; Tumkin, I.I.; Mereshchenko, A.S.; Nikolaev, D.M.; Vasin, A.V.; Ryazantsev, M.N. Au–Ru Composite for Enzyme-Free Epinephrine Sensing. Chemosensors 2022, 10, 513. https://doi.org/10.3390/chemosensors10120513
Panov MS, Zakharov AP, Khairullina EM, Tumkin II, Mereshchenko AS, Nikolaev DM, Vasin AV, Ryazantsev MN. Au–Ru Composite for Enzyme-Free Epinephrine Sensing. Chemosensors. 2022; 10(12):513. https://doi.org/10.3390/chemosensors10120513
Chicago/Turabian StylePanov, Maxim S., Alexey P. Zakharov, Evgenia M. Khairullina, Ilya I. Tumkin, Andrey S. Mereshchenko, Dmitrii M. Nikolaev, Andrey V. Vasin, and Mikhail N. Ryazantsev. 2022. "Au–Ru Composite for Enzyme-Free Epinephrine Sensing" Chemosensors 10, no. 12: 513. https://doi.org/10.3390/chemosensors10120513
APA StylePanov, M. S., Zakharov, A. P., Khairullina, E. M., Tumkin, I. I., Mereshchenko, A. S., Nikolaev, D. M., Vasin, A. V., & Ryazantsev, M. N. (2022). Au–Ru Composite for Enzyme-Free Epinephrine Sensing. Chemosensors, 10(12), 513. https://doi.org/10.3390/chemosensors10120513









